Nuclear Medicine Imaging in Neuroblastoma: Current Status and New Developments
Abstract
1. Introduction
1.1. Neuroblastoma
1.2. Diagnostic Imaging
1.3. Advances in Nuclear Imaging
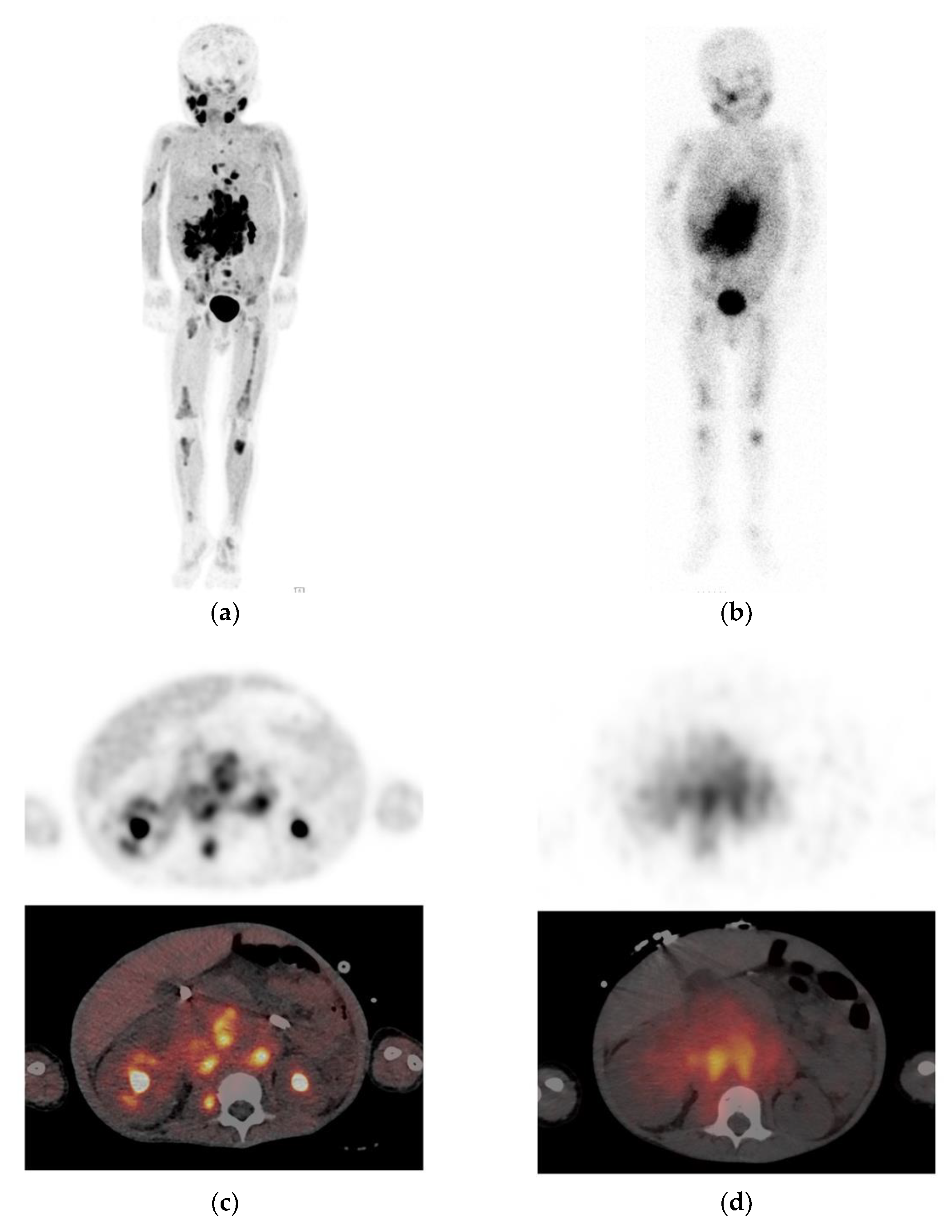
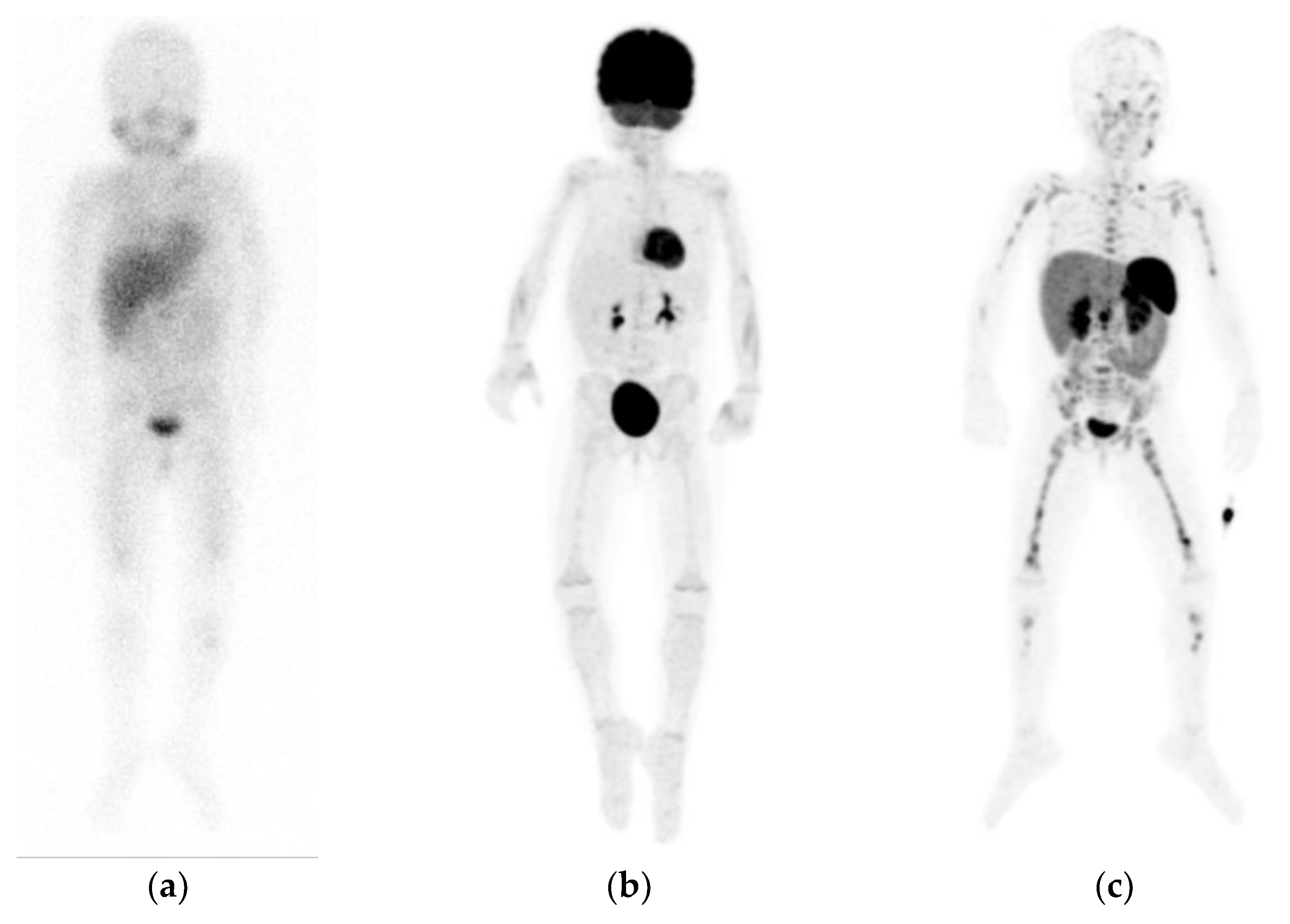
2. [123I]mIBG
2.1. Uptake and Biodistribution
2.2. Indication
2.3. Preparation
2.4. Image Interpretation
2.5. Prognostic Scoring Systems
2.6. Diagnostic Accuracy
2.7. Advantages and Limitations
3. [124I]mIBG
3.1. Uptake and Biodistribution
3.2. Indication
3.3. Preparation
3.4. Diagnostic Accuracy
3.5. Advantages and Limitations
4. [18F]mFBG
4.1. Uptake and Biodistribution
4.2. Indication
4.3. Preparation
4.4. Diagnostic Accuracy
4.5. Advantages and Limitations
5. [18F]FDG
5.1. Uptake and Biodistribution
5.2. Indication
5.3. Preparation
5.4. Diagnostic Accuracy
5.5. Advantages and Limitations
6. [68Ga]Ga-DOTA Peptides
6.1. Uptake and Biodistribution
6.2. Indication
6.3. Preparation
6.4. Diagnostic Accuracy
6.5. Advantages and Limitations
7. [18F]F-DOPA
7.1. Uptake and Biodistribution
7.2. Indication
7.3. Preparation
7.4. Diagnostic Accuracy
7.5. Advantages and Limitations
8. Other
8.1. [11C]mHED
8.2. Other Benzylguanidine Analogues
8.3. Potential Theranostics
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Tracer | Molecular Target | Radioisotope (Half-Life) | Advantages | Limitations |
|---|---|---|---|---|
| [123I]mIBG | norepinephrine transporter | iodine-123 (13 h) |
|
|
| [124I]mIBG | norepinephrine transporter | iodine-124 (100 h) |
|
|
| [18F]mFBG | norepinephrine transporter | fluorine-18 (110 min) |
|
|
| [18F]FDG | glucose metabolism | fluorine-18 (110 min) |
|
|
| [18F]F-DOPA | catecholamine metabolism | fluorine-18 (110 min) |
|
|
| [68Ga]Ga-DOTA peptides | somatostatin receptors | gallium-68 (68 min) |
|
|
| [11C]mHED | norepinephrine transporter | carbon-11 (20 min) |
|
|
References
- Irwin, M.S.; Park, J.R. Neuroblastoma. Pediatric Clin. N. Am. 2015, 62, 225–256. [Google Scholar] [CrossRef]
- Tas, M.L.; Reedijk, A.M.J.; Karim-Kos, H.E.; Kremer, L.C.M.; van de Ven, C.P.; Dierselhuis, M.P.; van Eijkelenburg, N.K.A.; van Grotel, M.; Kraal, K.C.J.M.; Peek, A.M.L.; et al. Neuroblastoma between 1990 and 2014 in the Netherlands: Increased incidence and improved survival of high-risk neuroblastoma. Eur. J. Cancer 2020, 124, 47–55. [Google Scholar] [CrossRef]
- Morgenstern, D.A.; London, W.B.; Stephens, D.; Volchenboum, S.L.; Simon, T.; Nakagawara, A.; Shimada, H.; Schleiermacher, G.; Matthay, K.K.; Cohn, S.L.; et al. Prognostic significance of pattern and burden of metastatic disease in patients with stage 4 neuroblastoma: A study from the International Neuroblastoma Risk Group database. Eur. J. Cancer 2016, 65, 1–10. [Google Scholar] [CrossRef]
- Monclair, T.; Brodeur, G.M.; Ambros, P.F.; Brisse, H.J.; Cecchetto, G.; Holmes, K.; Kaneko, M.; London, W.B.; Matthay, K.K.; Nuchtern, J.G.; et al. The International Neuroblastoma Risk Group (INRG) staging system: An INRG task force report. J. Clin. Oncol. 2009, 27, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.M.; Pritchard, J.; Berthold, F.; Carlsen, N.L.T.; Castel, V.; Castleberry, R.P.; de Bernardi, B.; Evans, A.E.; Favrot, M.; Hedborg, F.; et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 1993, 11, 1466–1477. [Google Scholar] [CrossRef]
- Cohn, S.L.; Pearson, A.D.J.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG task force report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Hero, B.; Benz-Bohm, G.; von Schweinitz, D.; Berthold, F. Review of image defined risk factors in localized neuroblastoma patients: Results of the GPOH NB97 trial. Pediatric Blood Cancer 2008, 50, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Maris, J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Ladenstein, R.; Lambert, B.; Pötschger, U.; Castellani, M.R.; Lewington, V.; Bar-Sever, Z.; Oudoux, A.; Śliwińska, A.; Taborska, K.; Biassoni, L.; et al. Validation of the MIBG skeletal SIOPEN scoring method in two independent high-risk neuroblastoma populations: The SIOPEN/HR-NBL1 and COG-A3973 trials. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 292–305. [Google Scholar] [CrossRef]
- Matthay, K.K.; Reynolds, C.P.; Seeger, R.C.; Shimada, H.; Adkins, E.S.; Haas-Kogan, D.; Gerbing, R.B.; London, W.B.; Villablanca, J.G. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A children’s oncology group study. J. Clin. Oncol. 2009, 27, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- London, W.B.; Castel, V.; Monclair, T.; Ambros, P.F.; Pearson, A.D.J.; Cohn, S.L.; Berthold, F.; Nakagawara, A.; Ladenstein, R.L.; Iehara, T.; et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: A report from the International Neuroblastoma Risk Group project. J. Clin. Oncol. 2011, 29, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.; Rubie, H.; Varo, A.; Le Deley, M.C.; Amoroso, L.; Chevance, A.; Garaventa, A.; Gambart, M.; Bautista, F.; Valteau-Couanet, D.; et al. Outcome of children with relapsed or refractory neuroblastoma: A meta-analysis of ITCC/SIOPEN European phase II clinical trials. Pediatric Blood Cancer 2017, 64, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Brisse, H.J.; McCarville, M.B.; Granata, C.; Krug, K.B.; Wootton-Gorges, S.L.; Kanegawa, K.; Giammarile, F.; Schmidt, M.; Shulkin, B.L.; Matthay, K.K.; et al. Guidelines for imaging and staging of neuroblastic tumors: Consensus report from the International Neuroblastoma Risk Group Project. Radiology 2011, 261, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.J.; Jaju, A. MR imaging of neuroblastic masses. Magn. Reson. Imaging Clin. N. Am. 2008, 16, 499–513. [Google Scholar] [CrossRef]
- Rozovsky, K.; Koplewitz, B.Z.; Krausz, Y.; Revel-Vilk, S.; Weintraub, M.; Chisin, R.; Klein, M. Added value of SPECT/CT for correlation of MIBG scintigraphy and diagnostic CT in neuroblastoma and pheochromocytoma. Am. J. Roentgenol. 2008, 190, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, M.; Taki, J.; Mochizuki, T.; Kinuya, S. Comparison of diagnostic value of I-123 MIBG and high-dose I-131 MIBG scintigraphy including incremental value of SPECT/CT over planar image in patients with malignant pheochromocytoma/paraganglioma and neuroblastoma. Clin. Nucl. Med. 2011, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Servaes, S.; Zhuang, H. SPECT/CT MIBG imaging is crucial in the follow-up of the patients with high-risk neuroblastoma. Clin. Nucl. Med. 2018, 43, 232–238. [Google Scholar] [CrossRef]
- Bar-Sever, Z.; Biassoni, L.; Shulkin, B.; Kong, G.; Hofman, M.S.; Lopci, E.; Manea, I.; Koziorowski, J.; Castellani, R.; Boubaker, A.; et al. Guidelines on nuclear medicine imaging in neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2009–2024. [Google Scholar] [CrossRef]
- Biermann, M.; Schwarzlmüller, T.; Fasmer, K.E.; Reitan, B.C.; Johnsen, B.; Rosendahl, K. Is there a role for PET-CT and SPECT-CT in pediatric oncology? Acta Radiol. 2013, 54, 1037–1045. [Google Scholar] [CrossRef]
- Sharp, S.E.; Trout, A.T.; Weiss, B.D.; Gelfand, M.J. MIBG in neuroblastoma diagnostic imaging and therapy. Radiographics 2016, 36, 258–278. [Google Scholar] [CrossRef]
- Vallabhajosula, S.; Nikolopoulou, A. Radioiodinated metaiodobenzylguanidine (MIBG): Radiochemistry, biology, and pharmacology. Semin. Nucl. Med. 2011, 41, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Smets, L.A.; Loesberg, C.; Janssen, M.; Metwally, E.A.; Huiskamp, R. Active uptake and extravesicular storage of M-iodobenzylguanidine in human neuroblastoma SK-N-SH cells. Cancer Res. 1989, 49, 2941–2944. [Google Scholar] [PubMed]
- Bombardieri, E.; Giammarile, F.; Aktolun, C.; Baum, R.P.; Bischof Delaloye, A.; Maffioli, L.; Moncayo, R.; Mortelmans, L.; Pepe, G.; Reske, S.N.; et al. 131I/123I-metaiodobenzylguanidine (MIBG) scintigraphy: Procedure guidelines for tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Vik, T.A.; Pfluger, T.; Kadota, R.; Castel, V.; Tulchinsky, M.; Farto, J.C.A.; Heiba, S.; Serafini, A.; Tumeh, S.; Khutoryansky, N.; et al. (123)I-MIBG scintigraphy in patients with known or suspected neuroblastoma: Results from a prospective multicenter trial. Pediatric Blood Cancer 2009, 52, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K.; Shulkin, B.; Ladenstein, R.; Michon, J.; Giammarile, F.; Lewington, V.; Pearson, A.D.J.; Cohn, S.L. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: A report for the International Neuroblastoma Risk Group (INRG) task force. Br. J. Cancer 2010, 102, 1319–1326. [Google Scholar] [CrossRef]
- Pfluger, T.; Schmied, C.; Porn, U.; Leinsinger, G.; Vollmar, C.; Dresel, S.; Schmid, I.; Hahn, K. Integrated imaging using MRI and 123I metaiodobenzylguanidine scintigraphy to improve sensitivity and specificity in the diagnosis of pediatric neuroblastoma. AJR Am. J. Roentgenol. 2003, 181, 1115–1124. [Google Scholar] [CrossRef]
- Matthay, K.K.; Edeline, V.; Lumbroso, J.; Tanguy, M.L.; Asselain, B.; Zucker, J.M.; Valteau-Couanet, D.; Hartmann, O.; Michon, J. Correlation of early metastatic response by 123I- metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J. Clin. Oncol. 2003, 21, 2486–2491. [Google Scholar] [CrossRef]
- Lewington, V.; Lambert, B.; Poetschger, U.; Sever, Z.B.; Giammarile, F.; McEwan, A.J.B.; Castellani, R.; Lynch, T.; Shulkin, B.; Drobics, M.; et al. 123I-MIBG scintigraphy in neuroblastoma: Development of a SIOPEN semi-quantitative reporting, method by an international panel. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 234–241. [Google Scholar] [CrossRef]
- Decarolis, B.; Schneider, C.; Hero, B.; Simon, T.; Volland, R.; Roels, F.; Dietlein, M.; Berthold, F.; Schmidt, M. Iodine-123 metaiodobenzylguanidine scintigraphy scoring allows prediction of outcome in patients with stage 4 neuroblastoma: Results of the cologne interscore comparison study. J. Clin. Oncol. 2013, 31, 944–951. [Google Scholar] [CrossRef]
- Yanik, G.A.; Parisi, M.T.; Shulkin, B.L.; Naranjo, A.; Kreissman, S.G.; London, W.B.; Villablanca, J.G.; Maris, J.M.; Park, J.R.; Cohn, S.L.; et al. Semiquantitative MIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: A report from the children’s oncology group. J. Nucl. Med. 2013, 54, 541–548. [Google Scholar] [CrossRef]
- Yanik, G.A.; Parisi, M.T.; Naranjo, A.; Nadel, H.; Gelfand, M.J.; Park, J.R.; Ladenstein, R.L.; Poetschger, U.; Boubaker, A.; Valteau-Couanet, D.; et al. Validation of postinduction curie scores in high-risk neuroblastoma: A children’s oncology group and SIOPEN group report on SIOPEN/HR-NBL1. J. Nucl. Med. 2018, 59, 502–508. [Google Scholar] [CrossRef]
- Bleeker, G.; Tytgat, G.A.M.; Adam, J.A.; Caron, H.N.; Kremer, L.C.M.; Hooft, L.; van Dalen, E.C. 123I-MIBG scintigraphy and 18F-FDG-PET imaging for diagnosing neuroblastoma. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Melzer, H.I.; Walz, C.; von Schweinitz, D.; Coppenrath, E.; Schmid, I.; Bartenstein, P.; Pfluger, T. High 123I-MIBG uptake in neuroblastic tumours indicates unfavourable histopathology. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Biasotti, S.; Garaventa, A.; Villavecchia, G.P.; Cabria, M.; Nantron, M.; de Bernardi, B. False-negative metaiodobenzylguanidine scintigraphy at diagnosis of neuroblastoma. Med. Pediatric Oncol. 2000, 35, 153–155. [Google Scholar] [CrossRef]
- DuBois, S.G.; Geier, E.; Batra, V.; Yee, S.W.; Neuhaus, J.; Segal, M.; Martinez, D.; Pawel, B.; Yanik, G.; Naranjo, A.; et al. Evaluation of norepinephrine transporter expression and metaiodobenzylguanidine avidity in neuroblastoma: A report from the children’s oncology group. Int. J. Mol. Imaging 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- DuBois, S.G.; Mody, R.; Naranjo, A.; van Ryn, C.; Russ, D.; Oldridge, D.; Kreissman, S.; Baker, D.L.; Parisi, M.; Shulkin, B.L.; et al. MIBG avidity correlates with clinical features, tumor biology, and outcomes in neuroblastoma: A report from the children’s oncology group. Pediatric Blood Cancer 2017, 64, 139–148. [Google Scholar] [CrossRef]
- Gains, J.E.; Sebire, N.J.; Moroz, V.; Wheatley, K.; Gaze, M.N. Immunohistochemical evaluation of molecular radiotherapy target expression in neuroblastoma tissue. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 402–411. [Google Scholar] [CrossRef]
- Colavolpe, C.; Guedj, E.; Cammilleri, S.; Taïeb, D.; Mundler, O.; Coze, C. Utility of FDG-PET:CT in the follow-up of neuroblastoma which became MIBG-negative. Pediatric Blood Cancer 2008, 51, 828–831. [Google Scholar] [CrossRef]
- Mc Dowell, H.; Losty, P.; Barnes, N.; Kokai, G. Utility of FDG-PET/CT in the follow-up of neuroblastoma which became MIBG-negative. Pediatric Blood Cancer 2009, 52, 552. [Google Scholar] [CrossRef]
- Schwarz, K.B.; Driver, I.; Lewis, I.J.; Taylor, R.E. Positive MIBG scanning at the time of relapse in neuroblastoma which was MIBG negative at diagnosis. Br. J. Radiol. 1997, 70, 90–92. [Google Scholar] [CrossRef]
- Marachelian, A.; Shimada, H.; Sano, H.; Jackson, H.; Stein, J.; Sposto, R.; Matthay, K.K.; Baker, D.; Villablanca, J.G. The significance of serial histopathology in a residual mass for outcome of intermediate risk stage 3 neuroblastoma. Pediatric Blood Cancer 2012, 58, 675–681. [Google Scholar] [CrossRef]
- Decarolis, B.; Simon, T.; Krug, B.; Leuschner, I.; Vokuhl, C.; Kaatsch, P.; von Schweinitz, D.; Klingebiel, T.; Mueller, I.; Schweigerer, L.; et al. Treatment and outcome of ganglioneuroma and ganglioneuroblastoma intermixed. BMC Cancer 2016, 16, 542. [Google Scholar] [CrossRef] [PubMed]
- Rault, E.; Vandenberghe, S.; van Holen, R.; de Beenhouwer, J.; Staelens, S.; Lemahieu, I. Comparison of image quality of different iodine isotopes (I-123, I-124, and I-131). Cancer Biother. Radiopharm. 2007, 22, 423–430. [Google Scholar] [CrossRef]
- Beijst, C.; de Keizer, B.; Lam, M.G.E.H.; Janssens, G.O.; Tytgat, G.A.M.; de Jong, H.W.A.M. A Phantom study: Should 124 I-MIBG PET/CT replace 123 I-MIBG SPECT/CT? Med. Phys. 2017, 44, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Cistaro, A.; Quartuccio, N.; Caobelli, F.; Piccardo, A.; Paratore, R.; Coppolino, P.; Sperandeo, A.; Arnone, G.; Ficola, U. 124I-MIBG: A new promising positron-emitting radiopharmaceutical for the evaluation of neuroblastoma. Nucl. Med. Rev. 2015, 18, 102–106. [Google Scholar] [CrossRef]
- Huang, S.Y.; Bolch, W.E.; Lee, C.; van Brocklin, H.F.; Pampaloni, M.H.; Hawkins, R.A.; Sznewajs, A.; DuBois, S.G.; Matthay, K.K.; Seo, Y. Patient-specific dosimetry using pretherapy [124I]m-iodobenzylguanidine ([124I]MIBG) dynamic PET/CT imaging before [131I]MIBG targeted radionuclide therapy for neuroblastoma. Mol. Imaging Biol. 2015, 17, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Aboian, M.; Huang, S.; Pampaloni, M.H.; Hawkins, R.A.; Huh, Y.; Vo, K.; Gustafson, C.; Matthay, K.; Seo, Y. 124 I-MIBG PET-CT to monitor metastatic disease in children with relapsed neuroblastoma. J. Nucl. Med. 2020, 62, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-L.; Wahnishe, H.; Sayre, G.A.; Cho, H.-M.; Kim, H.-J.; Hernandez-Pampaloni, M.; Hawkins, R.A.; Dannoon, S.F.; VanBrocklin, H.F.; Itsara, M.; et al. Radiation dose estimation using preclinical imaging with 124I-metaiodobenzylguanidine (MIBG) PET. Med. Phys. 2010, 37, 4861–4867. [Google Scholar] [CrossRef]
- Lassmann, M.; Treves, S.T. Paediatric radiopharmaceutical administration: Harmonization of the 2007 EANM paediatric dosage card (Version 1.5.2008) and the 2010 North American consensus guidelines. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1036–1041. [Google Scholar] [CrossRef]
- Seo, Y.; Gustafson, W.C.; Dannoon, S.F.; Nekritz, E.A.; Lee, C.L.; Murphy, S.T.; van Brocklin, H.F.; Hernandez-Pampaloni, M.; Haas-Kogan, D.A.; Weiss, W.A.; et al. Tumor dosimetry using [124I]miodobenzylguanidine micropet/CT for [131I]m-iodobenzylguanidine treatment of neuroblastoma in a murine xenograft model. Mol. Imaging Biol. 2012, 14, 735–742. [Google Scholar] [CrossRef]
- Pentlow, K.S.; Graham, M.C.; Lambrecht, R.M.; Daghighian, F.; Bacharach, S.L.; Bendriem, B.; Finn, R.D.; Jordan, K.; Kalaigian, H.; Karp, J.S.; et al. Quantitative imaging of iodine-124 with PET. J. Nucl. Med. 1996, 37, 1557–1562. [Google Scholar]
- Zhang, H.; Huang, R.; Cheung, N.K.V.; Guo, H.; Zanzonico, P.B.; Thaler, H.T.; Lewis, J.S.; Blasberg, R.G. Imaging the norepinephrine transporter in neuroblastoma: A comparison of [18F]-MFBG and 123I-MIBG. Clin. Cancer Res. 2014, 20, 2182–2191. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; Zanzonico, P.; Staton, K.D.; Carrasquillo, J.A.; Reidy-Lagunes, D.; Lyashchenko, S.; Burnazi, E.; Zhang, H.; Lewis, J.S.; Blasberg, R.; et al. Biodistribution and dosimetry of 18 F-meta-fluorobenzylguanidine: A first-in-human PET/CT imaging study of patients with neuroendocrine malignancies. J. Nucl. Med. 2018, 59, 147–153. [Google Scholar] [CrossRef]
- Pauwels, E.; Celen, S.; Vandamme, M.; Leysen, W.; Baete, K.; Bechter, O.; Bex, M.; Serdons, K.; van Laere, K.; Bormans, G.; et al. Improved resolution and sensitivity of [18F]MFBG PET compared with [123I]MIBG SPECT in a patient with a norepinephrine transporter–expressing tumour. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef]
- Plathow, C.; Weber, W.A. Tumor cell metabolism imaging. J. Nucl. Med. 2008, 49, 43S–63S. [Google Scholar] [CrossRef]
- Shammas, A.; Lim, R.; Charron, M. Pediatric FDG PET/CT: Physiologic uptake, normal variants, and benign conditions. Radiogr. Rev. Publ. Radiol. Soc. North Am. Inc. 2009, 29, 1467–1486. [Google Scholar] [CrossRef]
- Sharp, S.E.; Shulkin, B.L.; Gelfand, M.J.; Salisbury, S.; Furman, W.L. 123I-MIBG scintigraphy and 18F-FDG PET in neuroblastoma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2009, 50, 1237–1243. [Google Scholar] [CrossRef]
- Shulkin, B.L.; Sisson, C.; Castle, P.; Hutchinson, R.J.; Castle, V.P.; Yanik, G.A.; Shapiro, B.; Sisson, J.C. Neuroblastoma: Positron emission tomography with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose compared with metaiodobenzylguanidine scintigraphy. Radiology 1996, 199, 743–750. [Google Scholar] [CrossRef]
- Melzer, H.I.; Coppenrath, E.; Schmid, I.; Albert, M.H.; von Schweinitz, D.; Tudball, C.; Bartenstein, P.; Pfluger, T. 123I-MIBG scintigraphy/SPECT versus 18F-FDG PET in paediatric neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1648–1658. [Google Scholar] [CrossRef]
- Papathanasiou, N.D.; Gaze, M.N.; Sullivan, K.; Aldridge, M.; Waddington, W.; Almuhaideb, A.; Bomanji, J.B. 18F-FDG PET/CT and 123I-metaiodobenzylguanidine imaging in high-risk neuroblastoma: Diagnostic comparison and survival analysis. J. Nucl. Med. 2011, 52, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Gil, T.Y.; Lee, D.K.; Lee, J.M.; Yoo, E.S.; Ryu, K.H. Clinical experience with 18F-fluorodeoxyglucose positron emission tomography and 123I-metaiodobenzylguanine scintigraphy in pediatric neuroblastoma: Complementary roles in follow-up of patients. Korean J. Pediatrics 2014, 57, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; Yeung, H.W.D.; Larson, S.M.; Kramer, K.; Cheung, N.K.V. Extending positron emission tomography scan utility to high-risk neuroblastoma: Fluorine-18 fluorodeoxyglucose positron emission tomography as sole imaging modality in follow-up of patients. J. Clin. Oncol. 2001, 19, 3397–3405. [Google Scholar] [CrossRef] [PubMed]
- Orr, K.E.; McHugh, K. The new international neuroblastoma response criteria. Pediatric Radiol. 2019, 49, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Bagatell, R.; Cohn, S.L.; Pearson, A.D.; Villablanca, J.G.; Berthold, F.; Burchill, S.; Boubaker, A.; McHugh, K.; Nuchtern, J.G.; et al. Revisions to the international neuroblastoma response criteria: A consensus statement from the National Cancer Institute clinical trials planning meeting. J. Clin. Oncol. 2017, 35, 2580–2587. [Google Scholar] [CrossRef]
- Stauss, J.; Franzius, C.; Pfluger, T.; Juergens, K.U.; Biassoni, L.; Begent, J.; Kluge, R.; Amthauer, H.; Voelker, T.; Højgaard, L.; et al. Guidelines for 18F-FDG PET and PET-CT imaging in paediatric oncology. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1581–1588. [Google Scholar] [CrossRef]
- Taggart, D.R.; Han, M.M.; Quach, A.; Groshen, S.; Ye, W.; Villablanca, J.G.; Jackson, H.A.; Aparici, C.M.; Carlson, D.; Maris, J.; et al. Comparison of iodine-123 metaiodobenzylguanidine (MIBG) scan and [ 18F]fluorodeoxyglucose positron emission tomography to evaluate response after iodine-131 MIBG therapy for relapsed neuroblastoma. J. Clin. Oncol. 2009, 27, 5343–5349. [Google Scholar] [CrossRef]
- Tolboom, N.; Servaes, S.E.; Zhuang, H. Neuroblastoma presenting as non-MIBG-avid widespread soft tissue metastases without bone involvement revealed by FDG PET/CT imaging. Clin. Nucl. Med. 2017, 42, 643–644. [Google Scholar] [CrossRef]
- Wartski, M.; Jehanno, N.; Michon, J.; de Labriolle-Vaylet, C.; Montravers, F. Weak uptake of 123I-MIBG and 18F-FDOPA contrasting with high 18F-FDG uptake in stage i neuroblastoma. Clin. Nucl. Med. 2015, 40, 969–970. [Google Scholar] [CrossRef]
- Codreanu, I.; Zhuang, H. Disparities in uptake pattern of (123)I-MIBG, (18)F-FDG, and (99m)Tc-MDP within the same primary neuroblastoma. Clin. Nucl. Med. 2014, 39, 184–186. [Google Scholar] [CrossRef]
- Sato, Y.; Kurosawa, H.; Sakamoto, S.; Kuwashima, S.; Hashimoto, T.; Okamoto, K.; Tsuchioka, T.; Fukushima, K.; Arisaka, O.; Segura, M. Usefulness of 18F-fluorodeoxyglucose positron emission tomography for follow-up of 13-cis-retinoic acid treatment for residual neuroblastoma after myeloablative chemotherapy. Medicine 2015, 94, e1290. [Google Scholar] [CrossRef]
- Garcia, J.R.; Bassa, P.; Soler, M.; Jaramillo, A.; Ortiz, S.; Riera, E. Benign differentiation of treated neuroblastoma as a cause of false positive by 123I-MIBG SPECT/CT. Usefulness of 18F-FDG PET/CT. Rev. Española De Med. Nucl. E Imagen Mol. (Engl. Ed.) 2019, 38, 389–390. [Google Scholar] [CrossRef]
- Liu, C.-J.; Lu, M.-Y.; Liu, Y.-L.; Ko, C.-L.; Ko, K.-Y.; Tzen, K.-Y.; Chang, H.-H.; Yang, Y.-L.; Jou, S.-T.; Hsu, W.-M.; et al. Risk stratification of pediatric patients with neuroblastoma using volumetric parameters of 18F-FDG and 18F-DOPA PET/CT. Clin. Nucl. Med. 2017, 42, e142–e148. [Google Scholar] [CrossRef]
- Kang, S.Y.; Rahim, M.K.; Kim, Y.-l.; Cheon, G.J.; Kang, H.J.; Shin, H.Y.; Kang, K.W.; Chung, J.K.; Kim, E.E.; Lee, D.S. Clinical significance of pretreatment FDG PET/CT in MIBG-avid pediatric neuroblastoma. Nucl. Med. Mol. Imaging 2017, 51, 154–160. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Chen, S.; Huang, S.; Wu, S.; Zhang, L.; Zhang, F.; Wang, H. Prognostic value of metabolic indices and bone marrow uptake pattern on preoperative 18F–FDG PET/CT in pediatric patients with neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 306–315. [Google Scholar] [CrossRef]
- Lee, J.W.; Cho, A.; Yun, M.; Lee, J.D.; Lyu, C.J.; Kang, W.J. Prognostic value of pretreatment FDG PET in pediatric neuroblastoma. Eur. J. Radiol. 2015, 84, 2633–2639. [Google Scholar] [CrossRef]
- Pauwels, E.; Cleeren, F.; Bormans, G.; Deroose, C.M. Somatostatin receptor PET ligands—The next generation for clinical practice. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 311–331. [Google Scholar]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601. [Google Scholar] [CrossRef]
- Hofman, M.S.; Eddie Lau, W.F.; Hicks, R.J. Somatostatin receptor imaging With68Ga DOTATATE PET/CT: Clinical utility, normal patterns, pearls, and pitfalls in interpretation1. Radiographics 2015, 35, 500–516. [Google Scholar] [CrossRef]
- Abongwa, C.; Mott, S.; Schafer, B.; McNeely, P.; Abusin, G.; O’Dorisio, T.; Zamba, G.; O’Dorisio, M.S.; Menda, Y. Safety and accuracy of 68Ga-DOTATOC PET/CT in children and young adults with solid tumors. Am. J. Nucl. Med. Mol. Imaging 2017, 7, 228–235. [Google Scholar] [PubMed]
- Poeppel, T.D.; Binse, I.; Petersenn, S.; Lahner, H.; Schott, M.; Antoch, G.; Brandau, W.; Bockisch, A.; Boy, C. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2011, 52, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Kabasakal, L.; Demirci, E.; Ocak, M.; Decristoforo, C.; Araman, A.; Ozsoy, Y.; Uslu, I.; Kanmaz, B. Comparison of 68Ga-DOTATATE and 68Ga-DOTANOC PET/CT imaging in the same patient group with neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- O’Dorisio, M.S.; Chen, F.; O’Dorisio, T.M.; Wray, D.; Qualman, S.J. Characterization of somatostatin receptors on human neuroblastoma tumors. Cell Growth Differ. 1994, 5, 1–8. [Google Scholar] [PubMed]
- Georgantzi, K.; Tsolakis, A.V.; Stridsberg, M.; Jakobson, A.; Christofferson, R.; Janson, E.T. Differentiated expression of somatostatin receptor subtypes in experimental models and clinical neuroblastoma. Pediatric Blood Cancer 2011, 56, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Albers, A.R.; O’Dorisio, M.S.; Balster, D.A.; Caprara, M.; Gosh, P.; Chen, F.; Hoeger, C.; Rivier, J.; Wenger, G.D.; O’Dorisio, T.M.; et al. Somatostatin receptor gene expression in neuroblastoma. Regul. Pept. 2000, 88, 61–73. [Google Scholar] [CrossRef]
- Kroiss, A.; Putzer, D.; Uprimny, C.; Decristoforo, C.; Gabriel, M.; Santner, W.; Kranewitter, C.; Warwitz, B.; Waitz, D.; Kendler, D.; et al. Functional imaging in phaeochromocytoma and neuroblastoma with 68Ga-DOTA-Tyr3-octreotide positron emission tomography and 123I-metaiodobenzylguanidine. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 865–873. [Google Scholar] [CrossRef]
- Kong, G.; Hofman, M.S.; Murray, W.K.; Wilson, S.; Wood, P.; Downie, P.; Super, L.; Hogg, A.; Eu, P.; Hicks, R.J. Initial experience with gallium-68 DOTA-octreotate PET/CT and peptide receptor radionuclide therapy for pediatric patients with refractory metastatic neuroblastoma. J. Pediatric Hematol. Oncol. 2016, 38, 87–96. [Google Scholar] [CrossRef]
- Gains, J.E.; Aldridge, M.D.; Mattoli, M.V.; Bomanji, J.B.; Biassoni, L.; Shankar, A.; Gaze, M.N. 68Ga-DOTATATE and 123I-MIBG as imaging biomarkers of disease localisation in metastatic neuroblastoma: Implications for molecular radiotherapy. Nucl. Med. Commun. 2020, 2, 1169–1177. [Google Scholar] [CrossRef]
- Telli, T.; Lay Ergün, E.; Volkan Salanci, B.; Özgen Kiratli, P. The complementary role of 68Ga-DOTATATE PET/CT in neuroblastoma. Clin. Nucl. Med. 2020, 45, 326–329. [Google Scholar] [CrossRef]
- Torun, N. 68Ga-DOTA-TATE in neuroblastoma with marrow involvement. Clin. Nucl. Med. 2019, 44, 467–468. [Google Scholar] [CrossRef]
- Alexander, N.; Marrano, P.; Thorner, P.; Naranjo, A.; van Ryn, C.; Martinez, D.; Batra, V.; Zhang, L.; Irwin, M.S.; Baruchel, S. Prevalence and clinical correlations of somatostatin receptor-2 (SSTR2) expression in neuroblastoma. J. Pediatric Hematol. Oncol. 2019, 41, 222–227. [Google Scholar] [CrossRef]
- Machado, J.S.; Beykan, S.; Herrmann, K.; Lassmann, M. Recommended administered activities for 68Ga-labelled peptides in paediatric nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2036–2039. [Google Scholar] [CrossRef]
- Campana, D.; Ambrosini, V.; Pezzilli, R.; Fanti, S.; Labate, A.M.M.; Santini, D.; Ceccarelli, C.; Nori, F.; Franchi, R.; Corinaldesi, R.; et al. Standardized uptake values Of68Ga-DOTANOC PET: A promising prognostic tool in neuroendocrine tumors. J. Nucl. Med. 2010, 51, 353–359. [Google Scholar] [CrossRef]
- Kim, Y.-l.; Yoo, C.; Oh, S.J.; Lee, S.J.; Kang, J.; Hwang, H.S.; Hong, S.M.; Ryoo, B.Y.; Ryu, J.S. Tumour-to-liver ratio determined by [68Ga]Ga-DOTA-TOC PET/CT as a prognostic factor of lanreotide efficacy for patients with well-differentiated gastroenteropancreatic-neuroendocrine tumours. Ejnmmi Res. 2020, 10, 63. [Google Scholar] [CrossRef]
- Zhang, L.; Vines, D.C.; Scollard, D.A.; McKee, T.; Komal, T.; Ganguly, M.; Do, T.; Wu, B.; Alexander, N.; Vali, R.; et al. Correlation of somatostatin receptor-2 expression with gallium-68-DOTA-TATE uptake in neuroblastoma xenograft models. Contrast Media Mol. Imaging 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Orlando, C.; Raggi, C.C.; Bagnoni, L.; Sestini, R.; Briganti, V.; La Cava, G.; Bernini, G.; Tonini, G.; Pazzagli, M.; Serio, M.; et al. Somatostatin receptor type 2 gene expression in neuroblastoma, measured by competitive RT-PCR, is related to patient survival and to somatostatin receptor imaging by indium -111-pentetreotide. Med. Pediatric Oncol. 2001, 36. [Google Scholar] [CrossRef]
- Moertel, C.L.; Reubi, J.C.; Scheithauer, B.S.; Schaid, D.J.; Kvols, L.K. Expression of somatostatin receptors in childhood neuroblastoma. Am. J. Clin. Pathol. 1994, 102. [Google Scholar] [CrossRef] [PubMed]
- Briganti, V.; Sestini, R.; Orlando, C.; Bernini, G.; La Cava, G.; Tamburini, A.; Raggi, C.C.; Serio, M.; Maggi, M. Imaging of somatostatin receptors by indium-111-pentetreotide correlates with quantitative determination of somatostatin receptor type 2 gene expression in neuroblastoma tumors. Clin. Cancer Res. 1997, 3, 2385–2391. [Google Scholar]
- Gains, J.E.; Bomanji, J.B.; Fersht, N.L.; Sullivan, T.; D’Souza, D.; Sullivan, K.P.; Aldridge, M.; Waddington, W.; Gaze, M.N. 177Lu-DOTATATE molecular radiotherapy for childhood neuroblastoma. J. Nucl. Med. 2011, 52, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Menda, Y.; O’Dorisio, M.S.; Kao, S.; Khanna, G.; Michael, S.; Connolly, M.; Babich, J.; O’Dorisio, T.; Bushnell, D.; Madsen, M. Phase I trial of 90Y-DOTATOC therapy in children and young adults with refractory solid tumors that express somatostatin receptors. J. Nucl. Med. 2010, 51, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Gains, J.E.; Moroz, V.; Aldridge, M.D.; Wan, S.; Wheatley, K.; Laidler, J.; Peet, C.; Bomanji, J.B.; Gaze, M.N. A Phase IIa trial of molecular radiotherapy with 177-lutetium DOTATATE in children with primary refractory or relapsed high-risk neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Jager, P.L.; Chirakal, R.; Marriott, C.J.; Brouwers, A.H.; Koopmans, K.P.; Gulenchyn, K.Y. 6-L-18F-fluorodihydroxyphenylalanine PET in neuroendocrine tumors: Basic aspects and emerging clinical applications. J. Nucl. Med. 2008, 49, 573–586. [Google Scholar] [CrossRef]
- Koopmans, K.P.; Neels, O.N.; Kema, I.P.; Elsinga, P.H.; Links, T.P.; de Vries, E.G.E.; Jager, P.L. Molecular imaging in neuroendocrine tumors: Molecular uptake mechanisms and clinical results. Crit. Rev. Oncol. Hematol. 2009, 71, 199–213. [Google Scholar] [CrossRef]
- Lu, M.Y.; Liu, Y.L.; Chang, H.H.; Jou, S.T.; Yang, Y.L.; Lin, K.H.; Lin, D.T.; Lee, Y.L.; Lee, H.; Wu, P.Y.; et al. Characterization of neuroblastic tumors using 18F-FDOPA PET. J. Nucl. Med. 2013, 54, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E.; Piccardo, A.; Nanni, C.; Altrinetti, V.; Garaventa, A.; Pession, A.; Cistaro, A.; Chiti, A.; Villavecchia, G.; Fanti, S. 18F-DOPA PET/CT in neuroblastoma: Comparison of conventional imaging with CT/MR. Clin. Nucl. Med. 2012, 37, e73–e78. [Google Scholar] [CrossRef]
- Lopci, E.; D’Ambrosio, D.; Nanni, C.; Chiti, A.; Pession, A.; Marengo, M.; Fanti, S. Feasibility of carbidopa premedication in pediatric patients: A pilot study. Cancer Biother. Radiopharm. 2012, 27, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, A.; Lopci, E.; Conte, M.; Garaventa, A.; Foppiani, L.; Altrinetti, V.; Nanni, C.; Bianchi, P.; Cistaro, A.; Sorrentino, S.; et al. Comparison of 18F-dopa PET/CT and 123I-MIBG scintigraphy in stage 3 and 4 neuroblastoma: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 57–71. [Google Scholar] [CrossRef]
- Piccardo, A.; Morana, G.; Puntoni, M.; Campora, S.; Sorrentino, S.; Zucchetta, P.; Ugolini, M.; Conte, M.; Cistaro, A.; Ferrarazzo, G.; et al. Diagnosis, treatment response, and prognosis: The role of 18F-DOPA PET/CT in children affected by neuroblastoma in comparison with 123I-MIBG scan: The first prospective study. J. Nucl. Med. 2020, 61, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, A.; Puntoni, M.; Lopci, E.; Conte, M.; Foppiani, L.; Sorrentino, S.; Morana, G.; Naseri, M.; Cistaro, A.; Villavecchia, G.; et al. Prognostic value of 18F-DOPA PET/CT at the time of recurrence in patients affected by neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1046–1056. [Google Scholar] [CrossRef]
- Rischpler, C.; Fukushima, K.; Isoda, T.; Javadi, M.S.; Dannals, R.F.; Abraham, R.; Wahl, R.; Bengel, F.M.; Higuchi, T. Discrepant uptake of the radiolabeled norepinephrine analogues hydroxyephedrine (HED) and metaiodobenzylguanidine (MIBG) in rat hearts. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Shulkin, B.L.; Wieland, D.M.; Baro, M.E.; Ungar, D.R.; Mitchell, D.S.; Dole, M.G.; Rawwas, J.B.; Castle, V.P.; Sisson, J.C.; Hutchinson, R.J. PET hydroxyephedrine imaging of neuroblastoma. J. Nucl. Med. 1996, 37, 16–21. [Google Scholar]
- Franzius, C.; Hermann, K.; Weckesser, M.; Kopka, K.; Juergens, K.U.; Vormoor, J.; Schober, O. Whole-body PET/CT with 11C-meta-hydroxyephedrine in tumors of the sympathetic nervous system: Feasibility study and comparison with 123I-MIBG SPECT/CT. J. Nucl. Med. 2006, 47, 1635–1642. [Google Scholar] [PubMed]
- Chen, X.; Kudo, T.; Lapa, C.; Buck, A.; Higuchi, T. Recent advances in radiotracers targeting norepinephrine transporter: Structural development and radiolabeling improvements. J. Neural Transm. 2020, 127, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.; Park, H.J.; Choi, H.S.; So, Y.; Lee, B.C.; Lee, W.W. Case report of PET/CT imaging of a patient with neuroblastoma using18f-FPBG. Pediatrics 2014, 134, e1731–e1734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, R.; Pillarsetty, N.V.K.; Thorek, D.L.J.; Vaidyanathan, G.; Serganova, I.; Blasberg, R.G.; Lewis, J.S. Synthesis and evaluation of 18F-labeled benzylguanidine analogs for targeting the human norepinephrine transporter. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 322–332. [Google Scholar] [CrossRef][Green Version]
- Yu, M.; Bozek, J.; Lamoy, M.; Guaraldi, M.; Silva, P.; Kagan, M.; Yalamanchili, P.; Onthank, D.; Mistry, M.; Lazewatsky, J.; et al. Evaluation of LMI1195, a novel 18F-labeled cardiac neuronal PET imaging agent, in cells and animal models. Circ. Cardiovasc. Imaging 2011, 4, 435–443. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; McDougald, D.; Koumarianou, E.; Choi, J.; Hens, M.; Zalutsky, M.R. Synthesis and evaluation of 4-[18F]fluoropropoxy-3-iodobenzylguanidine ([18F]FPOIBG): A novel 18F-labeled analogue of MIBG. Nucl. Med. Biol. 2015, 42, 673–684. [Google Scholar] [CrossRef]
- Hampel, T.; Bruns, M.; Bayer, M.; Handgretinger, R.; Bruchelt, G.; Brückner, R. Synthesis and biological effects of new hybrid compounds composed of benzylguanidines and the alkylating group of busulfan on neuroblastoma cells. Bioorganic Med. Chem. Lett. 2014, 24, 2728–2733. [Google Scholar] [CrossRef]
- Kortylewicz, Z.P.; Coulter, D.W.; Han, G.; Baranowska-Kortylewicz, J. Radiolabeled (R)-(–)-5-Iodo-3′-O-[2-(ε-guanidinohexanoyl)-2-phenylacetyl]-2′-deoxyuridine: A new theranostic for neuroblastoma. J. Label. Compd. Radiopharm. 2020, 63, 312–324. [Google Scholar] [CrossRef]
- Liu, D.; Li, M.; Bellizzi, A.; Menda, Y.; Lee, D.; Nourmahnad, K.; Ghobrial, S.; Schultz, M.; O’Dorisio, M.S. Preclinical evaluation of CXCR4 as novel radio-theranostic target for high grade neuroendocrine and neuroblastoma tumors. J. Nucl. Med. 2018, 59, 1313. [Google Scholar]
- Butch, E.; Mishra, J.; Amador-Diaz, V.; Vavere, A.; Snyder, S. Selective detection of GD2-positive pediatric solid tumors using 89Zr-dinutuximab PET to facilitate anti-GD2 immunotherapy. J. Nucl. Med. 2018, 59, 170. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samim, A.; Tytgat, G.A.M.; Bleeker, G.; Wenker, S.T.M.; Chatalic, K.L.S.; Poot, A.J.; Tolboom, N.; van Noesel, M.M.; Lam, M.G.E.H.; de Keizer, B. Nuclear Medicine Imaging in Neuroblastoma: Current Status and New Developments. J. Pers. Med. 2021, 11, 270. https://doi.org/10.3390/jpm11040270
Samim A, Tytgat GAM, Bleeker G, Wenker STM, Chatalic KLS, Poot AJ, Tolboom N, van Noesel MM, Lam MGEH, de Keizer B. Nuclear Medicine Imaging in Neuroblastoma: Current Status and New Developments. Journal of Personalized Medicine. 2021; 11(4):270. https://doi.org/10.3390/jpm11040270
Chicago/Turabian StyleSamim, Atia, Godelieve A.M. Tytgat, Gitta Bleeker, Sylvia T.M. Wenker, Kristell L.S. Chatalic, Alex J. Poot, Nelleke Tolboom, Max M. van Noesel, Marnix G.E.H. Lam, and Bart de Keizer. 2021. "Nuclear Medicine Imaging in Neuroblastoma: Current Status and New Developments" Journal of Personalized Medicine 11, no. 4: 270. https://doi.org/10.3390/jpm11040270
APA StyleSamim, A., Tytgat, G. A. M., Bleeker, G., Wenker, S. T. M., Chatalic, K. L. S., Poot, A. J., Tolboom, N., van Noesel, M. M., Lam, M. G. E. H., & de Keizer, B. (2021). Nuclear Medicine Imaging in Neuroblastoma: Current Status and New Developments. Journal of Personalized Medicine, 11(4), 270. https://doi.org/10.3390/jpm11040270






