Adherence to Anti-Osteoporotic Treatment and Clinical Implications after Hip Fracture: A Systematic Review
Abstract
1. Introduction
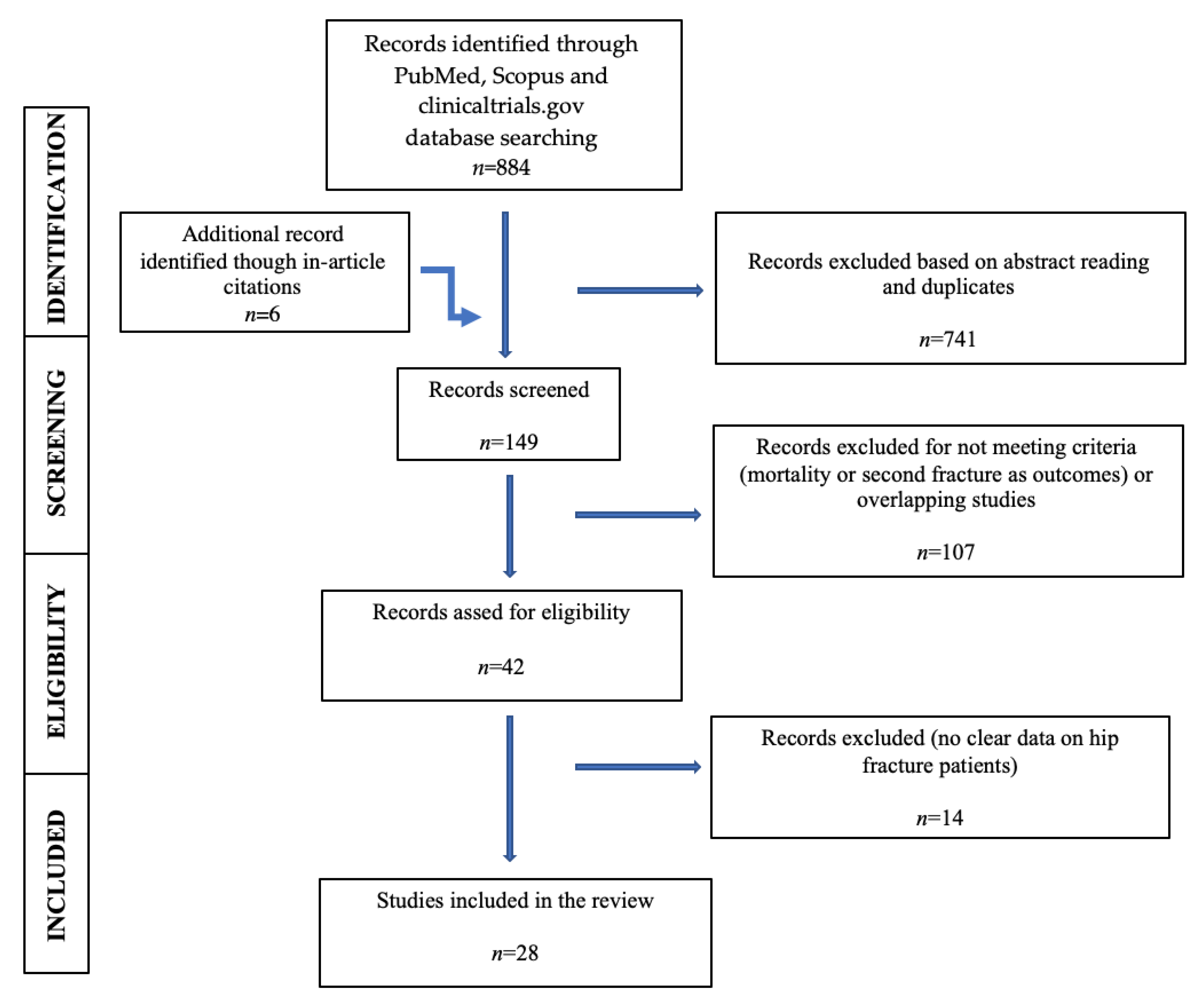
2. Materials and Methods
3. Adherence to Treatment
4. Mortality
5. Second Fracture
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Åkesson, K.; IOF Fracture Working Group; Marsh, D.; Mitchell, P.J.; McLellan, A.R.; Stenmark, J.; Pierroz, D.D.; Kyer, C.; Cooper, C. Capture the Fracture: A best practice framework and global campaign to break the fragility fracture cycle. Osteoporos. Int. 2013, 24, 2135–2152. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johansson, H.; Odén, A.; Harvey, N.C.; Gudnason, V.; Sanders, K.M.; Sigurdsson, G.; Siggeirsdottir, K.; Fitzpatrick, L.A.; Borgström, F.; et al. Characteristics of recurrent fractures. Osteoporos. Int. 2018, 29, 1747–1757. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A.; Odén, A.; Sernbo, I.; Redlund-Johnell, I.; Petterson, C.; De Laet, C.; Jönsson, B. Fracture risk following an osteoporotic fracture. Osteoporos. Int. 2004, 15, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Siggeirsdóttir, K.; Harvey, N.C.; Odén, A.; Gudnason, V.; McCloskey, E.; Sigurdsson, G.; A Kanis, J. Imminent risk of fracture after fracture. Osteoporos. Int. 2017, 28, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Haleem, S.; Lutchman, L.; Mayahi, R.; Grice, J.; Parker, M. Mortality following hip fracture: Trends and geographical variations over the last 40 years. Injury 2008, 39, 1157–1163. [Google Scholar] [CrossRef]
- Farahmand, B.Y.; Michaëlsson, K.; Ahlbom, A.; Ljunghall, S.; Baron, J.A.; Swedish Hip Fracture Study Group. Survival after hip fracture. Osteoporos. Int. 2005, 16, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Haentjens, P.; Magaziner, J.; Colón-Emeric, C.S.; Vanderschueren, D.; Milisen, K.; Velkeniers, B.; Boonen, S. Meta-analysis: Excess mortality after hip fracture among older women and men. Ann. Intern. Med. 2010, 152, 380–390. [Google Scholar] [CrossRef]
- Boudou, L.; Gerbay, B.; Chopin, F.; Ollagnier, E.; Collet, P.; Thomas, T. Management of osteoporosis in fracture liaison service associated with long-term adherence to treatment. Osteoporos. Int. 2011, 22, 2099–2106. [Google Scholar] [CrossRef]
- Kanis, J.A.; Svedbom, A.; Harvey, N.; McCloskey, E.V. The osteoporosis treatment gap. J. Bone Miner. Res. 2014, 29, 1926–1928. [Google Scholar] [CrossRef]
- McLellan, A.R.; Wolowacz, S.E.; Zimovetz, E.A.; Beard, S.M.; Lock, S.; McCrink, L.; Adekunle, F.; Roberts, D. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: A cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos. Int. 2011, 22, 2083–2098. [Google Scholar] [CrossRef]
- Cree, M.W.; Juby, A.G.; Carriere, K.C. Mortality and morbidity associated with osteoporosis drug treatment following hip fracture. Osteoporos. Int. 2003, 14, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Lyles, K.W.; Colón-Emeric, C.S.; Magaziner, J.S.; Adachi, J.D.; Pieper, C.F.; Mautalen, C.; Hyldstrup, L.; Recknor, C.; Nordsletten, L.; Moore, K.A.; et al. HORIZON recurrent fracture trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N. Engl. J. Med. 2007, 357, 1799–1809. [Google Scholar] [CrossRef]
- Behanova, M.; Reichardt, B.; Stamm, T.A.; Zwerina, J.; Klaushofer, K.; Kocijan, R. treatment effects of bisphosphonates and denosumab on survival and refracture from real-world data of hip-fractured patients. Calcif. Tissue Int. 2019, 105, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Bondo, L.; Eiken, P.A.; Abrahamsen, B. Analysis of the association between bisphosphonate treatment survival in Danish hip fracture patients—A nationwide register-based open cohort study. Osteoporos. Int. 2013, 24, 245–252. [Google Scholar] [CrossRef]
- Bergman, J.; Nordström, A.; Hommel, A.; Kivipelto, M.; Nordström, P. Bisphosphonates and mortality: Confounding in observational studies? Osteoporos. Int. 2019, 30, 1973–1982. [Google Scholar] [CrossRef]
- Sambrook, P.N.; Cameron, I.D.; Chen, J.S.; March, L.M.; Simpson, J.M.; Cumming, R.G.; Seibel, M.J. Oral bisphosphonates are associated with reduced mortality in frail older people: A prospective five-year study. Osteoporos. Int. 2011, 22, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Brozek, W.; Reichardt, B.; Zwerina, J.; Dimai, H.P.; Klaushofer, K.; Zwettler, E. Antiresorptive therapy and risk of mortality and refracture in osteoporosis-related hip fracture: A nationwide study. Osteoporos. Int. 2016, 27, 387–396. [Google Scholar] [CrossRef]
- Peng, J.; Liu, Y.; Chen, L.; Peng, K.; Xu, Z.; Zhang, D.; Xiang, Z. Bisphosphonates can prevent recurrent hip fracture and reduce the mortality in osteoporotic patient with hip fracture: A meta-analysis. Pak. J. Med. Sci. 1969, 32, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Cobden, A.; Cobden, S.B.; Camurcu, Y.; Ucpunar, H.; Duman, S.; Sofu, H. Effects of postoperative osteoporosis treatment on subsequent fracture and the 5-year survival rates after hemiarthroplasty for hip fracture. Arch. Osteoporos. 2019, 14, 100. [Google Scholar] [CrossRef]
- Van Geel, T.A.C.M.; Bliuc, D.; Geusens, P.P.M.; Center, J.R.; Dinant, G.-J.; Tran, T.; van den Bergh, J.P.W.; McLellan, A.R.; Eisman, J.A. Reduced mortality and subsequent fracture risk associated with oral bisphosphonate recommendation in a fracture liaison service setting: A prospective cohort study. PLoS ONE 2018, 13, e0198006. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Zhuang, H.; Yu, H.; Cai, S.; Xu, H.; Chen, Z.; Lin, J.; Yao, X. Anti-osteoporosis medications associated with decreased mortality after hip fracture. Orthop. Surg. 2019, 11, 777–783. [Google Scholar] [CrossRef]
- Abtahi, S.; Burden, A.M.; Geusens, P.; van den Bergh, J.P.; Van Staa, T.; De Vries, F. The association of oral bisphosphonate use with mortality risk following a major osteoporotic fracture in the United Kingdom: Population-based cohort study. J. Am. Med. Dir. Assoc. 2020, 21, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Boonen, S.; Orwoll, E.; Magaziner, J.; Colón-Emeric, C.S.; Adachi, J.D.; Bucci-Rechtweg, C.; Haentjens, P.; Kaufman, J.-M.; Rizzoli, R.; Vanderschueren, D.; et al. HORIZON recurrent fracture trial. Once-yearly zoledronic acid in older men compared with women with recent hip fracture. J. Am. Geriatr. Soc. 2011, 59, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Alhambra, D.; Judge, A.; Arden, N.K.; Cooper, C.; Lyles, K.W.; Javaid, M.K. Fracture prevention in patients with cognitive impairment presenting with a hip fracture: Secondary analysis of data from the HORIZON Recurrent Fracture Trial. Osteoporos. Int. 2013, 25, 77–83. [Google Scholar] [CrossRef]
- Sing, C.-W.; Wong, A.Y.; Kiel, D.P.; Cheung, E.Y.; Lam, J.K.; Cheung, T.T.; Chan, E.W.; Kung, A.W.; Wong, I.C.; Cheung, C.-L. Association of alendronate and risk of cardiovascular events in patients with hip fracture. J. Bone Miner. Res. 2018, 33, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Nordström, P.; Toots, A.; Gustafson, Y.; Thorngren, K.-G.; Hommel, A.; Nordström, A. Bisphosphonate use after hip fracture in older adults: A nationwide retrospective cohort study. J. Am. Med. Dir. Assoc. 2017, 18, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Center, J.R.; Bliuc, D.; Nguyen, N.D.; Nguyen, T.V.; Eisman, J.A. Osteoporosis medication and reduced mortality risk in elderly women and men. J. Clin. Endocrinol. Metab. 2011, 96, 1006–1014. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Sinigaglia, L.; Rossini, M.; Adami, S.; Cagnoni, C.; Magliaro, C.; Veronesi, C.; Buda, S.; Minisola, S. Adherence to therapeutic and diagnostic recommendations in patients with femur fracture and at risk of re-fracture or death: Results of an analysis of administrative databases. Reumatismo 2012, 64, 18–26. [Google Scholar] [CrossRef]
- Han, S.; Wen, S.-M.; Zhao, Q.-P.; Huang, H.; Wang, H.; Cong, Y.-X.; Shang, K.; Ke, C.; Zhuang, Y.; Zhang, B.-F. The efficacy of teriparatide in improving fracture healing in hip fractures: A systematic review and meta-analysis. BioMed Res. Int. 2020, 2020, 591450. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Chen, H.-M.; Chen, H.-J.; Chou, M.-Y.; Wang, Y.-C.; Hsu, Y.-H.; Liang, C.-K.; Chu, C.-S. A national study on long-term osteoporosis therapy and risk of recurrent fractures in patients with hip fracture. Arch. Gerontol. Geriatr. 2020, 88, 104021. [Google Scholar] [CrossRef]
- Lebanon, O.L.T.; Netzer, D.; Yaacobi, E.; Berner, Y.; Spiegel, D.; Bacharach, R.; Nabriski, D.; Nyska, M.; Brin, Y.; Rotman-Pikielny, P. Virtual orthopedic-rehabilitation-metabolic collaboration for treating osteoporotic HIP fractures. Endocr. Pract. 2020, 26, 332–339. [Google Scholar] [CrossRef]
- González-Quevedo, D.; Bautista-Enrique, D.; Pérez-Del-Río, V.; Bravo-Bardají, M.; García-De-Quevedo, D.; Tamimi, I. Fracture liaison service and mortality in elderly hip fracture patients: A prospective cohort study. Osteoporos. Int. 2019, 31, 77–84. [Google Scholar] [CrossRef]
- Rotman-Pikielny, P.; Frankel, M.; Lebanon, O.T.; Yaacobi, E.; Tamar, M.; Netzer, D.; Nabriski, D.; Nyska, M.; Brin, Y.S. Orthopedic-metabolic collaborative management for osteoporotic hip fracture. Endocr. Pract. 2018, 24, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-H.; Huang, K.-C.; Tsai, Y.-H.; Yang, T.-Y.; Lee, M.S.; Ueng, S.W.; Hsu, R.W. Risk analysis for second hip fracture in patients after hip fracture surgery: A nationwide population-based study. J. Am. Med. Dir. Assoc. 2014, 15, 725–731. [Google Scholar] [CrossRef]
- Palacios, S.; Kalouche-Khalil, L.; Rizzoli, R.; Zapalowski, C.; Resch, H.; Adachi, J.D.; Gallagher, J.C.; Feldman, R.G.; Kendler, D.L.; Wang, A.; et al. Treatment with denosumab reduces secondary fracture risk in women with postmenopausal osteoporosis. Climacteric 2015, 18, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Osaki, M.; Tatsuki, K.; Hashikawa, T.; Norimatsu, T.; Chiba, K.; Motokawa, S.; Furuichi, I.; Doiguchi, Y.; Aoyagi, K.; Shindo, H. Beneficial effect of risedronate for preventing recurrent hip fracture in the elderly Japanese women. Osteoporos. Int. 2012, 23, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, C.; Mao, Y.; Liu, K.; Liang, B.; Wu, L.; Shi, X. Study on zoledronic acid reducing acute bone loss and fracture rates in elderly postoperative patients with intertrochanteric fractures. Orthop. Surg. 2019, 11, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-K.; Ha, Y.-C.; Choi, H.J.; Jang, S.; Park, C.; Lim, Y.-T.; Shin, C.S. Bisphosphonate use and subsequent hip fracture in South Korea. Osteoporos. Int. 2013, 24, 2887–2892. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kung, P.; Chou, W.; Tsai, W. Alendronate medication possession ratio and the risk of second hip fracture: An 11-year population-based cohort study in Taiwan. Osteoporos. Int. 2020, 31, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Makras, P.; Babis, G.C.; Chronopoulos, E.; Karachalios, T.; Kazakos, K.; Paridis, D.; Potoupnis, M.; Tzavellas, A.-N.; Valkanis, C.; Kosmidis, C. Experience gained from the implementation of the fracture liaison service in Greece. Arch. Osteoporos. 2020, 15, 12. [Google Scholar] [CrossRef]
- Benjamin, B.; Benjamin, M.A.; Swe, M.; Sugathan, S. Review on the comparison of effectiveness between denosumab and bisphosphonates in post-menopausal osteoporosis. Osteoporos. Sarcopenia 2016, 2, 77–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saito, T.; Sterbenz, J.M.; Malay, S.; Zhong, L.; MacEachern, M.P.; Chung, K.C. Effectiveness of anti-osteoporotic drugs to prevent secondary fragility fractures: Systematic review and meta-analysis. Osteoporos. Int. 2017, 28, 3289–3300. [Google Scholar] [CrossRef] [PubMed]
- Goessl, C.; Katz, L.; Dougall, W.C.; Kostenuik, P.J.; Zoog, H.B.; Braun, A.; Dansey, R.; Wagman, R.B. The development of denosumab for the treatment of diseases of bone loss and cancer-induced bone destruction. Ann. N. Y. Acad. Sci. 2012, 1263, 29–40. [Google Scholar] [CrossRef]
- Austin, M.; Yang, Y.; Vittinghoff, E.; Adami, S.; Boonen, S.; Bauer, D.C.; Bianchi, G.; A Bolognese, M.; Christiansen, C.; Eastell, R.; et al. FREEDOM Trial. Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J. Bone Miner. Res. 2012, 27, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Fahrleitner-Pammer, A.; Papaioannou, N.; Gielen, E.; Tepie, M.F.; Toffis, C.; Frieling, I.; Geusens, P.; Makras, P.; Boschitsch, E.; Callens, J.; et al. Factors associated with high 24-month persistence with denosumab: Results of a real-world, non-interventional study of women with postmenopausal osteoporosis in Germany, Austria, Greece, and Belgium. Arch. Osteoporos. 2017, 12, 1–13. [Google Scholar] [CrossRef]
- Hadji, P.; Papaioannou, N.; Gielen, E.; Tepie, M.F.; Zhang, E.; Frieling, I.; Geusens, P.; Makras, P.; Resch, H.; Moller, G.L.; et al. Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos. Int. 2015, 26, 2479–2489. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Abrahamsen, B.; Al-Daghri, N.M.; Brandi, M.L.; Cannata-Andia, J.; Cortet, B.; Dimai, H.P.; Ferrari, S.; et al. European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Identification and management of patients at increased risk of osteoporotic fracture: Outcomes of an ESCEO expert consensus meeting. Osteoporos. Int. 2017, 28, 2023–2034. [Google Scholar] [CrossRef]
- National Healthcare Service. National Tariff Payment System 2017/2018 and 2018/2019. Annex A: National Tariff Workbook; NHS: Leeds, UK, 2018. [Google Scholar]
- Shah, A.; The REFRESH Study Team. Prieto-Alhambra, D.; Hawley, S.; Delmestri, A.; Lippett, J.; Cooper, C.; Judge, A.; Javaid, M.K. Geographic variation in secondary fracture prevention after a hip fracture during 1999–2013: A UK study. Osteoporos. Int. 2017, 28, 169–178. [Google Scholar] [CrossRef]
- Jennings, L.A.; Auerbach, A.D.; Maselli, J.; Pekow, P.S.; Lindenauer, P.K.; Lee, S.J. Missed opportunities for osteoporosis treatment in patients hospitalized for hip fracture. J. Am. Geriatr. Soc. 2010, 58, 650–657. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshida, H. Low bone mineral density at femoral neck is a predictor of increased mortality in elderly Japanese women. Osteoporos. Int. 2009, 21, 71–79. [Google Scholar] [CrossRef]
- Nguyen, N.D.; Center, J.R.; Eisman, J.A.; Nguyen, T.V. Bone loss, weight loss, and weight fluctuation predict mortality risk in elderly men and women. J. Bone Miner. Res. 2007, 22, 1147–1154. [Google Scholar] [CrossRef]
- Qu, X.; Huang, X.; Jin, F.; Wang, H.; Hao, Y.; Tang, T.; Dai, K. Bone mineral density and all-cause, cardiovascular and stroke mortality: A meta-analysis of prospective cohort studies. Int. J. Cardiol. 2013, 166, 385–393. [Google Scholar] [CrossRef]
- Ahmadi, N.; Mao, S.S.; Hajsadeghi, F.; Arnold, B.; Kiramijyan, S.; Gao, Y.; Flores, F.; Azen, S.; Budoff, M. The relation of low levels of bone mineral density with coronary artery calcium and mortality. Osteoporos. Int. 2018, 29, 1609–1616. [Google Scholar] [CrossRef]
- Cummings, S.R.; Lui, L.-Y.; Eastell, R.; Allen, I.E. Association between drug treatments for patients with osteoporosis and overall mortality rates. JAMA Intern. Med. 2019, 179, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Horne, A.M.; Mihov, B.; Stewart, A.; Bastin, S.; Gamble, G.D. Effect of zoledronate on lower respiratory infections in older women: Secondary analysis of a randomized controlled trial. Calcif. Tissue Int. 2021, 1–5. [Google Scholar] [CrossRef]
- Sing, C.; Kiel, D.P.; Hubbard, R.B.; Lau, W.C.; Li, G.H.; Kung, A.W.; Wong, I.C.; Cheung, C. Nitrogen-containing bisphosphonates are associated with reduced risk of pneumonia in patients with hip fracture. J. Bone Miner. Res. 2020, 35, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Joeris, A.; Hurtado-Chong, A.; Hess, D.; Kalampoki, V.; Blauth, M. Evaluation of the geriatric co-management for patients with fragility fractures of the proximal femur (Geriatric Fracture Centre (GFC) concept): Protocol for a prospective multicentre cohort study. BMJ Open 2017, 7, e014795. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, P.N.; Chen, C.J.; March, L.; Cameron, I.D.; Cumming, R.G.; Lord, S.R.; Simpson, J.M.; Seibel, M.J. High bone turnover is an independent predictor of mortality in the frail elderly. J. Bone Miner. Res. 2006, 21, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Martini, F.; Fratto, M.E.; Galluzzo, S.; Vincenzi, B.; Agrati, C.; Turchi, F.; Piacentini, P.; Rocci, L.; Manavalan, J.S.; et al. In vivo effects of zoledronic acid on peripheral γδ T lymphocytes in early breast cancer patients. Cancer Immunol. Immunother. 2008, 58, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.D.; Warrington, K.J.; Barrow, K.D.; Pugazhenthi, M.; Watsky, M.A.; Somes, G.; Ingels, J.; Postlethwaite, A.E. Pamidronate infusion in patients with systemic sclerosis results in changes in blood mononuclear cell cytokine profiles. Clin. Exp. Immunol. 2006, 146, 371–380. [Google Scholar] [CrossRef]
- Sawalha, S.; Parker, M.J. Characteristics and outcome in patients sustaining a second contralateral fracture of the hip. J. Bone Jt. Surg. Br. Vol. 2012, 94, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.; Oden, A.; Johnell, O.; De Laet, C.; Jonsson, B.; Oglesby, A. The components of excess mortality after hip fracture. Bone 2003, 32, 468–473. [Google Scholar] [CrossRef]
- Senohradski, K.; Markovic-Denic, L.; Lešić, A.; Bumbasirevic, V.; Bumbasirevic, M. Trends in the incidence of hip fractures. Osteoporos. Int. 2013, 24, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
| Authors/Year | Agent Investigated | Study Type | n | Main Results | Limitations |
|---|---|---|---|---|---|
| Mortality | |||||
| Behanova et al., 2019 [13] | oBP iBP DMAB | retrospective | 54,145 | - 17% lower risk of dying in treated women (HR 0.83, 0.71–0.98, p = 0.023) statistically significant only for intravenous bisphosphonates | - lack of criteria associated with treatment prescription - no data regarding compliance with treatment |
| Bondo et al., 2013 [14] | BP | cohort study | 42,076 | - lower 3-month mortality in BP-treated patients before fracture (OR 0.68, 0.59–0.77, OR 0.73, 0.61–0.88) for treatment initiation after the fracture | - possible confounders: lower mortality even in patients who filled only one prescription (OR = 0.84, CI 95% 0.73–0.95) |
| Bergman et al., 2019 [15] | BP | retrospective, cohort | 49,765 | - 15% lower mortality (HR 0.85, 0.79–0.91) in BP-treated patients | -possible confounders: the risk was lower starting from day 6 of treatment |
| Sambrook et al., 2011 [16] | BP | randomized prospective | 220 | - BP treatment was associated with reduced mortality after hip fracture (aHR of 0.92 per month treated) 8% relative reduction per month and 63% per year of treatment | -relatively young and healthy hip fracture cohort -small number of patients |
| Brozek et al., 2016 [17] | BP | retrospective | 31,668 | - lower risk of mortality with BP treatment before and after the hip fracture (lowest HR of 0.43 for treatment started after hip fracture) | -no data regarding compliance to treatment |
| Peng et al., 2016 [18] | BP | metanalysis of 4 trials (2 randomized and 2 prospective matched controlled studies) | 3088 | - lower mortality risk in BP-treated patients (OR 0.66, 0.52–0.85, p = 0.001) | - few studies, mixed randomized and non-randomized studies no data regarding the type of BP, dose, duration - no statistical heterogeneity (I2 = 35%) -dominated results by the HORIZON Recurrent Fracture Trial [12] |
| Cobden et al., 2019 [19] | BP | retrospective multicenter study | 562 | - 5-year survival rate was 16% for BP-treated patients compared to 5% for non-treated ones (p = 0.002); | - retrospective study no predictive risk factors for mortality included in the analysis - only patients after hemiarthroplasty were investigated -small number of patients |
| van Geel et al., 2018 [20] | BP | prospective cohort study | 5011 (2534 on BP) | - lower mortality rate for BP treated patients (HR 0.79, 0.64–0.97) in 8 years of follow-up | - no data regarding adherence to treatment -patients included in the Fracture Liaison Services with more medical interventions included than anti-osteoporosis treatment -all major fracture included |
| Wang et al., 2019 [21] | BP | retrospective | 690 | - lower mortality for BP and non-BP osteoporosis medication (HR 0.35, 0.19–0.64) and (HR 0.49, 0.34–0.69) | - retrospective study -only 57% of the fractures were followed -small number of patients |
| Abtani et al., 2020 [22] | BP | population-based cohort study | 163,273 | - 28% lower mortality after hip fracture in current BP-treated patients - 42% lower mortality after hip fracture in patients with past BP exposure | - no data regarding adherence to treatment |
| Boonen et al., 2011 [23] | Zoledronic Acid | randomized, placebo-controlled, double-blind trial | 508 men (248/260) 1619 women | - lower mortality after 5 mg of Zoledronic (HR 0.71, 0.46–1.31 in men) and (HR 0.74, 0.54–1.02 in women) | - short median follow-up of 1.9 years |
| Prieto-Alhambra et al., 2014 [24] | Zoledronic Acid | secondary analysis from HORIZON randomized controlled trial | 4093 1966/2127 | - lower mortality rates in the treatment arm (6.2% compared to 10.5%, in the placebo arm, p < 0.001) - smaller difference in mortality rates between the two groups in cognitive impaired patients (23.2% compared to 26.9%) | - study design with main focus on cognitive impaired patients |
| Sing et al., 2018 [25] | BP | retrospective cohort study | 4594/13,568 | - lower cardiovascular mortality rate (HR 0.33, 0.17–0.65) for alendronate and (HR 0.35, 0.2–0.63) for other BPs at 1 year -the lower realtive risk is mantained up to ten years of follow-up for alendronate (HR 0.59 0.44–0.79, p) or other BPs (HR 0.58, 0.44–0.75) | - no clear criteria for treatment recommendation - only one prescription needed for inclusion - possible confounding cardiovascular risk factor not included |
| Nordstrom et al., 2017 [26] | BP | restropective cohort study | 5845/15,518 | - decreased risk of death adjusted for all covariates (HR 0.79, 0.73–0.85) | -observational study -high mean time between the hip fracture and initiation of BP (331 days, rage of 1 to 2770 |
| Center et al., 2011 [27] | BP | prospective cohort Dubbo study | 1223 women/819 men (429 with fractures) | -lower risk of mortality in women (HR 0.33, 0.16–0.66) in the multivariate analysis | -no additional data regarding fracture type or the number of hip fractures included -observational study - lack of treatment recommendation criteria |
| Degli Esposti et al., 2012 [28] | Mainly BP | retrospective cohort study | 5636 (187 pre-fracture/651 post fracture) | - 62.3% lower risk of death (−73.4%–−46.6%) in treated patients, (HR 0.377, 0.266–0.534, p < 0.001) | -retrospective study with small number of patients -common analysis for all anti-osteoporosis agents -all hip fractures regardless of traumatic event |
| Han et al., 2020 [29] | Teriparatide | metanalysis of 6 studies (2 randomized and 4 retraospective studies) | 607 (269/338) | - lower mortality in the teriparatide group in the fixed model (OR 0.34, 0.13–0.88, p = 0.03) but the random-effect was not significant (OR 0.37, 0.12–1.09, p = 0.07) | - no heterogeneity (I2 of 4%) -observational studies that can exaggerate the effects -inconstant treatment duration -confounding factors not assessed |
| Hsu et al., 2020 [30] | BP/Raloxifene/Teriparatide/Denosumab | retrospective cohort study | 946 (210/736) | - lower risk of mortality in the persistence group (HR 0.83, 0.62–1.11), p = 0.21 for all anti-osteoporosis treatment - higher risk of mortality in patients aged 70–79 (aHR = 1.29, 1.08–4.86, p = 0.031) and >80 (aHR = 3.11, 1.49–6.5, p = 0.003) in persistence group | - small number of patients - no separate analysis for the non-BP agents - certain confounding factors not assessed |
| Lebanon et al., 2020 [31] | Zoledronic acid (46.3%)/Denosumab (34.1%)/Teriparatide | prospective cohort study | 253 (85/168) | -mortality rate in treated patients was 5.1% at one year compared to 26.3% in naive patients (p < 0.001) | - possible effects of calcium and vitamin D - small number of patients |
| Gonzalez-Quevedo et al., 2020 [32] | any treatment | prospective cohort study | 724 | -all-cause mortality was lower in the FLS-treated group (HR 0.66, 0.47–0.94) compared to all patients before FLS - treatment after the implementation of the FLS associated higher mortality risk compared to treatment initiated before (1.75, 0.54–5.49) | - small number of patients - no cause-and-effect relationtioship between mortality and FLS - small number of patients - patients included in the FLS with more medical interventions included than anti-osteoporosis treatment |
| Rotman-Pikielny et al., 2018 [33] | any treatment | prospective cohort study | 218/219 | - lower mortality rates in treated patients (4.3% versus 21.8%) with a 53% decrease in mortality risk in female sex (HR 0.47, 0.30-0.72, p < 0.001) - | - small number of patients - outcomes observed in a multidiscliplinary team with probability of secondary unrelated effects in compliant patients - confounding factors not assessed |
| Second Fracture | |||||
| Shen et al., 2014 [34] | BP | nationwide population-based longitudinal observational study | 87,415 | - BP treatment after hip fracture had a negative risk association with second fracture (20.8% versus 32.3%, p = 0.023), aOR = 2.24, 1.38–2.90, p = 0.017 | - register-based study |
| Palacios et al., 2015 [35] | DMAB | randomized post hoc analysis FREEDOM | 7808 | - 39% relative risk reduction for a secondary fragility fracture in denosumab treated patients (10.5% compared to 17.3% in the non-treated group, p < 0.0001) | - post hoc analysis |
| Behanova et al., 2019 [13] | oBP iBP DMAB | retrospective | 54,145 1919 oBP 1870 iBP 555 DMAB 42,795 untreated | - higher risk of subsequent hip fracture in patients with antiresorptive treatment: men with oBP (HR 2.89, 1.58–5.30), women on DMAB (HR 1.77, 1.08–2.91), and iBP (HR 1.81, 1.35–2.41) | - lack of data regarding adherence to treatment - short follow-up period - data regarding criteria for treatment recommendation |
| Sambrook et al., 2011 [16] | BP | randomized prospective | 220 | - additional fractures were observed in 53% without treatment and 26% BP treatment before the event | - relatively young and healthy hip fracture cohort -small number of patients |
| Brozek et al., 2016 [17] | BP | retrospective | 31,668 | - higher hip refracture rates in BP treated patients, before or after the index hip fracture, regardless of age - OR 1.87, 1.32–2.66 of second fracture in BP users age 70–84, after 1-year post fracture | - no data regarding compliance to treatment |
| Peng et al., 2016 [18] | BP | metanalysis of 4 trials (2 randomized and 2 prospective matched controlled studies) | 3088 | - second hip fracture different rate between the BP group and the control group (mean difference of 0.6, 0.39–0.93, p = 0.02) | - few studies, mixed randomized and non-randomized studies no data regarding the type of BP, dose, duration - no statistical heterogeneity (I2 = 35%) |
| Cobden et al., 2019 [19] | any treatment | retrospective multicenter study | 562 | - no significant differences in second fracture risk | -small number of patients - only patients after hemiarthroplasty were investigated |
| van Geel et al., 2018 [20] | BP | prospective cohort study | 5011 (2534 on BP) | - lower risk for subsequent fractures (HR 0.60, 0.49–0.73) | - no data regarding adherence to treatment -patients included in the Fracture Liaison Services with more medical interventions included than anti-osteoporosis treatment -all major fracture included |
| Osaki et al., 2012 [36] | Risedronate | prospective matched cohort study | 529 (173/356) | -HR 0.31, 0.12–0.79 in univariate and 0.218, 0.074–0.63 in multivariate analysis for subsequent fracture risk in risedronate treated patients | -no randomization -history of BP treatment in the control group -small number of patients |
| Liu et al., 2019 [37] | Zoledronic Acid | randomized controlled trial | 482 (353/129) | - lower refracture rate in the treatment group (5.9% compared to 8.5%, p < 0.01) | -short observation time -small number of patients |
| Prieto-Alhambra et al., 2014 [24] | Zoledronic Acid | secondary analysis from HORIZON randomized controlled trial | 4093 1966/2127 | - no significant correlation between treatment arm and placebo arm regarding re-fracture (p > 0.05) | - study design with main focus on cognitively impaired patients |
| Lee et al., 2013 [38] | BP | retrospective | 59,782 | - lower incidence of second hip fracture in compliant patients compared to non-compliant (0.8% versus 2.3%, p < 0.001) - lower incidence of second hip fracture in persistent users compared to non-persistent (0.9% versus 2.4%, p < 0.001) | -retrospective study -all hip fractures included regardless of the traumatic event |
| Boonen et al., 2011 [23] | Zoledronic Acid | randomized, placebo-controlled, double-blind trial | 508 men (248/260) 1619 women | - 8.9% rate of new fractures in zoledronic acid treated compared to 15.6% in placebo-treated women | - short median follow-up of 1.9 years |
| Nordstrom et al., 2017 [26] | BP | restropective cohort study | 5845/15,518 | - after BP initiation, the risk of hip fracture was lower (HR 0.76, 0.65–0.90) - BP users of more than 90 days had a lower risk of new hip fracture (HR 0.69, 0.54–0.87) - effect on second hip fracture risk decrease was seen even in later BP users with OR of 2.22 compared to 2.63 in never users - a decrease for any subsequent fracture was seen (HR 0.90, 0.78–1.04) | -observational study - new fracture data based on the national registry entries -high mean time between the hip fracture and the initiation of BP (331 days, rage of 1–2770) |
| Han et al., 2020 [29] | Teriparatide | metanalysis of 6 studies (2 randomized and 4 retraospective studies) | 607 (269/338) | - no significant difference in subsequent fracture risk between the two groups (OR 0.60, 0.30–1.18, p = 0.14) | - no heterogeneity (I2 of 0%) -observational studies that can exaggerate the effects -inconstant treatment duration -confounding factors not assessed |
| Degli Esposti et al., 2012 [28] | Mainly BP | retrospective cohort study | 5636 (187 pre-fracture/651 post fracture) | - lower risk of re-fracture of -53.3% (−67.3% – −33.2%), p < 0.001 | -retrospective study with small number of patients -common analysis for all anti-osteoporosis agents -all hip fractures regardless of the traumatic event |
| Hsu et al., 2020 [30] | BP/Raloxifene/Teriparatide/Denosumab | retrospective cohort study | 946 (210/736) | - lower rate of recurrent fractures in the persistence group for all agents (aHR 0.64, 0.49–0.99, p = 0.043) -persistence BP users with lower recurrent fracture rates (aHR 0.54, 0.32–0.90, p = 0.018) | - small number of patients -no separate analysis for the non-BP agent- certain confounding factors not assessed |
| Gonzalez-Quevedo et al., 2020 [32] | any treatment | prospective cohort study | 724 | - no statistically significant difference between groups | - small number of patients;- no cause-and-effect relationtioship between mortality and FLS - small number of patients - patients included in the FLS with more medical interventions included than anti-osteoporosis treatment |
| Chen et al., 2020 [39] | Alendronate | population-based cohort study | 88,320 (9278/79,042 | -lowest risk of second fracture in the MPR 75–100% (aHR 0.61, 0.47–0.78, p < 0.001) | - certain confounding factors not assessed - incomplete treatment adherence data - lack of criteria choice for aledronate treatment |
| Makras et al., 2020 [40] | any treatment | multicenter prospective study | 392 | - significant increase in new fractures in patients not receiving anti-osteoporosis treatment, p < 0.001 | -small number of patients -small period of treatment- effects possibly related to the FLS interventions besides treatment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobre, R.; Niculescu, D.A.; Petca, R.-C.; Popescu, R.-I.; Petca, A.; Poiană, C. Adherence to Anti-Osteoporotic Treatment and Clinical Implications after Hip Fracture: A Systematic Review. J. Pers. Med. 2021, 11, 341. https://doi.org/10.3390/jpm11050341
Dobre R, Niculescu DA, Petca R-C, Popescu R-I, Petca A, Poiană C. Adherence to Anti-Osteoporotic Treatment and Clinical Implications after Hip Fracture: A Systematic Review. Journal of Personalized Medicine. 2021; 11(5):341. https://doi.org/10.3390/jpm11050341
Chicago/Turabian StyleDobre, Ramona, Dan Alexandru Niculescu, Răzvan-Cosmin Petca, Răzvan-Ionuț Popescu, Aida Petca, and Cătălina Poiană. 2021. "Adherence to Anti-Osteoporotic Treatment and Clinical Implications after Hip Fracture: A Systematic Review" Journal of Personalized Medicine 11, no. 5: 341. https://doi.org/10.3390/jpm11050341
APA StyleDobre, R., Niculescu, D. A., Petca, R.-C., Popescu, R.-I., Petca, A., & Poiană, C. (2021). Adherence to Anti-Osteoporotic Treatment and Clinical Implications after Hip Fracture: A Systematic Review. Journal of Personalized Medicine, 11(5), 341. https://doi.org/10.3390/jpm11050341







