Targeted Physical Therapy Combined with Spasticity Management Changes Motor Development Trajectory for a 2-Year-Old with Cerebral Palsy
Abstract
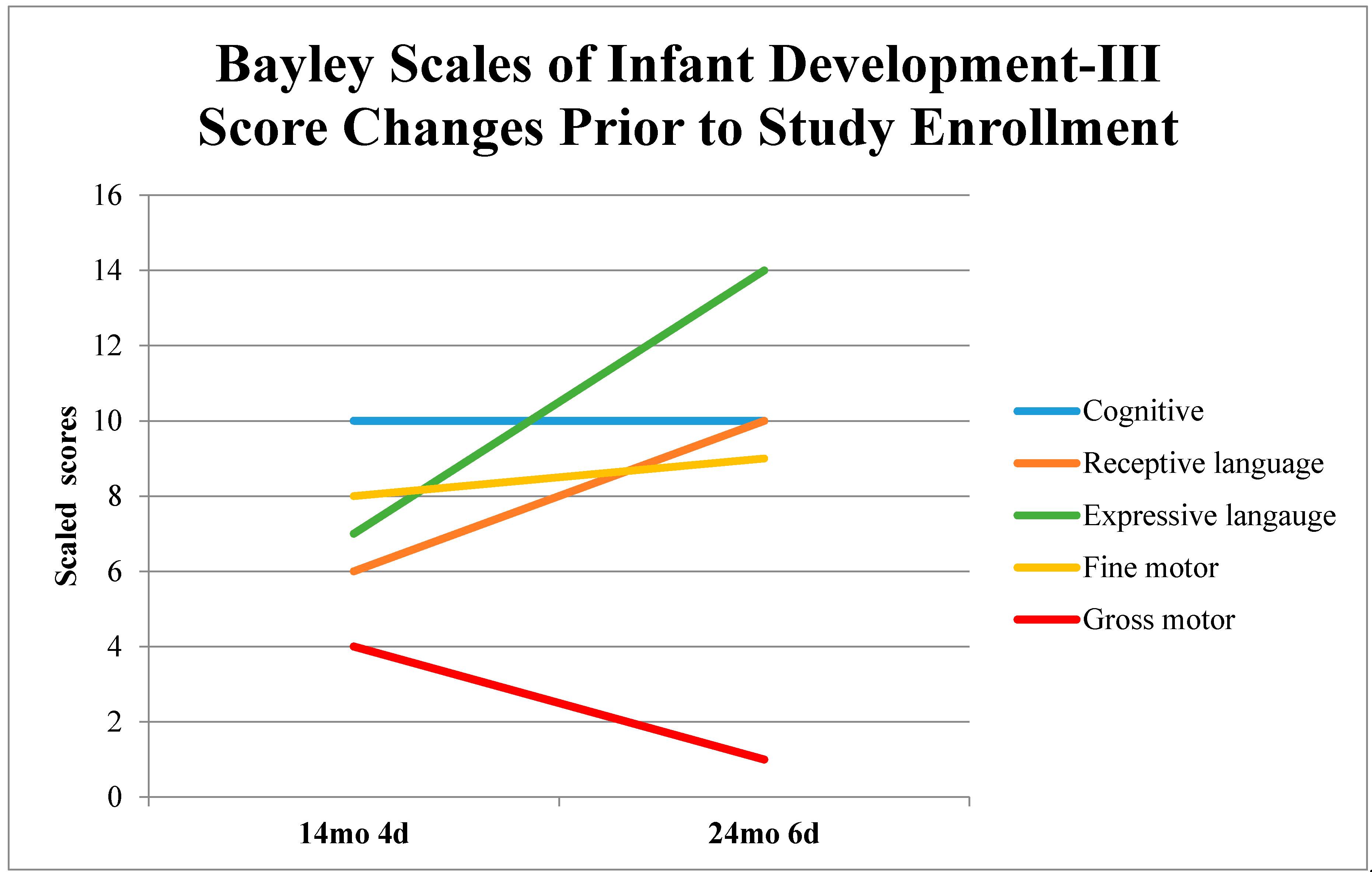
1. Introduction
2. Methods
2.1. Participant Description
2.2. Study Design and Measures
2.3. Interventions
2.3.1. Botulinum Toxin-A and Phenol
2.3.2. START-Play
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, C.; Darrah, J.; Gordon, A.M.; Harbourne, R.; Spittle, A.; Johnson, R.; Fetters, L. Effectiveness of motor interventions in infants with cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2016, 58, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Karch, D.; Heinemann, K. Physiotherapeutic Interventions: Bobath, Vojta, and Motor Learning Approaches. In Cerebral Palsy; Springer: Cham, Switzerland, 2018; pp. 155–164. [Google Scholar]
- Lobo, M.A.; Harbourne, R.T.; Dusing, S.C.; McCoy, S.W. Grounding Early Intervention: Physical Therapy Cannot Just Be About Motor Skills Anymore. Phys. Ther. 2013, 93, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Morgan, C.; Fahey, M.; Finch-Edmondson, M.; Galea, C.; Hines, A.; Langdon, K.; Mc Namara, M.; Paton, M.C.; Popat, H. State of the evidence traffic lights 2019: Systematic review of interventions for preventing and treating children with cerebral palsy. Curr. Neurol. Neurosci. Rep. 2020, 20, 1–21. [Google Scholar] [CrossRef]
- McManus, B.M.; Richardson, Z.; Schenkman, M.; Murphy, N.; Morrato, E.H. Timing and intensity of early intervention service use and outcomes among a safety-net population of children. JAMA Netw. Open 2019, 2, e187529. [Google Scholar] [CrossRef]
- Morgan, C.; Novak, I.; Badawi, N. Enriched Environments and Motor Outcomes in Cerebral Palsy: Systematic Review and Meta-analysis. Pediatrics (Evanston) 2013, 132, e735–e746. [Google Scholar] [CrossRef]
- Shonkoff, J.P.; Bales, S.N. Science does not speak for itself: Translating child development research for the public and its policymakers. Child Dev. 2011, 82, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Individuals with Disabilities Education Act Amendments of 1997. Available online: https://www2.ed.gov/policy/speced/leg/idea/idea.pdf (accessed on 20 February 2021).
- Woods, J.; Kashinath, S.; Goldstein, H. Effects of Embedding Caregiver-Implemented Teaching Strategies in Daily Routines on Children’s Communication Outcomes. J. Early Interv. 2004, 26, 175–193. [Google Scholar] [CrossRef]
- Dunst, C.J.; Hamby, D.; Trivette, C.M.; Raab, M.; Bruder, M.B. Everyday Family and Community Life and Children’s Naturally Occurring Learning Opportunities. J. Early Interv. 2000, 23, 151–164. [Google Scholar] [CrossRef]
- Hadders-Algra, M.; Boxum, A.G.; Hielkema, T.; Hamer, E.G. Effect of early intervention in infants at very high risk of cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2017, 59, 246–258. [Google Scholar] [CrossRef]
- Mahoney, G.; Robinson, C.; Perales, F. Early motor intervention: The need for new treatment paradigms. Infants Young Child. 2004, 17, 291–300. [Google Scholar] [CrossRef]
- Palisano, R.J. Research on the Effectiveness of Neurodevelopmental Treatment. Pediatric Phys. Ther. 1991, 3, 141–148. [Google Scholar] [CrossRef]
- Agarwal, A.; Verma, I. Cerebral palsy in children: An overview. J. Clin. Orthop. Trauma 2012, 3, 77–81. [Google Scholar] [CrossRef]
- Ward, R.; Reynolds, J.E.; Bear, N.; Elliott, C.; Valentine, J. What is the evidence for managing tone in young children with, or at risk of developing, cerebral palsy: A systematic review. Disabil. Rehabil. 2017, 39, 619–630. [Google Scholar] [CrossRef]
- Tedroff, K.; Löwing, K.; Haglund-Åkerlind, Y.; Gutierrez-Farewik, E.; Forssberg, H. Botulinumtoxin A treatment in toddlers with cerebral palsy. Acta Paediatr. 2010, 99, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Ploypetch, T.; Kwon, J.-Y.; Armstrong, H.F.; Kim, H. A Retrospective Review of Unintended Effects after Single-Event Multi-Level Chemoneurolysis With Botulinum Toxin-A and Phenol in Children with Cerebral Palsy. PM&R 2015, 7, 1073–1080. [Google Scholar] [CrossRef]
- Gooch, J.L.; Patton, C.P. Combining botulinum toxin and phenol to manage spasticity in children. Arch. Phys. Med. Rehabil. 2004, 85, 1121–1124. [Google Scholar] [CrossRef]
- Spira, R. Management of Spasticity in Cerebral Palsied Children by Peripheral Nerve Block with Phenol. Dev. Med. Child Neurol. 1971, 13, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.L.; Singh, U.; Dureja, G.P.; Singh, K.K.; Chaturvedi, S. Phenol block in the management of spastic cerebral palsy. Indian J. Pediatrics 1994, 61, 249–255. [Google Scholar] [CrossRef]
- Gonnade, N.; Lokhande, V.; Ajij, M.; Gaur, A.; Shukla, K. Phenol Versus Botulinum Toxin A Injection in Ambulatory Cerebral Palsy Spastic Diplegia: A Comparative Study. J. Pediatric Neurosci. 2017, 12, 338–343. [Google Scholar] [CrossRef]
- Baker, R.; Jasinski, M.; Maciag-Tymecka, I.; Michalowska-Mrozek, J.; Bonikowski, M.; Carr, L.; MacLean, J.; Lin, J.; Lynch, B.; Theologis, T. Botulinum toxin treatment of spasticity in diplegic cerebral palsy: A randomized, double-blind, placebo-controlled, dose-ranging study. Dev. Med. Child Neurol. 2002, 44, 666–675. [Google Scholar] [CrossRef]
- Love, S.; Novak, I.; Kentish, M.; Desloovere, K.; Heinen, F.; Molenaers, G.; O’flaherty, S.; Graham, H. Botulinum toxin assessment, intervention and after-care for lower limb spasticity in children with cerebral palsy: International consensus statement. Eur. J. Neurol. 2010, 17, 9–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Shin, H.I.; Kwon, B.S.; Kim, S.J.; Jung, I.Y.; Bang, M.S. Neuronox versus BOTOX for spastic equinus gait in children with cerebral palsy: A randomized, double-blinded, controlled multicentre clinical trial. Dev. Med. Child Neurol. 2011, 53, 239–244. [Google Scholar] [CrossRef]
- Galli, M.; Crivellini, M.; Santambrogio, G.C.; Fazzi, E.; Motta, F. Short-term effects of “botulinum toxin a” as treatment for children with cerebral palsy: Kinematic and kinetic aspects at the ankle joint. Funct. Neurol. 2001, 16, 317–323. [Google Scholar] [PubMed]
- Park, E.S.; Park, C.I.; Kim, D.Y.; Kim, Y.R. The effect of spasticity on cortical somatosensory-evoked potentials: Changes of cortical somatosensory-evoked potentials after botulinum toxin type A injection. Arch. Phys. Med. Rehabil. 2002, 83, 1592–1596. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Heinen, F.; Schenkel, A.; Doll, B.; Ebersbach, G.; Müller, J.; Poewe, W. Botulinum Toxin A in the Management of Spastic Gait Disorders in Children and Young Adults with Cerebral Palsy: A Randomized, Double-Blind Study of. Neuropediatrics 1999, 30, 120–124. [Google Scholar] [CrossRef]
- Koman, L.A.; Mooney, J.F., 3rd; Smith, B.P.; Walker, F.; Leon, J.M. Botulinum toxin type A neuromuscular blockade in the treatment of lower extremity spasticity in cerebral palsy: A randomized, double-blind, placebo-controlled trial. BOTOX Study Group. J. Pediatric Orthop. 2000, 20, 108–115. [Google Scholar] [CrossRef]
- Bourseul, J.S.; Molina, A.; Lintanf, M.; Houx, L.; Chaléat-Valayer, E.; Pons, C.; Brochard, S. Early Botulinum Toxin Injections in Infants with Musculoskeletal Disorders: A Systematic Review of Safety and Effectiveness. Arch. Phys. Med. Rehabil. 2018, 99, 1160–1176.e1165. [Google Scholar] [CrossRef] [PubMed]
- Harbourne, R.T.; Dusing, S.C.; Lobo, M.A.; McCoy, S.W.; Koziol, N.A.; Hsu, L.-Y.; Willett, S.; Marcinowski, E.C.; Babik, I.; Cunha, A.B.; et al. START-Play Physical Therapy Intervention Impacts Motor and Cognitive Outcomes in Infants With Neuromotor Disorders: A Multisite Randomized Clinical Trial. Phys. Ther. 2020. [Google Scholar] [CrossRef]
- Smith, L.B.; Thelen, E. Development as a dynamic system. Trends Cogn. Sci. 2003, 7, 343–348. [Google Scholar] [CrossRef]
- Spencer, J.P.; Perone, S.; Buss, A.T. Twenty years and going strong: A dynamic systems revolution in motor and cognitive development. Child Dev. Perspect. 2011, 5, 260–266. [Google Scholar] [CrossRef]
- Gibson, E.J.; Pick, A.D. An Ecological Approach to Perceptual Learning and Development; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Rahlin, M.; Barnett, J.; Becker, E.; Fregosi, C.M. Development through the Lens of a Perception-Action-Cognition Connection: Recognizing the Need for a Paradigm Shift in Clinical Reasoning. Phys. Ther. 2019, 99, 748–760. [Google Scholar] [CrossRef]
- Harbourne, R.T.; Berger, S.E. Embodied Cognition in Practice: Exploring Effects of a Motor-Based Problem-Solving Intervention. Phys. Ther. 2019, 99, 786–796. [Google Scholar] [CrossRef]
- Laakso, A. Embodiment and development in cognitive science. Cogn. Brain Behav. 2011, 15. [Google Scholar] [CrossRef]
- Bayley, N. Bayley scales of infant and toddler development, 3rd ed.; Harcourt Assessment: San Antonio, TX, USA, 2006. [Google Scholar]
- Campos, J.J.; Anderson, D.I.; Barbu-Roth, M.A.; Hubbard, E.M.; Hertenstein, M.J.; Witherington, D. Travel broadens the mind. Infancy 2000, 1, 149–219. [Google Scholar] [CrossRef] [PubMed]
- Harbourne, R.T.; Dusing, S.C.; Lobo, M.A.; Westcott-McCoy, S.; Bovaird, J.; Sheridan, S.; Galloway, J.C.; Chang, H.-J.; Hsu, L.-Y.; Koziol, N. Sitting together and reaching to play (START-play): Protocol for a multisite randomized controlled efficacy trial on intervention for infants with Neuromotor disorders. Phys. Ther. 2018, 98, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Hadders-Algra, M. Variation and Variability: Key Words in Human Motor Development. Phys. Ther. 2010, 90, 1823–1837. [Google Scholar] [CrossRef]
- Eames, N.W.; Baker, R.; Hill, N.; Graham, K.; Taylor, T.; Cosgrove, A. The effect of botulinum toxin A on gastrocnemius length: Magnitude and duration of response. Dev. Med. Child Neurol. 2007, 41, 226–232. [Google Scholar] [CrossRef]
- Romeiser-Logan, L.; Slaughter, R.; Hickman, R. Single-subject research designs in pediatric rehabilitation: A valuable step towards knowledge translation. Dev. Med. Child Neurol. 2017, 59, 574–580. [Google Scholar] [CrossRef]
- Beckers, L.W.M.E.; Stal, R.A.; Smeets, R.J.E.M.; Onghena, P.; Bastiaenen, C.H.G. Single-case Design Studies in Children with Cerebral Palsy: A Scoping Review. Dev. Neurorehabilit. 2020, 23, 73–105. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.-C. Early, accurate diagnosis and early intervention in cerebral palsy: Advances in diagnosis and treatment. JAMA Pediatrics 2017, 171, 897–907. [Google Scholar] [CrossRef]
- Palisano, R.J. GMFCS-E & R Gross Motor Function Classification System: Expanded and Revised; Canchild Centre for Childhood Disability Research: Hamilton, ON, Canada, 2007. [Google Scholar]
- Nourbakhsh, M.R.; Ottenbacher, K.J. The statistical analysis of single-subject data: A comparative examination. Phys. Ther. 1994, 74, 768–776. [Google Scholar] [CrossRef]
- Molinini, R.M.; Koziol, N.A.; Tripathi, T.; Harbourne, R.T.; McCoy, S.W.; Lobo, M.A.; Bovaird, J.; Dusing, S.C. Measuring Early Problem-Solving in Young Children with Motor Delays: A Validation Study. Phys. Occup. Ther. Pediatrics 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.J.; Rosenbaum, P.L.; Wright, M.; Avery, L.M. Clinics in Developmental Medicine. In Gross Motor Function Measure (GMFM-66 & GMFM-88), User’s Manual, 2nd ed.; Mac Keith Press: Plymouth, UK, 2013; p. 290. [Google Scholar] [CrossRef]
- Russell, D.J.; Avery, L.M.; Rosenbaum, P.L.; Raina, P.S.; Walter, S.D.; Palisano, R.J. Improved Scaling of the Gross Motor Function Measure for Children with Cerebral Palsy: Evidence of Reliability and Validity. Phys. Ther. 2000, 80, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Dusing, S.C.; Tripathi, T.; Marcinowski, E.C.; Thacker, L.R.; Brown, L.F.; Hendricks-Muñoz, K.D. Supporting play exploration and early developmental intervention versus usual care to enhance development outcomes during the transition from the neonatal intensive care unit to home: A pilot randomized controlled trial. BMC Pediatrics 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The coefficient of determination R 2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef]
- Harb, A.; Kishner, S. Modified Ashworth Scale. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554572/?report=printable (accessed on 23 January 2021).
- Bakheit, A.; Badwan, D.; McLellan, D. The effectiveness of chemical neurolysis in the treatment of lower limb muscle spasticity. Clin. Rehabil. 1996, 10, 40–43. [Google Scholar] [CrossRef]
- Karri, J.; Mas, M.F.; Francisco, G.E.; Li, S. Practice patterns for spasticity management with phenol neurolysis. J. Rehabil. Med. 2017, 49, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Gottman, J.M.; Leiblum, S.R. How to Do Psychotherapy and How to Evaluate It: A Manual for Beginners; Holt, Rinehart & Winston: New York, NY, USA, 1974. [Google Scholar] [CrossRef]
- Oeffinger, D.; Bagley, A.; Rogers, S.; Gorton, G.; Kryscio, R.; Abel, M.; Damiano, D.; Barnes, D.; Tylkowski, C. Outcome tools used for ambulatory children with cerebral palsy: Responsiveness and minimum clinically important differences. Dev. Med. Child Neurol. 2008, 50, 918–925. [Google Scholar] [CrossRef]
- Rosenbaum, D.A.; Carlson, R.A.; Gilmore, R.O. Acquisition of intellectual and perceptual-motor skills. Annu. Rev. Psychol. 2001, 52, 453–470. [Google Scholar] [CrossRef]
- Bruder, M.B.; Catalino, T.; Chiarello, L.A.; Mitchell, M.C.; Deppe, J.; Gundler, D.; Kemp, P.; LeMoine, S.; Long, T.; Muhlenhaupt, M. Finding a common lens: Competencies across professional disciplines providing early childhood intervention. Infants Young Child. 2019, 32, 280–293. [Google Scholar] [CrossRef]
- Morgan, C.; Novak, I.; Dale, R.C.; Badawi, N. Optimising motor learning in infants at high risk of cerebral palsy: A pilot study. BMC Pediatrics 2015, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Dusing, S.; Burnsed, J.; Brown, S.; Harper, A.; Hendricks-Munoz, K.; Stevenson, R.; Thacker, L.; Molinini, R.; Kane, A.; Khurana, S. Efficacy of Supporting Play Exploration and Early Development Intervention (SPEEDI) in the First Months of Life for Infants Born Very Preterm: 3-Arm Randomized Clinical Trial Protocol. Phys. Ther. 2020, 100, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B.; Muhammad, A. Harnessing the power of neuroplasticity for intervention. Front. Hum. Neurosci. 2014, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, K.; Sanders, M.R.; McKinlay, L.; Boyd, R.N. Parenting intervention combined with acceptance and commitment therapy: A trial with families of children with cerebral palsy. J. Pediatric Psychol. 2016, 41, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.A.; Kagan, S.H.; Corrigan, J.D. Research Design Options for Intervention Studies. Pediatric Phys. Ther. 2017, 29, S57. [Google Scholar] [CrossRef]



| Phase | Datapoint | Chronological Age (mo) | GMFM-66 | APSP |
|---|---|---|---|---|
| A | 1 | 24.2 | 46.1 | X |
| 2 | 25.4 | 45.4 | 202 | |
| 3 | 25.6 | 46.6 | 161 | |
| 4 | 26 | 45.4 | 167 | |
| B | 5 | 26.5 | 53.3 | 123 |
| 6 | 27 | 49 | 168 | |
| 7 | 27.2 | 50 | X | |
| 8 | 27.5 | 47.5 | X | |
| 9 | 27.9 | 46.6 | 230 | |
| 10 | 28.1 | 48 | X | |
| COVID-19 paused visits | ||||
| Post AB | 11 | 33.5 | 52.2 | 229.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuyvenberg, C.L.; Brown, S.E.; Inamdar, K.; Evans, M.; Hsu, L.-y.; Rolin, O.; Harbourne, R.T.; Westcott McCoy, S.; Lobo, M.A.; Koziol, N.A.; et al. Targeted Physical Therapy Combined with Spasticity Management Changes Motor Development Trajectory for a 2-Year-Old with Cerebral Palsy. J. Pers. Med. 2021, 11, 163. https://doi.org/10.3390/jpm11030163
Stuyvenberg CL, Brown SE, Inamdar K, Evans M, Hsu L-y, Rolin O, Harbourne RT, Westcott McCoy S, Lobo MA, Koziol NA, et al. Targeted Physical Therapy Combined with Spasticity Management Changes Motor Development Trajectory for a 2-Year-Old with Cerebral Palsy. Journal of Personalized Medicine. 2021; 11(3):163. https://doi.org/10.3390/jpm11030163
Chicago/Turabian StyleStuyvenberg, Corri L., Shaaron E. Brown, Ketaki Inamdar, Megan Evans, Lin-ya Hsu, Olivier Rolin, Regina T. Harbourne, Sarah Westcott McCoy, Michele A. Lobo, Natalie A. Koziol, and et al. 2021. "Targeted Physical Therapy Combined with Spasticity Management Changes Motor Development Trajectory for a 2-Year-Old with Cerebral Palsy" Journal of Personalized Medicine 11, no. 3: 163. https://doi.org/10.3390/jpm11030163
APA StyleStuyvenberg, C. L., Brown, S. E., Inamdar, K., Evans, M., Hsu, L.-y., Rolin, O., Harbourne, R. T., Westcott McCoy, S., Lobo, M. A., Koziol, N. A., & Dusing, S. C. (2021). Targeted Physical Therapy Combined with Spasticity Management Changes Motor Development Trajectory for a 2-Year-Old with Cerebral Palsy. Journal of Personalized Medicine, 11(3), 163. https://doi.org/10.3390/jpm11030163








