Sentinel Node Biopsy after Neoadjuvant Chemotherapy for Breast Cancer: Preliminary Experience with Clinically Node Negative Patients after Systemic Treatment
Abstract
1. Introduction
2. Materials and Methods
- “Overall Survival”: time from day of surgery to death from any cause or latest follow-up.
- “Distant Disease Free Survival”: time from day of surgery to distant recurrence.
- “Regional Disease Free Survival”: time from day of surgery to ipsilateral breast and/or axillary recurrence.
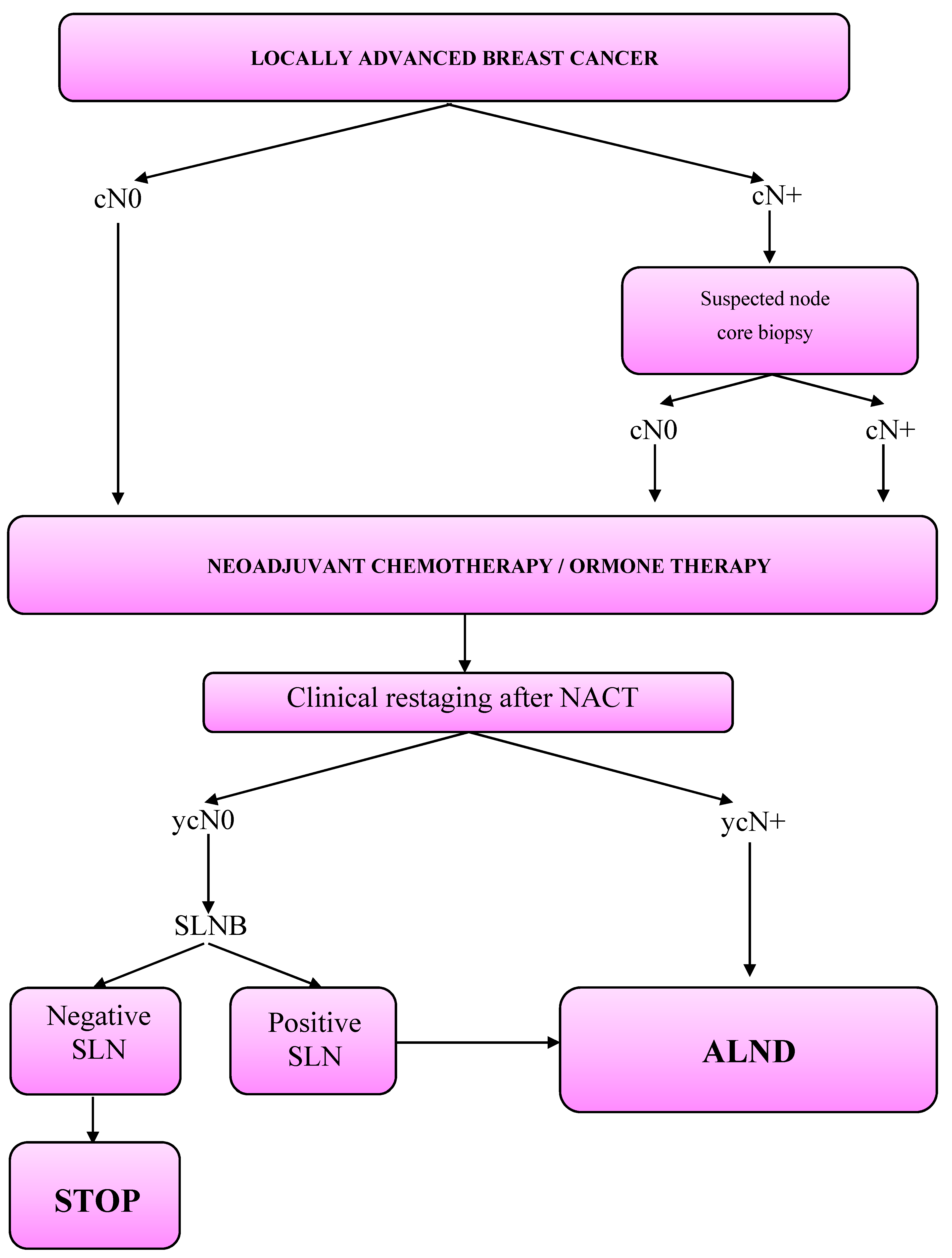
3. Clinical Workout
- Patients with locally advanced breast cancer;
- Patients with operable breast cancer and an unfavorable breast volume/tumor size ratio, in order to reduce the tumor diameter and achieve a conservative treatment instead of mastectomy;
- Patients with operable breast cancer and clinically involved lymph nodes (cN+), with the aim of ensuring a SLNB instead of a direct ALND;
- Young patients with unfavorable risk factors (triple negative tumor, Human Epidermal growth factor—2: HER2+, high Ki-67 rates), to provide prompt systemic treatment.
- HER2 negative patients:
- -
- Sequential scheme: Anthracyclines plus Cyclophosphamide on day 1 every 21 days for 4 cycles (4 AC); followed by docetaxel on day 1 every 21 days for 4 cycles or paclitaxel on day 1 every week for 12 cycles.
- -
- 6 TAC: docetaxel plus Doxorubicin plus Cyclophosphamide on day 1 every 21 days for 6 cycles.
- HER2 positive patients:
- -
- 6 TCH: docetaxel plus Carboplatin plus Herceptin on day 1 every 21 days for 6 cycles.
- -
- Sequential scheme: Anthracyclines plus Cyclophosphamide on day 1 every 21 days for 4 cycles (4 AC); followed by docetaxel on day 1 every 21 days for 4 cycles or paclitaxel on day 1 every week for 12 cycles plus Herceptin on day 1 every 21 days for 18 cycles.
- Level I oncoplastic breast surgery techniques—for resection of <20% of breast volume (peri-areolar, axillary or inframammary fold incisions).
- Level II oncoplastic surgery which involves resection of >20% of breast volume (round block, batwing and reduction mammoplasty techniques) [14].
- “Nipple Sparing Mastectomy” (NSM—removal of all the breast glandular tissue, while the nipple and areola are left in place along with breast skin) if tumor did not involve the nipple or tissue under the areola.
- “Skin Sparing Mastectomy” (removal of breast glandular tissue, nipple and areola while breast skin is kept intact) if tumor involved the nipple–areola complex.
- Simple mastectomy (removal of breast glandular tissue, nipple, areola and breast skin) if tumor involved breast skin.
- Anthracyclines and/or Taxanes were given to patients who did not receive them in the neoadjuvant regimen.
- Triple negative patients were given Capecitabine;
- HER2 positive cancers were treated with Trastuzumab emtansine (TDM-1).
- Cancers expressing hormone receptors (estrogen receptor, progesterone receptor) were treated with selective estrogen receptor modulators (Tamoxifen) or Luteinizing Hormone Release Hormone analogues (Enantone, Decapeptyl) if in premenopausal age while postmenopausal patients were given aromatase inhibitors (Anastrozole, Letrozole, Exemestane).
4. Statistical Analysis
5. Results
- Overall survival: During the entire follow-up, we reported the death of 15 (3.8%) women: two in the SN-negative group (OS 97.4%) and 13 in the SN-positive group (OS 82.7%)—p < 0.0001. Death was attributed to breast cancer in 92.5% of cases. Three-year OS was 94.3% overall, 95.5% in those initially cN0 and 93% in those initially cN1/N2.
- Distant disease free survival: Overall 36 (9%) patients developed distant metastases (DDFS 83.8%). According to SN-status we report six patients with distant metastasis in SN-negative group (DDFS 95.7%) and 30 patients in the SN-positive group (DDFS 67.9%)—p < 0.0001. Three-year DDFS was 92.2% in those initially cN0 and 84.8% in those initially cN1/2.
- Regional Disease Free survival: Overall, 24 patients developed a regional recurrence (RFS 89.4%): eight (2%) women had ipsilateral breast cancer recurrence, two (0.5%) had contralateral breast cancer, and 10 (2.5%) patients developed axillary recurrence. In four (1%) patients we diagnosed a synchronous recurrence in breast and axilla. RDFS was 96.5 % in patients with negative SLNB and 91.3% in those with positive SLNB (p = 0.007). Three-year RDFS was 94.2% in those initially cN0 and 87.9% in those initially cN1/2.
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franceschini, G.; Sanchez, A.M.; Di Leone, A.; Magno, S.; Moschella, F.; Accetta, C.; Masetti, R. Update on the surgical management of breast cancer. Ann. Ital. Chir. 2015, 86, 89–99. [Google Scholar]
- Classe, J.-M.; Loaec, C.; Alran, S.; Paillocher, N.; Tunon-Lara, C.; Gimbergues, P.; Faure-Virelizier, C.; Chauvet, M.-P.; Lasry, S.; Dupre, P.-F.; et al. Sentinel node detection after neoadjuvant chemotherapy in patient without previous axillary node involvement (GANEA 2 trial): Follow-up of a prospective multi-institutional cohort. SABCS 2016. [Google Scholar] [CrossRef]
- Currey, A.; Patten, C.R.; Bergom, C.; Wilson, J.F.; Kong, A.L. Management of the axilla after neo-adjuvant chemotherapy for breast cancer: Sentinel node biopsy and radiotherapy considerations. Breast J. 2018, 24, 902–910. [Google Scholar] [CrossRef]
- Shirzadi, A.; Mahmoodzadeh, H.; Qorbani, M. Assessment of sentinel lymph node biopsy after neoadjuvant chemotherapy for breast cancer in two subgroups: Initially node negative and node positive converted to node negative—A systemic review and meta-analysis. J. Res. Med. Sci. 2019, 24. [Google Scholar] [CrossRef]
- Classe, J.M.; Bordes, V.; Campion, L.; Mignotte, H.; Dravet, F.; Leveque, J.; Sagan, C.; Dupre, P.F.; Body, G.; Giard, S. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: Results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J. Clin. Oncol. 2009, 27, 726–732. [Google Scholar] [CrossRef]
- Hunt, K.K.; Yi, M.; Mittendorf, E.A.; Guerrero, C.; Babiera, G.V.; Bedrosian, I.; Hwang, R.F.; Kuerer, H.M.; Ross, M.I.; Meric-Bernstam, F. Sentinel Lymph Node Surgery After Neoadjuvant Chemotherapy is Accurate and Reduces the Need for Axillary Dissection in Breast Cancer Patients. Ann. Surg. 2009, 250, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Tan, V.K.M.; Goh, B.K.P.; Fook-Cong, S.; Kin, L.W.; Wong, W.-K.; Yong, W.S. The feasibility and Accuracy of Sentinel Lymph Node Biopsy in Clinically Node-Negative Patients after Neoadjuvant Chemotherapy for Breast Cancer—A Systematic Review and Meta-Analysis. J. Surg. Oncol. 2011, 104, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Pilewskie, M.; Morrow, M. Axillary Nodal Management Following Neoadjuvant Chemotherapy. JAMA Oncol. 2017, 3, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, M.; Franceschini, G.; Douek, M. New techniques for sentinel node biopsy in breast cancer. Transl. Cancer Res. 2018, 7, S405–S417. [Google Scholar] [CrossRef]
- Franceschini, G. Sentinel node biopsy after neoadjuvant chemotherapy for breast cancer in patients with pre-treatment node-positive: Recommendations to optimize the performance. Eur. J. Surg. Oncol. 2020, 46, 216–217. [Google Scholar] [CrossRef]
- El Hage, C.; Headon, H.; El Tokhy, O.; Heeney, J.; Kasem, A.; Mokbel, K. Is sentinel lymph node biopsy a viable alternative to complete axillary dissection following neoadjuvant chemotherapy in women with node-positive breast cancer at diagnosis? An updated meta-analysis involving 3,398 patients. Am. J. Surg. 2016, 212, 969–981. [Google Scholar] [CrossRef]
- Kantor, O.; James, T.A. ASO Author Reflections: Improving Patient Selection for Sentinel Lymph Node Biopsy After Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2018, 25, 640–641. [Google Scholar] [CrossRef]
- Galimberti, V.; Fontana, S.K.R.; Maisonneuve, P.; Steccanella, F.; Vento, A.R.; Intra, M.; Naninato, P.; Caldarella, P.; Iorfida, M.; Colleoni, M.; et al. Sentinel node biopsy after neoadjuvant treatment in breast cancer: Five-year follow-up of patients with clinically node-negative or node-positive disease before treatment. Eur. J. Surg. Oncol. 2016, 42, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Franceschini, G.; D’Archi, S.; De Lauretis, F.; Scardina, L.; Di Giorgio, D.; Accetta, C.; Masetti, R. Results obtained with level II oncoplastic surgery spanning 20 years of breast cancer treatment: Do we really need further demonstration of reliability? Breast J. 2019, 26, 125–132. [Google Scholar] [CrossRef]
- Franceschini, G.; Di Leone, A.; Natale, M.; Sanchez, A.M.; Masetti, R. Conservative surgery after neoadjuvant chemotherapy in patients with operable breast cancer. Ann. Ital. Chir. 2018, 89, 290. [Google Scholar] [PubMed]
- Sanchez, A.M.; Franceschini, G.; Orlandi, A.; Di Leone, A.; Masetti, R. New challenges in multimodal workout of locally advanced breast cancer. Surgeon 2017, 15, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; Von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A. Alliance for Clinical Trials in Oncology. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef]
- Simons, J.M.; van Nijnatten, T.J.A.; van der Pol, C.C.; Luiten, E.J.T.; Koppert, L.B.; Smidt, M.L. Diagnostic Accuracy of Different Surgical Procedures for Axillary Staging After Neoadjuvant Systemic Therapy in Node-positive Breast Cancer: A Systematic Review and Meta-analysis. Ann. Surg. 2019, 269, 432–442. [Google Scholar] [CrossRef]
- Geng, C.; Chen, X.; Pan, X.; Li, J. The Feasibility and Accuracy of Sentinel Lymph Node Biopsy in Initially Clinically Node-Negative Breast Cancer after Neoadjuvant Chemotherapy: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0162605. [Google Scholar] [CrossRef]
- Tee, S.R.; Devane, L.A.; Evoy, D.; Rothwell, J.; Geraghty, J.; Prichard, R.S.; McDermott, E.W. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br. J. Surg. 2018, 105, 1541–1552. [Google Scholar] [CrossRef]
- Fringuelli, F.M.; Lima, G.; Bottini, A.; Aguggini, S.; Allevia, G.; Bonardi, S. Lymphoscintigraphy: The experience of the Cremona breast unit. Internet communication. 2012. [Google Scholar]
- Zhang, G.-C.; Liao, N.; Guo, Z.-B.; Qian, X.-K.; Ren, C.-Y.; Yao, M.; Li, X.-R.; Wang, K.; Zu, J. Accuracy and axilla sparing potentials of sentinel lymph node biopsy with methylene blue alone performed before versus after neoadjuvant chemotherapy in breast cancer: A single institution experience. Clin. Transl. Oncol. 2012, 15, 79–84. [Google Scholar] [CrossRef]

| cN0 | cN1/2 | |
|---|---|---|
| All | 219 (54.8%) | 180 (45.2%) |
| Age (years) | ||
| <35 | 21 (9.6%) | 12 (6.7%) |
| 35–49 | 105 (47.9%) | 89 (49.4%) |
| 50–69 | 80 (36.5%) | 70 (38.9%) |
| >70 | 13 (5.9%) | 9 (5%) |
| Breast Related Cancer Antigens (BRCA) mutations | 29 (13.2%) | 16 (47.2%) |
| Menopausal status | 103 (47%) | 85 (47.2%) |
| Grading | ||
| G1 | 3 (1.4%) | 2 (1.1%) |
| G2 | 78 (35.6%) | 66 (36.7%) |
| G3 | 118 (53.9%) | 96 (53.3%) |
| Unknown | 20 (9.1%) | 16 (8.9%) |
| Tumor subtype | ||
| Luminal A | 8 (3.7%) | 3 (1.7%) |
| Luminal B | 152 (69.4%) | 133 (73.9%) |
| HER 2 positive | 17 (7.8%) | 16 (8.9%) |
| Triple negative | 42 (19.2%) | 28 (15.6%) |
| Clinical T | ||
| cT1 | 28 (12.8%) | 27 (15%) |
| cT2 | 146 (66.7%) | 105 (58.3%) |
| cT3 | 29 (13.2%) | 33 (18.3%) |
| cT4 | 16 (7.3%) | 15 (8.3%) |
| Multifocality/multicentricity | 91 (41.6%) | 92 (51.1%) |
| cN0 | cN1/2 | |
|---|---|---|
| All | 219 (54.8%) | 180 (45.2%) |
| Neoadjuvant treatment | ||
| Hormone Therapy | 23 (10.5%) | 1 (0.6%) |
| Chemotherapy | 196 (89.5%) | 179 (99.4%) |
| Neoadjuvant chemotherapy | ||
| Anthracycline and/or Taxane | 5 (2.6%) | 4 (2.2%) |
| Anthracycline + Taxane | 159 (81%) | 141 (78.8%) |
| Other | 32 (16.4%) | 34 (19%) |
| Herceptin containing regimen | 64 (29.2%) | 59 (32.8%) |
| Clinical response | ||
| Complete response | 77 (35.2%) | 67 (37.2%) |
| Partial response | 125 (57%) | 103 (57.3%) |
| No response | 8 (3.7%) | 4 (2.2%) |
| Progression | 9 (4.1%) | 6 (3.3%) |
| cN0 | cN1/2 | |
|---|---|---|
| All | 219 (54.8%) | 180 (45.2%) |
| Surgery | ||
| Conservative surgery | 142 (64.8%) | 104 (57.8%) |
| Conservative mastectomy | 68 (31.1%) | 66 (36.7%) |
| Simple mastectomy | 9 (4.1%) | 10 (5.6%) |
| RT after conservative surgery | ||
| No treatment * | 6 (4.2%) | 3 (2.9%) |
| Radiotherapy | 136 (95.8%) | 101 (97.1%) |
| RT after mastectomy | ||
| No treatment | 48 (62.3%) | 16 (21.1%) |
| Radiotherapy | 29 (37.7%) | 60 (78.9%) |
| cN0 | cN1/2 | |
|---|---|---|
| All | 219 (54.8%) | 180 (45.2%) |
| ypT | ||
| ypT0 | 66 (30.2%) | 66 (36.7%) |
| ypTmic | 20 (9.1%) | 24 (13.3%) |
| ypT1 | 92 (42%) | 64 (35.5%) |
| ypT2 | 36 (16.4%) | 21 (11.7%) |
| ypT3 | 3 (1.4%) | 3 (1.7%) |
| ypT4 | 2 (0.9%) | 2 (1.1%) |
| Multifocality/multicentricity | 55 (25.1%) | 44 (24.4%) |
| ypN | ||
| ypN0 | 149 (68%) | 86 (47.8%) |
| ypNi+ * | 13 (5.9%) | 11 (6.1%) |
| ypNmic ** | 18 (8.2%) | 10 (5.6%) |
| ypN1 | 34 (15.5%) | 55 (30.6%) |
| ypN2 | 5 (2.3%) | 17 (9.4%) |
| ypN3 | 0 (0%) | 1 (0.6%) |
| ER | ||
| Positive | 112 (51.1%) | 80 (44.5%) |
| Negative | 30 (13.7%) | 17 (9.4%) |
| Not evaluable *** | 77 (35.2%) | 83 (46.1%) |
| PR | ||
| Positive | 83 (37.9%) | 50 (27.8%) |
| Negative | 59 (26.9%) | 47 (26.1%) |
| Not evaluable *** | 77 (35.2%) | 83 (46.1%) |
| Ki-67 | ||
| <24% | 94 (42.9%) | 64 (35.6%) |
| ≥25% | 48 (21.9%) | 33 (18.3%) |
| Not evaluable *** | 77 (35.2%) | 83 (46.1%) |
| Tumor subtype | ||
| Luminal A | 46 (21%) | 29 (16.1%) |
| Luminal B | 70 (32%) | 51 (28.4%) |
| HER2 | 4 (1.8%) | 6 (3.3%) |
| Triple negative | 22 (10%) | 11 (6.1%) |
| Not evaluable *** | 77 (35.2%) | 83 (46.1%) |
| All Patients | cN0 | cN1/2 | ||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |
| Clinical Characteristics | ||||||
| Menopausal status | 0.804 | / | 0.861 | / | 0.139 | / |
| BRCA1/2 mutation | 0.430 | / | 0.460 | / | 0.996 | / |
| Multifocality ad the diagnosis | 0.288 | / | 0.430 | / | 0.811 | / |
| Luminal HER2 + | 0.524 | / | 0.720 | / | 0.504 | / |
| Triple Negative | 1.231 (0.002) | 1.879 (0.0001) | 1.394 (0.023) | 2.606 (0.0001) | 1.668 (0.027) | 1.888 (0.002) |
| Pathological Characteristics | ||||||
| ypT2, ypT3, ypT4 | 0.873 (0.014) | 0.767 | 0.071 | / | 0.925 (0.040) | 0.417 |
| LS + (ypN+(sn)) | 2.048 (0.0001) | 1.977 (0.0001) | 2.502 (0.001) | 2.807 (0.001) | 1.540 (0.005) | 1.213 (0.045) |
| ypN2, ypN3 | 1.946 (0.0001) | 1.370 (0.003) | 2.759 (0.0001) | 2.157 (0.004) | 1.237 (0.0027) | 0.121 |
| pCR on T | −1.815 (0.003) | 0.331 | −3.704 (0.142) | / | −1.432 (0.020) | 0.457 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, A.M.; Terribile, D.; Franco, A.; Martullo, A.; Orlandi, A.; Magno, S.; Di Leone, A.; Moschella, F.; Natale, M.; D’Archi, S.; et al. Sentinel Node Biopsy after Neoadjuvant Chemotherapy for Breast Cancer: Preliminary Experience with Clinically Node Negative Patients after Systemic Treatment. J. Pers. Med. 2021, 11, 172. https://doi.org/10.3390/jpm11030172
Sanchez AM, Terribile D, Franco A, Martullo A, Orlandi A, Magno S, Di Leone A, Moschella F, Natale M, D’Archi S, et al. Sentinel Node Biopsy after Neoadjuvant Chemotherapy for Breast Cancer: Preliminary Experience with Clinically Node Negative Patients after Systemic Treatment. Journal of Personalized Medicine. 2021; 11(3):172. https://doi.org/10.3390/jpm11030172
Chicago/Turabian StyleSanchez, Alejandro Martin, Daniela Terribile, Antonio Franco, Annamaria Martullo, Armando Orlandi, Stefano Magno, Alba Di Leone, Francesca Moschella, Maria Natale, Sabatino D’Archi, and et al. 2021. "Sentinel Node Biopsy after Neoadjuvant Chemotherapy for Breast Cancer: Preliminary Experience with Clinically Node Negative Patients after Systemic Treatment" Journal of Personalized Medicine 11, no. 3: 172. https://doi.org/10.3390/jpm11030172
APA StyleSanchez, A. M., Terribile, D., Franco, A., Martullo, A., Orlandi, A., Magno, S., Di Leone, A., Moschella, F., Natale, M., D’Archi, S., Scardina, L., Mason, E. J., De Lauretis, F., Marazzi, F., Masetti, R., & Franceschini, G. (2021). Sentinel Node Biopsy after Neoadjuvant Chemotherapy for Breast Cancer: Preliminary Experience with Clinically Node Negative Patients after Systemic Treatment. Journal of Personalized Medicine, 11(3), 172. https://doi.org/10.3390/jpm11030172















