A Novel Precision Approach to Overcome the “Addiction Pandemic” by Incorporating Genetic Addiction Risk Severity (GARS) and Dopamine Homeostasis Restoration
Abstract
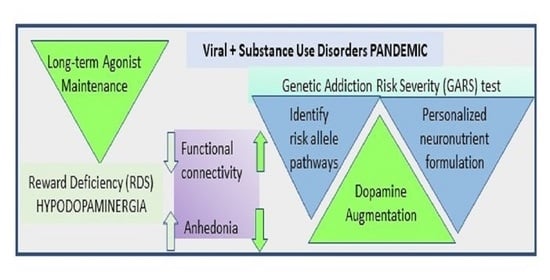
:1. Introduction
The Reward Deficiency Syndrome (RDS) Concept
2. Snapshot of Evidence: Biochemical and Genetic Dysfunctions That Are Evident in the Context of RDS
3. Reward Deficiency Syndrome (RDS); A Behavioral Octopus
4. The Cascade of Neurotransmission—The Blueprint
5. Measuring the Neurogenetics of Addiction Risk and Developing the GARS Test
6. Genetic Polymorphisms of RDS: Case-Control Studies, for Alcoholism
7. Genetic Vulnerability: Clinical Implications
8. Induction of Dopamine Homeostasis
9. Quantitative Electroencephalogramo (qEEG) Studies
10. Functional Magnetic Resonant Imaging (fMRI) Study Evidence for Dopamine Homeostasis
11. Biphasic Approach to Addiction Treatment
12. Limitations and the Future
13. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oesterle, T.S.; Thusius, N.J.; Rummans, T.A.; Gold, M.S. Medication-Assisted Treatment for Opioid-Use Disorder. Mayo Clin. Proc. 2019, 94, 2072–2086. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Baron, D.; McLaughlin, T.; Gold, M.S. Molecular neurological correlates of endorphinergic/dopaminergic mechanisms in reward circuitry linked to endorphinergic deficiency syndrome (EDS). J. Neurol. Sci. 2020, 411, 116733. [Google Scholar] [CrossRef] [Green Version]
- Patterson Silver Wolf, D.A.; Gold, M. Treatment resistant opioid use disorder (TROUD): Definition, rationale, and recommendations. J. Neurol. Sci. 2020, 411, 116718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, M.S.; Baron, D.; Bowirrat, A.; Blum, K. Neurological correlates of brain reward circuitry linked to opioid use disorder (OUD): Do homo sapiens acquire or have a reward deficiency syndrome? J. Neurol. Sci. 2020, 418, 117137. [Google Scholar] [CrossRef]
- Downs, B.W.; Blum, K.; Baron, D.; Bowirrat, A.; Lott, L.; Brewer, R.; Boyett, B.; Siwicki, D.; Roy, A.K.; Podesta, A.; et al. Death by Opioids: Are there non-addictive scientific solutions? J. Syst. Integr. Neurosci. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Lott, L.; Baron, D.; Smith, D.E.; Badgaiyan, R.D.; Gold, M.S. Improving naltrexone compliance and outcomes with putative pro- dopamine regulator KB220, compared to treatment as usual. J. Syst. Integr. Neurosci. 2020, 7. [Google Scholar] [CrossRef]
- Morgan, J.R.; Schackman, B.R.; Leff, J.A.; Linas, B.P.; Walley, A.Y. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J. Subst. Abus. Treat. 2018, 85, 90–96. [Google Scholar] [CrossRef]
- Ooteman, W.; Naassila, M.; Koeter, M.W.; Verheul, R.; Schippers, G.M.; Houchi, H.; Daoust, M.; van den Brink, W. Predicting the effect of naltrexone and acamprosate in alcohol-dependent patients using genetic indicators. Addict. Biol. 2009, 14, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Cowen, M.S.; Adams, C.; Kraehenbuehl, T.; Vengeliene, V.; Lawrence, A.J. The acute anti-craving effect of acamprosate in alcohol-preferring rats is associated with modulation of the mesolimbic dopamine system. Addict. Biol. 2005, 10, 233–242. [Google Scholar] [CrossRef]
- Gold, M.S.; Blum, K.; Febo, M.; Baron, D.; Modestino, E.J.; Elman, I.; Badgaiyan, R.D. Molecular role of dopamine in anhedonia linked to reward deficiency syndrome (RDS) and anti- reward systems. Front. Biosci. (Sch. Ed.) 2018, 10, 309–325. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Thanos, P.K.; Wang, G.J.; Febo, M.; Demetrovics, Z.; Modestino, E.J.; Braverman, E.R.; Baron, D.; Badgaiyan, R.D.; Gold, M.S. The Food and Drug Addiction Epidemic: Targeting Dopamine Homeostasis. Curr. Pharm. Des. 2018, 23, 6050–6061. [Google Scholar] [CrossRef]
- Blum, K.; Febo, M.; Fahlke, C.; Archer, T.; Berggren, U.; Demetrovics, Z.; Dushaj, K.; Badgaiyan, R.D. Hypothesizing Balancing Endorphinergic and Glutaminergic Systems to Treat and Prevent Relapse to Reward Deficiency Behaviors: Coupling D-Phenylalanine and N-Acetyl-L-Cysteine (NAC) as a Novel Therapeutic Modality. Clin. Med. Rev. Case Rep. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.K.; Polimanti, R.; Johnson, E.C.; McClintick, J.N.; Adams, M.J.; Adkins, A.E.; Aliev, F.; Bacanu, S.A.; Batzler, A.; Bertelsen, S.; et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat. Neurosci. 2018, 21, 1656–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotyuk, E.; Magi, A.; Eisinger, A.; Király, O.; Vereczkei, A.; Barta, C.; Griffiths, M.D.; Székely, A.; Kökönyei, G.; Farkas, J.; et al. Co-occurrences of substance use and other potentially addictive behaviors: Epidemiological results from the Psychological and Genetic Factors of the Addictive Behaviors (PGA) Study. J. Behav. Addict. 2020, 9, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Pasman, J.A.; Verweij, K.J.H.; Gerring, Z.; Stringer, S.; Sanchez-Roige, S.; Treur, J.L.; Abdellaoui, A.; Nivard, M.G.; Baselmans, B.M.L.; Ong, J.S.; et al. GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat. Neurosci. 2018, 21, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Stringer, S.; Frei, O.; Umićević Mirkov, M.; de Leeuw, C.; Polderman, T.J.C.; van der Sluis, S.; Andreassen, O.A.; Neale, B.M.; Posthuma, D. A global overview of pleiotropy and genetic architecture in complex traits. Nat. Genet. 2019, 51, 1339–1348. [Google Scholar] [CrossRef]
- Blum, K.; Modestino, E.J.; Gondre-Lewis, M.C.; Baron, D.; Steinberg, B.; Thanos, P.K.; Downs, B.W.; Lott, L.; Braverman, E.R.; Moran, M.; et al. Pro-Dopamine Regulator (KB220) A Fifty Year Sojourn to Combat Reward Deficiency Syndrome (RDS): Evidence Based Bibliography (Annotated). CPQ Neurol. Psychol. 2018, 1. Available online: https://www.cientperiodique.com/journal/fulltext/CPQNP/1/2/13 (accessed on 7 January 2021).
- Blum, K.; Liu, Y.; Wang, W.; Wang, Y.; Zhang, Y.; Oscar-Berman, M.; Smolen, A.; Febo, M.; Han, D.; Simpatico, T.; et al. rsfMRI effects of KB220Z on neural pathways in reward circuitry of abstinent genotyped heroin addicts. Postgrad. Med. 2015, 127, 232–241. [Google Scholar] [CrossRef]
- Febo, M.; Blum, K.; Badgaiyan, R.D.; Perez, P.D.; Colon-Perez, L.M.; Thanos, P.K.; Ferris, C.F.; Kulkarni, P.; Giordano, J.; Baron, D.; et al. Enhanced functional connectivity and volume between cognitive and reward centers of naive rodent brain produced by pro-dopaminergic agent KB220Z. PloS ONE 2017, 12, e0174774. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, B.; Blum, K.; McLaughlin, T.; Lubar, J.; Febo, M.; Braverman, E.R.; Badgaiyan, R.D. Low-Resolution Electromagnetic Tomography (LORETA) of changed Brain Function Provoked by Pro-Dopamine Regulator (KB220z) in one Adult ADHD case. Open J. Clin. Med. Case Rep. 2016, 2, 1121. [Google Scholar] [PubMed]
- Mullier, E.; Roine, T.; Griffa, A.; Xin, L.; Baumann, P.S.; Klauser, P.; Cleusix, M.; Jenni, R.; Alemàn-Gómez, Y.; Gruetter, R.; et al. N-Acetyl-Cysteine Supplementation Improves Functional Connectivity Within the Cingulate Cortex in Early Psychosis: A Pilot Study. Int. J. Neuropsychopharmacol. Off. Sci. J. Coll. Int. Neuropsychopharmacol. (Cinp) 2019, 22, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Solanki, N.; Abijo, T.; Galvao, C.; Darius, P.; Blum, K.; Gondré-Lewis, M.C. Administration of a putative pro-dopamine regulator, a neuronutrient, mitigates alcohol intake in alcohol-preferring rats. Behav. Brain Res. 2020, 385, 112563, PMCID:PMC7244251. [Google Scholar] [CrossRef] [PubMed]
- Blum, K. Reward Deficiency Syndrome. In The SAGE Encyclopedia of Abnormal and Clinical Psychology; Wenzel, A., Ed.; Sage Publications, Inc.: Oaks, CA, USA, 2017; p. 4. [Google Scholar]
- Blum, K.; Gondre-Lewis, M.; Steinberg, B.; Elman, I.; Baron, D.; Modestino, E.J.; Badgaiyan, R.D.; Gold, M.S. Our evolved unique pleasure circuit makes humans different from apes: Reconsideration of data derived from animal studies. J. Syst. Integr. Neurosci. 2018, 4. [Google Scholar] [CrossRef]
- Willuhn, I.; Burgeno, L.M.; Groblewski, P.A.; Phillips, P.E. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat. Neurosci. 2014, 17, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Modestino, E.J.; Gondre-Lewis, M.; Downs, B.W.; Baron, D.; Steinberg, B.; Siwicki, D.; Giordano, J.; McLaughlin, T.; Neary, J.; et al. “Dopamine homeostasis” requires balanced polypharmacy: Issue with destructive, powerful dopamine agents to combat America’s drug epidemic. J. Syst. Integr. Neurosci. 2017, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, K.; Noble, E.P.; Sheridan, P.J.; Montgomery, A.; Ritchie, T.; Jagadeeswaran, P.; Nogami, H.; Briggs, A.H.; Cohn, J.B. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA 1990, 263, 2055–2060. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Baron, D.; Lott, L.; Ponce, J.V.; Siwicki, D.; Boyett, B.; Steinberg, B.; Modestino, E.J.; Fried, L.; Hauser, M.; et al. In Search of Reward Deficiency Syndrome (RDS)-free Controls: The “Holy Grail” in Genetic Addiction Risk Testing. Curr. Psychopharmacol. 2020, 9, 7–21. [Google Scholar] [CrossRef]
- Fried, L.; Modestino, E.J.; Siwicki, D.; Lott, L.; Thanos, P.K.; Baron, D.; Badgaiyan, R.D.; Ponce, J.V.; Giordano, J.; Downs, B.W.; et al. Hypodopaminergia and “Precision Behavioral Management” (PBM): It is a Generational Family Affair. Curr. Pharm. Biotechnol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hillemacher, T.; Frieling, H.; Buchholz, V.; Hussein, R.; Bleich, S.; Meyer, C.; John, U.; Bischof, A.; Rumpf, H.J. Alterations in DNA-methylation of the dopamine-receptor 2 gene are associated with abstinence and health care utilization in individuals with a lifetime history of pathologic gambling. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2015, 63, 30–34. [Google Scholar] [CrossRef]
- Blum, K.; Lott, L.; Siwicki, D.; Fried, L.; Hauser, M.; Simpatico, T.; Baron, D.; Howeedy, A.; Badgaiyan, R.D. Genetic Addiction Risk Score (GARS(™)) as a Predictor of Substance Use Disorder: Identifying Predisposition Not Diagnosis. Curr. Trends Med. Diagn. Methods 2018, 1. [Google Scholar] [CrossRef]
- Blum, K.; Badgaiyan, R.D.; Agan, G.; Fratantonio, J.; Simpatico, T.; Febo, M.; Haberstick, B.C.; Smolen, A.; Gold, M.S. Molecular Genetic Testing in Reward Deficiency Syndrome (RDS): Facts and Fiction. J. Reward. Defic. Syndr. 2015, 1, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Gondré-Lewis, M.C.; Baron, D.; Thanos, P.K.; Braverman, E.R.; Neary, J.; Elman, I.; Badgaiyan, R.D. Introducing Precision Addiction Management of Reward Deficiency Syndrome, the Construct That Underpins All Addictive Behaviors. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Chen, A.L.; Chen, T.J.; Braverman, E.R.; Reinking, J.; Blum, S.H.; Cassel, K.; Downs, B.W.; Waite, R.L.; Williams, L.; et al. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): A commentary. Biol. Med. Model. 2008, 5, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boundy, V.A.; Lu, L.; Molinoff, P.B. Differential coupling of rat D2 dopamine receptor isoforms expressed in Spodoptera frugiperda insect cells. J. Pharmacol. Exp. Ther. 1996, 276, 784. [Google Scholar]
- Boundy, V.A.; Pacheco, M.A.; Guan, W.; Molinoff, P.B. Agonists and antagonists differentially regulate the high affinity state of the D2L receptor in human embryonic kidney 293 cells. Mol. Pharmacol. 1995, 48, 956–964. [Google Scholar] [PubMed]
- Blum, K.; Chen, T.H.; Downs, B.W.; Meshkin, B.; Blum, S.H.; Martinez Pons, M.; Mengucci, J.F.; Waite, R.L.; Arcuri, V.; Varshofsiky, M.; et al. Synaptamine (SG8839),™ An Amino-Acid Enkephalinase Inhibition Nutraceutical Improves Recovery of Alcoholics, A Subtype of Reward Deficiency Syndrome (RDS). Trends Appl. Sci. Res. 2007, 2, 132–138. [Google Scholar]
- Blum, K.; Febo, M.; Smith, D.E.; Roy, A.K., 3rd; Demetrovics, Z.; Cronjé, F.J.; Femino, J.; Agan, G.; Fratantonio, J.L.; Pandey, S.C.; et al. Neurogenetic and epigenetic correlates of adolescent predisposition to and risk for addictive behaviors as a function of prefrontal cortex dysregulation. J. Child. Adolesc Psychopharmacol. 2015, 25, 286–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.K.; Bowirrat, A.; Manka, M.; Miller, M.; Stokes, S.; Manka, D.; Allen, C.; Gant, C.; Downs, B.W.; Smolen, A.; et al. Acute intravenous synaptamine complex variant KB220 “normalizes” neurological dysregulation in patients during protracted abstinence from alcohol and opiates as observed using quantitative electroencephalographic and genetic analysis for reward polymorphisms: Part 1, pilot study with 2 case reports. Postgrad. Med. 2010, 122, 188–213. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Oscar-Berman, M.; Stuller, E.; Miller, D.; Giordano, J.; Morse, S.; McCormick, L.; Downs, W.B.; Waite, R.L.; Barh, D.; et al. Neurogenetics and Nutrigenomics of Neuro-Nutrient Therapy for Reward Deficiency Syndrome (RDS): Clinical Ramifications as a Function of Molecular Neurobiological Mechanisms. J. Addict. Res. 2012, 3, 139. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.W.; Parekh, P.K.; Kaplan, G.N.; Becker-Krail, D.D.; Williams, W.P., 3rd; Yamaguchi, S.; Yoshino, J.; Shelton, M.A.; Zhu, X.; Zhang, H.; et al. NAD+ cellular redox and SIRT1 regulate the diurnal rhythms of tyrosine hydroxylase and conditioned cocaine reward. Mol. Psychiatry 2019, 24, 1668–1684. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; William, M.; Chu, X.P. Nicotinamide phosphoribosyltransferase contributes to cocaine addiction through sirtuin 1. Int. J. Physiol. Pathophysiol. Pharm. 2019, 11, 318–320. [Google Scholar]
- Witt, E.A.; Reissner, K.J. The effects of nicotinamide on reinstatement to cocaine seeking in male and female Sprague Dawley rats. Psychopharmacology 2020, 237, 669–680. [Google Scholar] [CrossRef]
- Braidy, N.; Villalva, M.D.; van Eeden, S. Sobriety and Satiety: Is NAD+ the Answer? Antioxidants 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Cronholm, T.; Jones, A.W.; Skagerberg, S. Mechanism and regulation of ethanol elimination in humans: Intermolecular hydrogen transfer and oxidoreduction in vivo. Alcohol. Clin. Exp. Res. 1988, 12, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.J.; Norton, W.H.; Trümbach, D.; Meijer, A.H.; Ninkovic, J.; Topp, S.; Heck, D.; Marr, C.; Wurst, W.; Theis, F.J.; et al. Zebrafish reward mutants reveal novel transcripts mediating the behavioral effects of amphetamine. Genome Biol. 2009, 10, R81. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Erdos, J.J.; Terwilliger, R.; Duman, R.S.; Tallman, J.F. Regulation of G proteins by chronic morphine in the rat locus coeruleus. Brain Res. 1989, 476, 230–239. [Google Scholar] [CrossRef]
- O’Hollaren, P. Diphosphopyridine nucleotide in the prevention, diagnosis and treatment of drug addiction. A preliminary report. West J. Surg. Obs. Gynecol. 1961, 69, 213–215. [Google Scholar]
- Tampier, L.; Quintanilla, M.E.; Mardones, J. Effect of nicotinamide administration on ethanol consumption and on liver and brain acetaldehyde oxidation rate, by UChB rats. Addict. Biol. 1999, 4, 191–195. [Google Scholar] [CrossRef]
- Leventelis, C.; Goutzourelas, N.; Kortsinidou, A.; Spanidis, Y.; Toulia, G.; Kampitsi, A.; Tsitsimpikou, C.; Stagos, D.; Veskoukis, A.S.; Kouretas, D. Buprenorphine and Methadone as Opioid Maintenance Treatments for Heroin-Addicted Patients Induce Oxidative Stress in Blood. Oxidative Med. Cell. Longev. 2019, 2019, 9417048. [Google Scholar] [CrossRef] [PubMed]
- Salarian, A.; Kadkhodaee, M.; Zahmatkesh, M.; Seifi, B.; Bakhshi, E.; Akhondzadeh, S.; Adeli, S.; Askari, H.; Arbabi, M. Opioid Use Disorder Induces Oxidative Stress and Inflammation: The Attenuating Effect of Methadone Maintenance Treatment. Iran J. Psychiatry 2018, 13, 46–54. [Google Scholar]
- Famitafreshi, H.; Karimian, M. Socialization Alleviates Burden of Oxidative-Stress in Hippocampus and Prefrontal Cortex in Morphine Addiction Period in Male Rats. Curr. Mol. Pharm. 2018, 11, 254–259. [Google Scholar] [CrossRef]
- Zahmatkesh, M.; Kadkhodaee, M.; Salarian, A.; Seifi, B.; Adeli, S. Impact of opioids on oxidative status and related signaling pathways: An integrated view. J. Opioid Manag. 2017, 13, 241–251. [Google Scholar] [CrossRef]
- Gutowicz, M.; Kaźmierczak, B.; Barańczyk-Kuźma, A. The influence of heroin abuse on glutathione-dependent enzymes in human brain. Drug. Alcohol. Depend. 2011, 113, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.; Shah, J.; Hodgson, N.; Byun, H.M.; Deth, R. Morphine induces redox-based changes in global DNA methylation and retrotransposon transcription by inhibition of excitatory amino acid transporter type 3-mediated cysteine uptake. Mol. Pharmacol. 2014, 85, 747–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Si, P.; Ruan, Z.; Ma, S.; Yan, X.; Sun, L.; Peng, F.; Yuan, H.; Cai, D.; Ding, D.; et al. Primary studies on heroin abuse and injury induced by oxidation and lipoperoxidation. Chin. Med. J. (Engl.) 2001, 114, 297–302. [Google Scholar] [PubMed]
- Wrona, M.Z.; Waskiewicz, J.; Han, Q.P.; Han, J.; Li, H.; Dryhurst, G. Putative oxidative metabolites of 1-methyl-6-hydroxy-1,2,3,4-tetrahydro-beta-carboline of potential relevance to the addictive and neurodegenerative consequences of ethanol abuse. Alcohol 1997, 14, 213–223. [Google Scholar] [CrossRef]
- Deng, Y.; Bu, Q.; Hu, Z.; Deng, P.; Yan, G.; Duan, J.; Hu, C.; Zhou, J.; Shao, X.; Zhao, J.; et al. (1) H-nuclear magnetic resonance-based metabonomic analysis of brain in rhesus monkeys with morphine treatment and withdrawal intervention. J. Neurosci. Res. 2012, 90, 2154–2162. [Google Scholar] [CrossRef]
- Xu, B.; Wang, Z.; Li, G.; Li, B.; Lin, H.; Zheng, R.; Zheng, Q. Heroin-administered mice involved in oxidative stress and exogenous antioxidant-alleviated withdrawal syndrome. Basic Clin. Pharm. Toxicol. 2006, 99, 153–161. [Google Scholar] [CrossRef]
- Blum, K.; Baron, D. Opioid Substitution Therapy: Achieving Harm Reduction While Searching for a Prophylactic Solution. Curr. Pharm. Biotechnol. 2019, 20, 180–182. [Google Scholar] [CrossRef]
- Blum, K.; Modestino, E.J.; Neary, J.; Gondré-Lewis, M.C.; Siwicki, D.; Moran, M.; Hauser, M.; Braverman, E.R.; Baron, D.; Steinberg, B.; et al. Promoting Precision Addiction Management (PAM) to Combat the Global Opioid Crisis. Biomed. J. Sci. Tech. Res. 2018, 2, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Swenson, S.; Blum, K.; McLaughlin, T.; Gold, M.S.; Thanos, P.K. The therapeutic potential of exercise for neuropsychiatric diseases: A review. J. Neurol. Sci. 2020, 412, 116763. [Google Scholar] [CrossRef]
- Diana, M.; Raij, T.; Melis, M.; Nummenmaa, A.; Leggio, L.; Bonci, A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat. Rev. Neurosci. 2017, 18, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Chen, Y.C.; Lee, C.H.; Cheng, C.M. Opioid Addiction, Genetic Susceptibility, and Medical Treatments: A Review. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vink, J.M. Genetics of Addiction: Future Focus on Gene × Environment Interaction? J. Stud. Alcohol. Drugs 2016, 77, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.; Ripke, S.; Als, T.D.; Mattheisen, M.; Walters, R.K.; Won, H.; Pallesen, J.; Agerbo, E.; Andreassen, O.A.; Anney, R.; et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 2019, 51, 431–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgson, K.; Coleman, J.R.I.; Hagenaars, S.P.; Purves, K.L.; Glanville, K.; Choi, S.W.; O’Reilly, P.; Breen, G.; Lewis, C.M. Cannabis use, depression and self-harm: Phenotypic and genetic relationships. Addiction 2020, 115, 482–492. [Google Scholar] [CrossRef]
- Andersen, A.M.; Pietrzak, R.H.; Kranzler, H.R.; Ma, L.; Zhou, H.; Liu, X.; Kramer, J.; Kuperman, S.; Edenberg, H.J.; Nurnberger, J.I., Jr.; et al. Polygenic Scores for Major Depressive Disorder and Risk of Alcohol Dependence. JAMA Psychiatry 2017, 74, 1153–1160. [Google Scholar] [CrossRef]
- Gurriarán, X.; Rodríguez-López, J.; Flórez, G.; Pereiro, C.; Fernández, J.M.; Fariñas, E.; Estévez, V.; Arrojo, M.; Costas, J. Relationships between substance abuse/dependence and psychiatric disorders based on polygenic scores. Genes Brain. Behav. 2019, 18, e12504. [Google Scholar] [CrossRef]
- Cabana-Domínguez, J.; Shivalikanjli, A.; Fernàndez-Castillo, N.; Cormand, B. Genome-wide association meta-analysis of cocaine dependence: Shared genetics with comorbid conditions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 94, 109667. [Google Scholar] [CrossRef]
- Davis, C.; Loxton, N.J.; Levitan, R.D.; Kaplan, A.S.; Carter, J.C.; Kennedy, J.L. ’Food addiction’ and its association with a dopaminergic multilocus genetic profile. Physiol. Behav. 2013, 118, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Leménager, T.; Streit, F.; Fauth-Bühler, M.; Frank, J.; Juraeva, D.; Witt, S.H.; Degenhardt, F.; Hofmann, A.; Heilmann-Heimbach, S.; et al. Genome-wide association study of pathological gambling. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2016, 36, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.S. The Role of Alcohol, Drugs, and Deaths of Despair in the U.S.’s Falling Life Expectancy. Mo Med 2020, 117, 99–101. [Google Scholar] [PubMed]
- Skolnick, P. The Opioid Epidemic: Crisis and Solutions. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 143–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, K.; Gold, M.S.; Jacobs, W.; McCall, W.V.; Febo, M.; Baron, D.; Dushaj, K.; Demetrovics, Z.; Badgaiyan, R.D. Neurogenetics of acute and chronic opiate/opioid abstinence: Treating symptoms and the cause. Front. Biosci. (Landmark Ed.) 2017, 22, 1247–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, K.; Oscar-Berman, M.; Jacobs, W.; McLaughlin, T.; Gold, M.S. Buprenorphine Response as a Function of Neurogenetic Polymorphic Antecedents: Can Dopamine Genes Affect Clinical Outcomes in Reward Deficiency Syndrome (RDS)? J. Addict. Res. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar]
- Baron, D.; Blum, K.; Chen, A.; Gold, M.; Badgaiyan, R.D. Conceptualizing Addiction From an Osteopathic Perspective: Dopamine Homeostasis. J. Am. Osteopath. Assoc. 2018, 118, 115–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borsook, D.; Linnman, C.; Faria, V.; Strassman, A.M.; Becerra, L.; Elman, I. Reward deficiency and anti-reward in pain chronification. Neurosci. Biobehav. Rev. 2016, 68, 282–297. [Google Scholar] [CrossRef]
- Luijten, M.; Schellekens, A.F.; Kühn, S.; Machielse, M.W.; Sescousse, G. Disruption of Reward Processing in Addiction: An Image-Based Meta-analysis of Functional Magnetic Resonance Imaging Studies. JAMA Psychiatry 2017, 74, 387–398. [Google Scholar] [CrossRef]
- Uhl, G.R.; Martinez, M.J.; Paik, P.; Sulima, A.; Bi, G.H.; Iyer, M.R.; Gardner, E.; Rice, K.C.; Xi, Z.X. Cocaine reward is reduced by decreased expression of receptor-type protein tyrosine phosphatase D (PTPRD) and by a novel PTPRD antagonist. Proc. Natl. Acad. Sci. USA 2018, 115, 11597–11602. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.X.; Huang, E.J. Dopaminergic Neurons and Brain Reward Pathways: From Neurogenesis to Circuit Assembly. Am. J. Pathol. 2016, 186, 478–488. [Google Scholar] [CrossRef] [Green Version]
- Bowirrat, A.; Oscar-Berman, M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. Am. J. Med Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2005, 132b, 29–37. [Google Scholar] [CrossRef]
- Fujita, M.; Ide, S.; Ikeda, K. Opioid and nondopamine reward circuitry and state-dependent mechanisms. Ann. N. Y. Acad. Sci. 2019, 1451, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, N.B.; Small, D.M. Fuel not fun: Reinterpreting attenuated brain responses to reward in obesity. Physiol. Behav. 2016, 162, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardi, R.E.; Olevska, A.; Morella, I.; Fasano, S.; Santos, E.; Brambilla, R.; Spanagel, R. The Inhibition of RasGRF2, But Not RasGRF1, Alters Cocaine Reward in Mice. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 6325–6338. [Google Scholar] [CrossRef]
- Mensen, A.; Poryazova, R.; Huegli, G.; Baumann, C.R.; Schwartz, S.; Khatami, R. The Roles of Dopamine and Hypocretin in Reward: A Electroencephalographic Study. PLoS ONE 2015, 10, e0142432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Zhang, Y.; Zhang, D.; Chang, S.; Jing, R.; Yue, W.; Lu, L.; Chen, D.; Sun, Y.; Fan, Y.; et al. GABRA2 rs279858-linked variants are associated with disrupted structural connectome of reward circuits in heroin abusers. Transl. Psychiatry 2018, 8, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, S.; Veit, R.; Sauer, H.; Enck, P.; Friederich, H.C.; Unholzer, T.; Bauer, U.M.; Linder, K.; Heni, M.; Fritsche, A.; et al. Dopamine Depletion Reduces Food-Related Reward Activity Independent of BMI. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016, 41, 1551–1559. [Google Scholar] [CrossRef] [Green Version]
- Ernst, M.; Luciana, M. Neuroimaging of the dopamine/reward system in adolescent drug use. Cns Spectr. 2015, 20, 427–441. [Google Scholar] [CrossRef] [Green Version]
- Steiner, N.; Rossetti, C.; Sakurai, T.; Yanagisawa, M.; de Lecea, L.; Magistretti, P.J.; Halfon, O.; Boutrel, B. Hypocretin/orexin deficiency decreases cocaine abuse liability. Neuropharmacology 2018, 133, 395–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hommer, D.W.; Bjork, J.M.; Gilman, J.M. Imaging brain response to reward in addictive disorders. Ann. N. Y. Acad. Sci. 2011, 1216, 50–61. [Google Scholar] [CrossRef]
- Blum, K.; Gardner, E.; Oscar-Berman, M.; Gold, M. “Liking” and “wanting” linked to Reward Deficiency Syndrome (RDS): Hypothesizing differential responsivity in brain reward circuitry. Curr. Pharm. Des. 2012, 18, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Hamida, S.; Mendonça-Netto, S.; Arefin, T.M.; Nasseef, M.T.; Boulos, L.J.; McNicholas, M.; Ehrlich, A.T.; Clarke, E.; Moquin, L.; Gratton, A.; et al. Increased Alcohol Seeking in Mice Lacking Gpr88 Involves Dysfunctional Mesocorticolimbic Networks. Biol. Psychiatry 2018, 84, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Wedekind, D. Possible role of a dysregulation of the endogenous opioid system in antisocial personality disorder. Hum. Psychopharmacol. 2015, 30, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Modestino, E.J.; Blum, K.; Oscar-Berman, M.; Gold, M.S.; Duane, D.D.; Sultan, S.G.S.; Auerbach, S.H. Reward Deficiency Syndrome: Attentional/Arousal Subtypes, Limitations of Current Diagnostic Nosology, and Future Research. J. Reward Defic. Syndr. 2015, 1, 6–9. [Google Scholar] [CrossRef] [Green Version]
- Edwards, D.; Roy, A.K., 3rd; Boyett, B.; Badgaiyan, R.D.; Thanos, P.K.; Baron, D.; Hauser, M.; Badgaiyan, S.; Brewer, R.; Siwicki, D.B.; et al. Addiction by Any Other Name is Still Addiction: Embracing Molecular Neurogenetic/Epigenetic Basis of Reward Deficiency. J. Addict. Sci. 2020, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Borsook, D.; Youssef, A.M.; Simons, L.; Elman, I.; Eccleston, C. When pain gets stuck: The evolution of pain chronification and treatment resistance. Pain 2018, 159, 2421–2436. [Google Scholar] [CrossRef]
- Elman, I.; Borsook, D.; Volkow, N.D. Pain and suicidality: Insights from reward and addiction neuroscience. Prog. Neurobiol. 2013, 109, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Simons, L.E.; Elman, I.; Borsook, D. Psychological processing in chronic pain: A neural systems approach. Neurosci. Biobehav. Rev. 2014, 39, 61–78. [Google Scholar] [CrossRef] [Green Version]
- Elman, I.; Borsook, D. The failing cascade: Comorbid post traumatic stress- and opioid use disorders. Neurosci. Biobehav. Rev. 2019, 103, 374–383. [Google Scholar] [CrossRef]
- Elman, I.; Upadhyay, J.; Langleben, D.D.; Albanese, M.; Becerra, L.; Borsook, D. Reward and aversion processing in patients with post-traumatic stress disorder: Functional neuroimaging with visual and thermal stimuli. Transl. Psychiatry 2018, 8, 240. [Google Scholar] [CrossRef]
- Moulton, E.A.; Elman, I.; Becerra, L.R.; Goldstein, R.Z.; Borsook, D. The cerebellum and addiction: Insights gained from neuroimaging research. Addict. Biol. 2014, 19, 317–331. [Google Scholar] [CrossRef] [Green Version]
- Green, C.L.; Nahhas, R.W.; Scoglio, A.A.; Elman, I. Post-traumatic stress symptoms in pathological gambling: Potential evidence of anti-reward processes. J. Behav. Addict. 2017, 6, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Elman, I.; Howard, M.; Borodovsky, J.T.; Mysels, D.; Rott, D.; Borsook, D.; Albanese, M. Metabolic and Addiction Indices in Patients on Opioid Agonist Medication-Assisted Treatment: A Comparison of Buprenorphine and Methadone. Sci. Rep. 2020, 10, 5617. [Google Scholar] [CrossRef]
- Wang, A.L.; Elman, I.; Lowen, S.B.; Blady, S.J.; Lynch, K.G.; Hyatt, J.M.; O’Brien, C.P.; Langleben, D.D. Neural correlates of adherence to extended-release naltrexone pharmacotherapy in heroin dependence. Transl. Psychiatry 2015, 5, e531. [Google Scholar] [CrossRef]
- Elman, I.; Becerra, L.; Tschibelu, E.; Yamamoto, R.; George, E.; Borsook, D. Yohimbine-induced amygdala activation in pathological gamblers: A pilot study. PloS ONE 2012, 7, e31118. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, J.; Maleki, N.; Potter, J.; Elman, I.; Rudrauf, D.; Knudsen, J.; Wallin, D.; Pendse, G.; McDonald, L.; Griffin, M.; et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain A J. Neurol. 2010, 133, 2098–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langleben, D.D.; Ruparel, K.; Elman, I.; Busch-Winokur, S.; Pratiwadi, R.; Loughead, J.; O’Brien, C.P.; Childress, A.R. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am. J. Psychiatry 2008, 165, 390–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breier, A.; Kestler, L.; Adler, C.; Elman, I.; Wiesenfeld, N.; Malhotra, A.; Pickar, D. Dopamine D2 receptor density and personal detachment in healthy subjects. Am. J. Psychiatry 1998, 155, 1440–1442. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.M.; Elman, I.; Weisenfeld, N.; Kestler, L.; Pickar, D.; Breier, A. Effects of acute metabolic stress on striatal dopamine release in healthy volunteers. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2000, 22, 545–550. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Chen, A.L.C.; Thanos, P.K.; Febo, M.; Demetrovics, Z.; Dushaj, K.; Kovoor, A.; Baron, D.; Smith, D.E.; Roy, A.K.; et al. Genetic addiction risk score (GARS) ™, a predictor of vulnerability to opioid dependence. Front. Biosci. (Elite Ed.) 2018, 10, 175–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| GENE | Risk Allele | Phenotype | # of Studies | Pat ** Case (N) Control (N) | Meta-Analysis *** (N) | Sig (p) | Comment |
|---|---|---|---|---|---|---|---|
| DRD1 | Rs4532 & specific haplotype rs686*T-rs4532*G within the DRD1 gene | Alcohol Use Disorder (AUD) and Aggression & Impulsivity | Three | Case (569) Cont (218) | NONE | <0.1–0.01 | POSITIVE for Alcohol Dependence and related phenotypes like aggression & Impulsivity |
| DRD2 | Rs1800497 | Severe alcoholism, long-term drinking, alcohol dependence, parental rule-setting, comparison severe vs. less severe alcoholics, relapse and ASI after 12 years in 12 step programs, Family linkage, heavy drinking, early-onset, stress, harm avoidance and antisocial behavior related to AUD, severe medical consequences, mortality hospitalization, Children of Alcoholics (C.O.A.s), parental history of alcoholism, & drinking in the general population, | Sixty–Two | Case (17,382) Cont (17,036) | Four (4) | <0.04–0.09 | POSITIVE for Alcohol Dependence and related phenotypes |
| DRD3 | DRD3 Ser9Gly polymorphism (rs6280) | Alcohol Dependence (AD), Anhedonia and Major –depressive disorder and Obsessive-Compulsive Drinking | Three | Case (545) Cont (156) | NONE | <0.001–0.008 | POSITIVE for Alcohol Dependence (AD), Anhedonia and Major –depressive disorder and Obsessive-Compulsive Drinking |
| DRD4 | Rs180095 48bP repeat VNTR | Risk Factor for Alcoholism, Alcohol Dependence Smoking Behavior, Polysubstance abuse, higher rates of novelty seeking, higher lifetime alcoholism, generalized addiction, increased influence of peer pressure to drink, problematic alcohol use, increase the risk for severity of alcoholism, blunted response to alcohol cues, increase in alcohol craving, increased risk for social bonding with fellow alcoholics. | Forty –Eight | Case (11,740) Cont (9365) | Two (2) | <0.06–0.05 | POSITIVE for many alcohol-related phenotypes |
| DAT1 | 9R allele compared to 10R. | Alcoholism, alcohol consumption, alcohol withdrawal symptoms (AWS) and delirium tremens (DT), number of drinking days, vulnerability to alcoholism, and families with alcoholism compared to families without alcoholism | Twenty–Four | Case (4644) Cont (3761) | Two (2) | <0.05–0.09 | POSITIVE for Alcoholism, alcohol consumption, alcohol withdrawal symptoms (AWS) and delirium tremens (DT), number of drinking days, vulnerability to alcoholism, and families with alcoholism compared to families without alcoholism |
| COMT | Rs4680 Catechol-O-methyl-transferase (COMT) Val158Met | Alcohol Dependence (AD), alcohol intake past year, generalized SUD, Alcohol & Tobacco consumption, drug abuse, in alcoholics reduced dopamine receptor sensitivity | Seventy–Five | Case (10,018) Cont (8861) | One (1) | <0.01–0.01 | POSITIVE for Alcohol Dependence (AD), alcohol intake past year, generalized SUD, Alcohol & Tobacco consumption, drug abuse, in alcoholics reduced dopamine receptor sensitivity |
| OPRM1 | OPRMI (rs1799971) | Alcohol Dependence (AD), Severity of AWS, sensitivity to dopamine receptors, alcohol consumption, depression, response to alcohol cues and relapse risk, alcohol sensitivity in adolescents, drinking frequency, vulnerability for alcohol to hijack the reward system, alcohol craving, alcohol-related hospital readmission, more readmissions, and fewer days until the first readmission | Fifteen | Case (6428) Cont (5196) | One (1) | <0.047–0.06 | POSITIVE for Alcohol Dependence (AD), Severity of AWS, sensitivity to dopamine receptors, alcohol consumption, depression, response to alcohol cues and relapse risk, alcohol sensitivity in adolescents, drinking frequency, vulnerability for alcohol to hijack the reward system, alcohol craving, alcohol-related hospital readmission, more readmissions, and fewer days until the first readmission |
| GABRB3 | Receptor beta3 subunit (GABRB3) 181 variant | The risk for Alcoholism, the onset of drug abuse in Children of Alcoholics (COAS), Parental transmission and alcoholism, hypodopaminergia, Mood-related alcohol expectancy (AE), drinking refusal self-efficacy (DRSE), depression, and prevalence in COAS | Four | Case (196) Cont () | NONE | <0.05–0.07 | POSITIVE for risk for Alcoholism, the onset of drug abuse in COAS, Parental transmission and alcoholism, hypodopaminergia, Mood-related alcohol expectancy (AE), drinking refusal self-efficacy (DRSE), depression, and prevalence in COAS |
| MAOA | 30 BP. VNTR-3.5R, 4R DN repeat polymorphisms | Alcohol Dependence, impulsivity, antisocial personality, susceptibility to alcoholism, smoking behavior, poor psychosocial environment, and lower age of onset of alcoholism. | Five | Case (731) Cont (1111) | NONE | <0.043–0 | POSITIVE for Alcohol Dependence, impulsivity, antisocial personality, susceptibility to alcoholism, smoking behavior, poor psychosocial environment, and lower age of onset of alcoholism |
| SLC6A4 (5HTTLPR) | promoter region (5-HTTLPR) (rs25531) | Alcohol Dependence, anxiety, age of onset, cue craving, lower socialization, depression, & polydrug abuse | Twenty–Seven | Case (13,328) Cont (2982) | Two (2) | <0.03–0.001 | Alcohol Dependence, anxiety, age of onset, cue craving, lower socialization, depression, & polydrug abuse |
| TOTAL | NA | NA | 268 | Case 65,581 Cont 48,686 | Ten (10) | <0.06–0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blum, K.; Kazmi, S.; Modestino, E.J.; Downs, B.W.; Bagchi, D.; Baron, D.; McLaughlin, T.; Green, R.; Jalali, R.; Thanos, P.K.; et al. A Novel Precision Approach to Overcome the “Addiction Pandemic” by Incorporating Genetic Addiction Risk Severity (GARS) and Dopamine Homeostasis Restoration. J. Pers. Med. 2021, 11, 212. https://doi.org/10.3390/jpm11030212
Blum K, Kazmi S, Modestino EJ, Downs BW, Bagchi D, Baron D, McLaughlin T, Green R, Jalali R, Thanos PK, et al. A Novel Precision Approach to Overcome the “Addiction Pandemic” by Incorporating Genetic Addiction Risk Severity (GARS) and Dopamine Homeostasis Restoration. Journal of Personalized Medicine. 2021; 11(3):212. https://doi.org/10.3390/jpm11030212
Chicago/Turabian StyleBlum, Kenneth, Shan Kazmi, Edward J. Modestino, Bill William Downs, Debasis Bagchi, David Baron, Thomas McLaughlin, Richard Green, Rehan Jalali, Panayotis K. Thanos, and et al. 2021. "A Novel Precision Approach to Overcome the “Addiction Pandemic” by Incorporating Genetic Addiction Risk Severity (GARS) and Dopamine Homeostasis Restoration" Journal of Personalized Medicine 11, no. 3: 212. https://doi.org/10.3390/jpm11030212
APA StyleBlum, K., Kazmi, S., Modestino, E. J., Downs, B. W., Bagchi, D., Baron, D., McLaughlin, T., Green, R., Jalali, R., Thanos, P. K., Elman, I., Badgaiyan, R. D., Bowirrat, A., & Gold, M. S. (2021). A Novel Precision Approach to Overcome the “Addiction Pandemic” by Incorporating Genetic Addiction Risk Severity (GARS) and Dopamine Homeostasis Restoration. Journal of Personalized Medicine, 11(3), 212. https://doi.org/10.3390/jpm11030212