Comprehensive NGS Panel Validation for the Identification of Actionable Alterations in Adult Solid Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Panel Design
2.2. Sample Selection
2.3. DNA Extraction and Quality Control
2.4. Library Preparation and Next-Generation Sequencing
2.5. Bioinformatic Pipeline
2.6. Variant Functional and Clinical Classification
3. Results
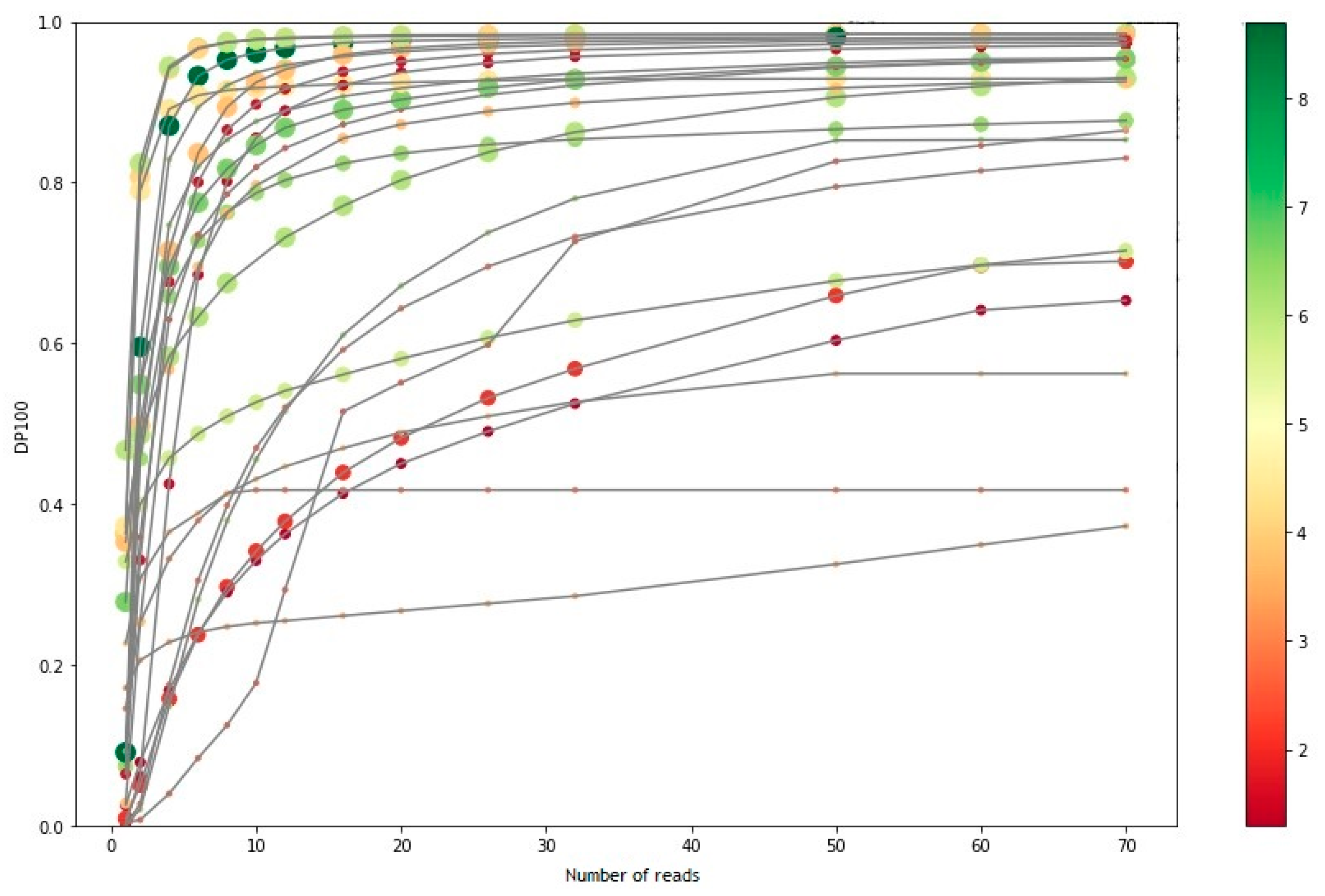
3.1. Sequencing Performance
3.2. Analytical Sensitivity and Specificity
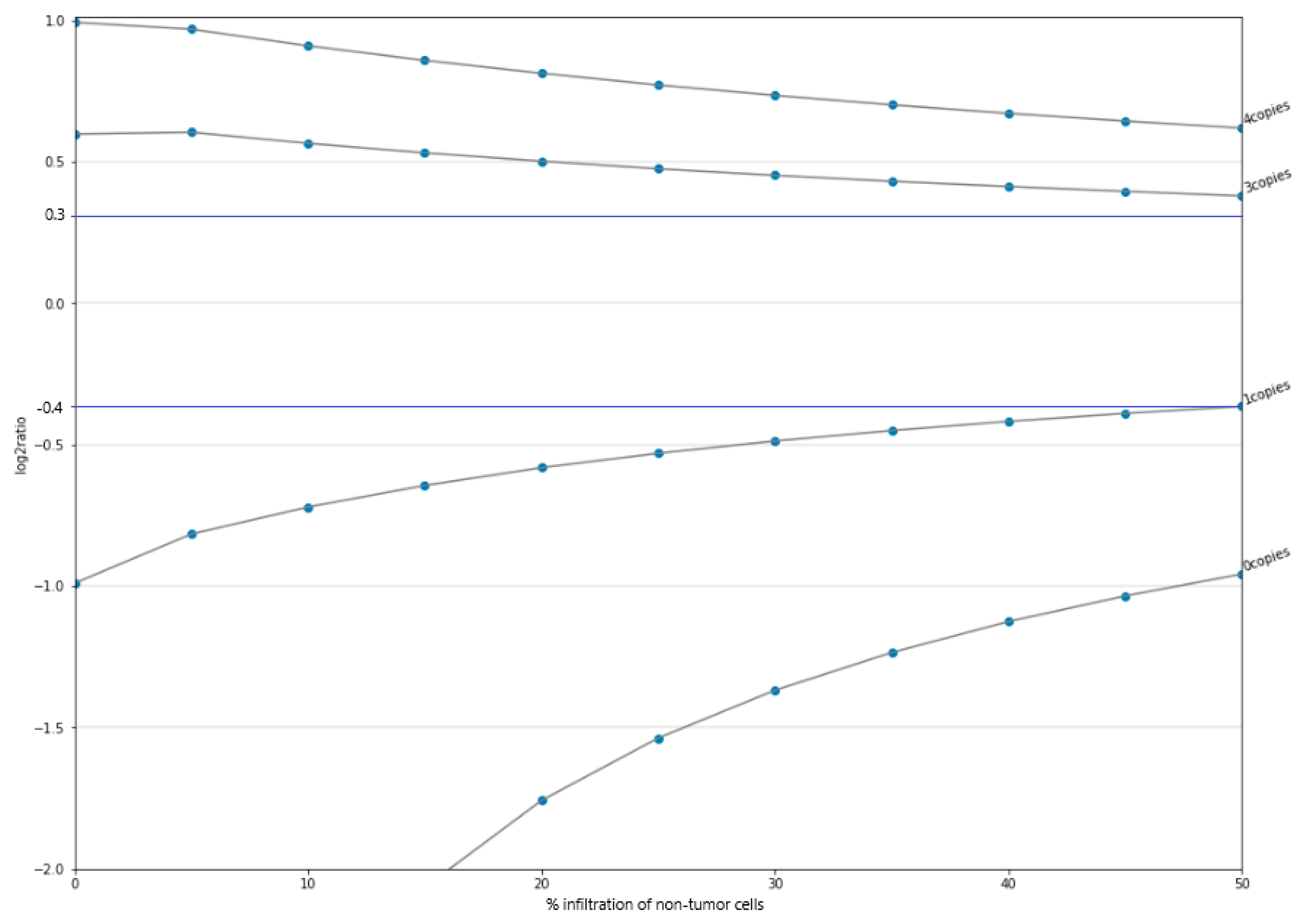
3.3. Limit-of-Detection
3.4. Repeatability and Reproducibility
3.5. Number of Samples per Run
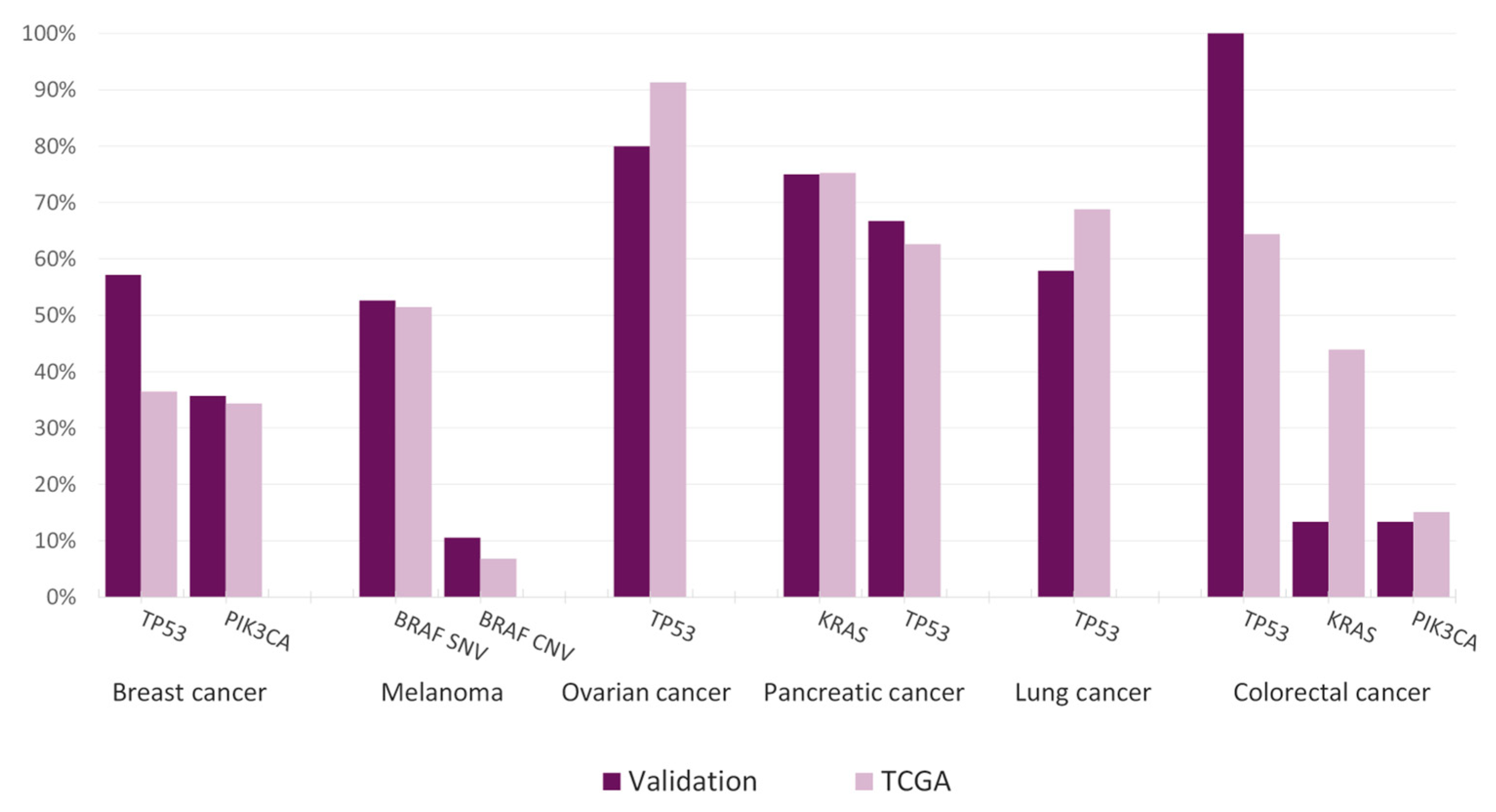
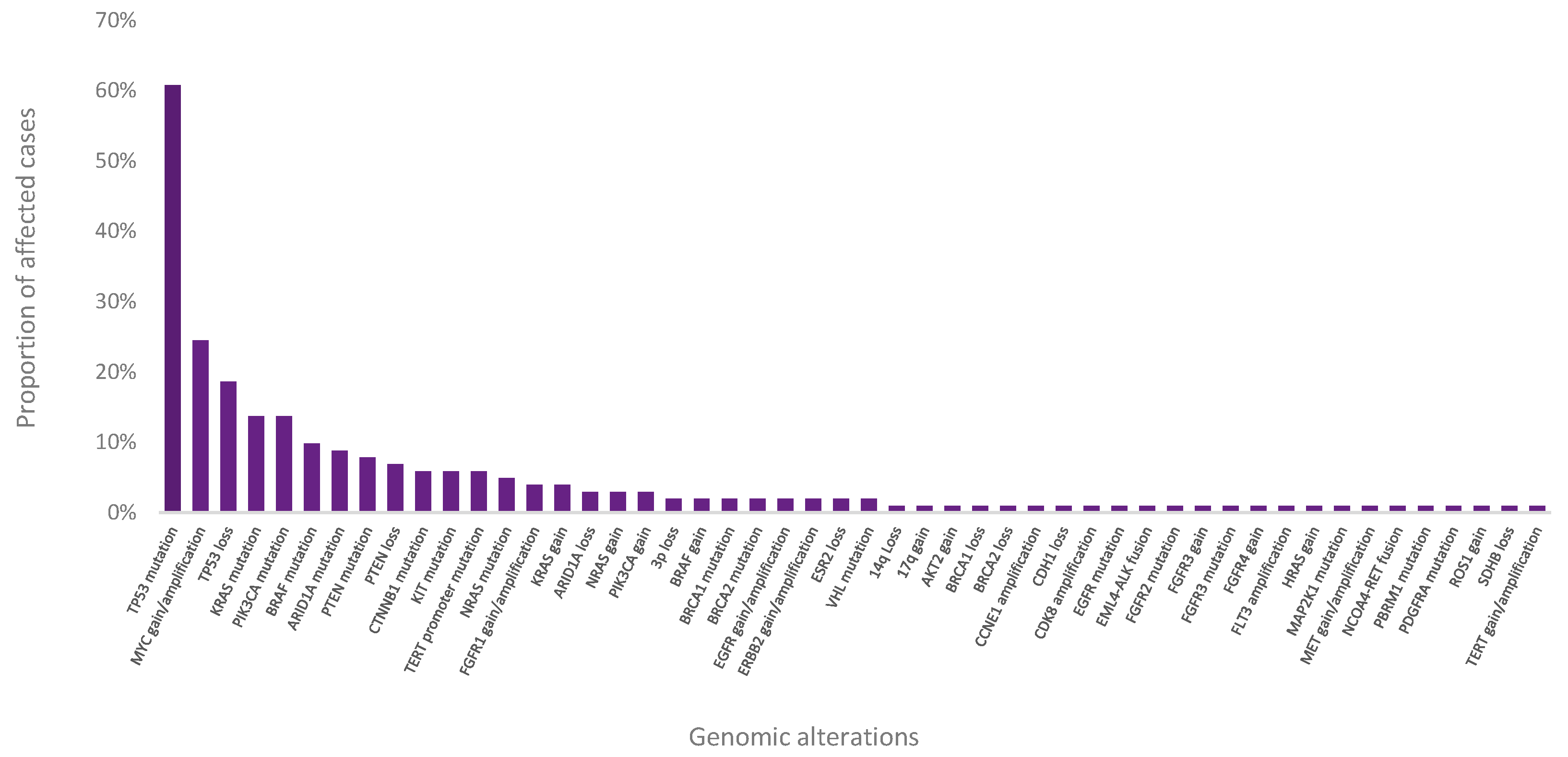
3.6. Clinical Feasibility
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular Targeted Therapy: Treating Cancer with Specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kamps, R.; Brandão, R.; Bosch, B.; Paulussen, A.; Xanthoulea, S.; Blok, M.; Romano, A. Next-Generation Sequencing in Oncology: Genetic Diagnosis, Risk Prediction and Cancer Classification. Int. J. Mol. Sci. 2017, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, N.; Roviello, G.; Corona, S.P.; Scaltriti, M.; Ianza, A.; Bortul, M.; Zanconati, F.; Generali, D. The Prognostic Value of PI3K Mutational Status in Breast Cancer: A Meta-analysis. J. Cell. Biochem. 2018, 119, 4287–4292. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Alpelisib: First Global Approval. Drugs 2019, 79, 1249–1253. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Huang, D.; Tang, L.; Yang, F.; Jin, J.; Guan, X. PIK3CA Mutations Contribute to Fulvestrant Resistance in ER-Positive Breast. Am. J. Transl. Res. 2019, 11, 6055. [Google Scholar]
- Rimawi, M.F.; De Angelis, C.; Contreras, A.; Pareja, F.; Geyer, F.C.; Burke, K.A.; Herrera, S.; Wang, T.; Mayer, I.A.; Forero, A.; et al. Low PTEN Levels and PIK3CA Mutations Predict Resistance to Neoadjuvant Lapatinib and Trastuzumab without Chemotherapy in Patients with HER2 Over-Expressing Breast Cancer. Breast Cancer Res. Treat 2018, 167, 731–740. [Google Scholar] [CrossRef]
- Kataoka, Y.; Mukohara, T.; Shimada, H.; Saijo, N.; Hirai, M.; Minami, H. Association between Gain-of-Function Mutations in PIK3CA and Resistance to HER2-Targeted Agents in HER2-Amplified Breast Cancer Cell Lines. Ann. Oncol. 2010, 21, 255–262. [Google Scholar] [CrossRef]
- Olivier, M.; Langer, A.; Carrieri, P.; Bergh, J.; Klaar, S.; Eyfjord, J.; Theillet, C.; Rodriguez, C.; Lidereau, R.; Bi, I.; et al. The Clinical Value of Somatic TP53 Gene Mutations in 1794 Patients with Breast Cancer. Clin. Cancer Res. 2006, 12, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Silwal-Pandit, L.; Vollan, H.K.M.; Chin, S.-F.; Rueda, O.M.; McKinney, S.; Osako, T.; Quigley, D.A.; Kristensen, V.N.; Aparicio, S.; Børresen-Dale, A.-L.; et al. TP53 Mutation Spectrum in Breast Cancer Is Subtype Specific and Has Distinct Prognostic Relevance. Clin. Cancer Res. 2014, 20, 3569–3580. [Google Scholar] [CrossRef]
- Xie, M.; Li, J.; Cai, Z.; Li, K.; Hu, B. Impact of Primary Colorectal Cancer Location on the KRAS Status and Its Prognostic Value. BMC Gastroenterol. 2019, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.H.; Tougeron, D.; Shi, Q.; Alberts, S.R.; Mahoney, M.R.; Nelson, G.D.; Nair, S.G.; Thibodeau, S.N.; Goldberg, R.M.; Sargent, D.J.; et al. KRAS Codon 12 and 13 Mutations in Relation to Disease-Free Survival in BRAF–Wild-Type Stage III Colon Cancers from an Adjuvant Chemotherapy Trial (N0147 Alliance). Clin. Cancer Res. 2014, 20, 3033–3043. [Google Scholar] [CrossRef] [PubMed]
- Marchand, A.; Tallet, A.; Collin, C.; Cormier, B.; Venel, Y.; Miquelestorena-Standley, E.; Machet, L. A Rare BRAF T599dup Mutation Conferring Sensitivity to BRAF Inhibitor in a Patient with Metastatic Melanoma. Br. J. Dermatol. 2018, 179, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Yang, K.; Hao, X.; Wang, Y.; Wang, L.; Liu, Y.; Lin, L.; Li, J.; Xing, P. Clinical Characteristics and Treatment Outcomes of 65 Patients With BRAF-Mutated Non-Small Cell Lung Cancer. Front. Oncol. 2020, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, R.D.; Lawrence, D.P.; Weber, J.S.; Gajewski, T.F.; Gonzalez, R.; Lutzky, J.; O’Day, S.J.; Hamid, O.; Wolchok, J.D.; Chapman, P.B.; et al. Phase II Study of Nilotinib in Melanoma Harboring KIT Alterations Following Progression to Prior KIT Inhibition. Clin. Cancer Res. 2015, 21, 2289–2296. [Google Scholar] [CrossRef]
- Balch, C.M.; Soong, S.; Gershenwald, J.E.; Thompson, J.F.; Coit, D.G.; Atkins, M.B.; Ding, S.; Cochran, A.J.; Eggermont, A.M.M.; Flaherty, K.T.; et al. Age as a Prognostic Factor in Patients with Localized Melanoma and Regional Metastases. Ann. Surg. Oncol. 2013, 20, 3961–3968. [Google Scholar] [CrossRef]
- Maurichi, A.; Miceli, R.; Camerini, T.; Mariani, L.; Patuzzo, R.; Ruggeri, R.; Gallino, G.; Tolomio, E.; Tragni, G.; Valeri, B.; et al. Prediction of Survival in Patients With Thin Melanoma: Results From a Multi-Institution Study. J. Clin. Oncol. 2014, 32, 2479–2485. [Google Scholar] [CrossRef]
- Eriksson, H.; Frohm-Nilsson, M.; Järås, J.; Kanter-Lewensohn, L.; Kjellman, P.; Månsson-Brahme, E.; Vassilaki, I.; Hansson, J. Prognostic Factors in Localized Invasive Primary Cutaneous Malignant Melanoma: Results of a Large Population-Based Study. Br. J. Dermatol. 2015, 172, 175–186. [Google Scholar] [CrossRef]
- Queirolo, P.; Spagnolo, F. Binimetinib for the Treatment of NRAS-Mutant Melanoma. Expert Rev. Anticancer Ther. 2017, 17, 985–990. [Google Scholar] [CrossRef]
- Dummer, R.; Schadendorf, D.; Ascierto, P.A.; Arance, A.; Dutriaux, C.; Di Giacomo, A.M.; Rutkowski, P.; Del Vecchio, M.; Gutzmer, R.; Mandala, M.; et al. Binimetinib versus Dacarbazine in Patients with Advanced NRAS-Mutant Melanoma (NEMO): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2017, 18, 435–445. [Google Scholar] [CrossRef]
- Sarkisian, S.; Davar, D. MEK Inhibitors for the Treatment of NRAS Mutant Melanoma. Drug Des. Dev. Ther. 2018, 12, 2553–2565. [Google Scholar] [CrossRef] [PubMed]
- Specenier, P. An Overview of Binimetinib for the Treatment of Melanoma. Expert Opin. Pharmacother. 2020, 21, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Buscail, L.; Bournet, B.; Cordelier, P. Role of Oncogenic KRAS in the Diagnosis, Prognosis and Treatment of Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, X.; Li, F.; Xiao, D.; Hou, Y.; Zhu, S.; Liu, D.; Ye, X.; Ye, M.; Yang, J.; et al. Frequent Alterations in Cytoskeleton Remodelling Genes in Primary and Metastatic Lung Adenocarcinomas. Nat. Commun. 2015, 6, 10131. [Google Scholar] [CrossRef] [PubMed]
- Scoccianti, C.; Vesin, A.; Martel, G.; Olivier, M.; Brambilla, E.; Timsit, J.-F.; Tavecchio, L.; Brambilla, C.; Field, J.K.; Hainaut, P.; et al. Prognostic Value of TP53, KRAS and EGFR Mutations in Nonsmall Cell Lung Cancer: The EUELC Cohort. Eur. Respir. J. 2012, 40, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeon, H.-S.; Hwangbo, Y.; Jeong, J.Y.; Park, J.Y.; Lee, E.J.; Jin, G.; Shin, K.M.; Yoo, S.S.; Lee, J.; et al. The Influence of TP53 Mutations on the Prognosis of Patients with Early Stage Non-Small Cell Lung Cancer May Depend on the Intratumor Heterogeneity of the Mutations: Intratumor heterogeneity of tp53 mutations in NSCLC. Mol. Carcinog. 2015, 54, 93–101. [Google Scholar] [CrossRef]
- Santos, C.; Sanz-Pamplona, R.; Salazar, R. RET-Fusions: A Novel Paradigm in Colorectal Cancer. Ann. Oncol. 2018, 29, 1340–1343. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, J.; Muñoz-Couselo, E.; Soberino, J.; Racca, F.; Cortes, J. Targeting FGFR Pathway in Breast Cancer. Breast 2018, 37, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef]
- Elbauomy Elsheikh, S.; Green, A.R.; Lambros, M.B.; Turner, N.C.; Grainge, M.J.; Powe, D.; Ellis, I.O.; Reis-Filho, J.S. FGFR1 Amplification in Breast Carcinomas: A Chromogenic in Situhybridisation Analysis. Breast Cancer Res. 2007, 9, R23. [Google Scholar] [CrossRef]
- Kraehn, G.M.; Utikal, J.; Udart, M.; Greulich, K.M.; Bezold, G.; Kaskel, P.; Leiter, U.; Peter, R.U. Extra C-Myc Oncogene Copies in High Risk Cutaneous Malignant Melanoma and Melanoma Metastases. Br. J. Cancer 2001, 84, 72–79. [Google Scholar] [CrossRef]
- Levva, S.; Kotoula, V.; Kostopoulos, I.; Manousou, K.; Papadimitriou, C.; Papadopoulou, K.; Lakis, S.; Koukoulias, K.; Karavasilis, V.; Pentheroudakis, G.; et al. Prognostic Evaluation of Epidermal Growth Factor Receptor (EGFR) Genotype and Phenotype Parameters in Triple-Negative Breast Cancers. Cancer Genom. Proteom. 2017, 14, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Gossage, L.; Eisen, T.; Maher, E.R. VHL, the Story of a Tumour Suppressor Gene. Nat. Rev. Cancer 2015, 15, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, P.T.; Drapkin, R. Pathogenesis and Heterogeneity of Ovarian Cancer. Curr. Opin. Obstet. Gynecol. 2017, 29, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Etemadmoghadam, D.; Weir, B.A.; Au-Yeung, G.; Alsop, K.; Mitchell, G.; George, J.; Australian Ovarian Cancer Study Group; Davis, S.; D’Andrea, A.D.; Simpson, K.; et al. Synthetic Lethality between CCNE1 Amplification and Loss of BRCA1. Proc. Natl. Acad. Sci. USA 2013, 110, 19489–19494. [Google Scholar] [CrossRef] [PubMed]
- De’ Angelis, G.L.; Bottarelli, L.; Azzoni, C.; de’ Angelis, N.; Leandro, G.; Di Mario, F.; Gaiani, F.; Negri, F. Microsatellite Instability in Colorectal Cancer. Acta Biomed. 2018, 89, 97–101. [Google Scholar] [CrossRef]
- Kubeček, O.; Kopecký, J. Microsatellite Instability in Melanoma: A Comprehensive Review. Melanoma Res. 2016, 26, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Baretti, M.; Le, D.T. DNA Mismatch Repair in Cancer. Pharmacol. Ther. 2018, 189, 45–62. [Google Scholar] [CrossRef]
- Stadler, Z.K.; Battaglin, F.; Middha, S.; Hechtman, J.F.; Tran, C.; Cercek, A.; Yaeger, R.; Segal, N.H.; Varghese, A.M.; Reidy-Lagunes, D.L.; et al. Reliable Detection of Mismatch Repair Deficiency in Colorectal Cancers Using Mutational Load in Next-Generation Sequencing Panels. J. Clin. Oncol. 2016, 34, 2141–2147. [Google Scholar] [CrossRef]
- Waalkes, A.; Smith, N.; Penewit, K.; Hempelmann, J.; Konnick, E.Q.; Hause, R.J.; Pritchard, C.C.; Salipante, S.J. Accurate Pan-Cancer Molecular Diagnosis of Microsatellite Instability by Single-Molecule Molecular Inversion Probe Capture and High-Throughput Sequencing. Clin. Chem. 2018, 64, 950–958. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.P.; Minkovsky, A.; Jia, Y.; Ducar, M.D.; Shivdasani, P.; Gong, X.; Ligon, A.H.; Sholl, L.M.; Kuo, F.C.; MacConaill, L.E.; et al. Validation of OncoPanel: A Targeted Next-Generation Sequencing Assay for the Detection of Somatic Variants in Cancer. Arch. Pathol. Lab. Med. 2017, 141, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Rankin, A.; Klempner, S.J.; Erlich, R.; Sun, J.X.; Grothey, A.; Fakih, M.; George, T.J.; Lee, J.; Ross, J.S.; Stephens, P.J.; et al. Broad Detection of Alterations Predicted to Confer Lack of Benefit from EGFR Antibodies or Sensitivity to Targeted Therapy in Advanced Colorectal Cancer. Oncologist 2016, 21, 1306–1314. [Google Scholar] [CrossRef]
- Boussemart, L.; Nelson, A.; Wong, M.; Ross, J.S.; Sosman, J.; Mehnert, J.; Daniels, G.; Kendra, K.; Ali, S.M.; Miller, V.A.; et al. Hybrid Capture-Based Genomic Profiling Identifies BRAF V600 and Non-V600 Alterations in Melanoma Samples Negative by Prior Testing. Oncologist 2019, 24, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Wang, L.; Arcila, M.E.; Balasubramanian, S.; Greenbowe, J.R.; Ross, J.S.; Stephens, P.; Lipson, D.; Miller, V.A.; Kris, M.G.; et al. Broad, Hybrid Capture–Based Next-Generation Sequencing Identifies Actionable Genomic Alterations in Lung Adenocarcinomas Otherwise Negative for Such Alterations by Other Genomic Testing Approaches. Clin. Cancer Res. 2015, 21, 3631–3639. [Google Scholar] [CrossRef]
- Ali, S.M.; Hensing, T.; Schrock, A.B.; Allen, J.; Sanford, E.; Gowen, K.; Kulkarni, A.; He, J.; Suh, J.H.; Lipson, D.; et al. Comprehensive Genomic Profiling Identifies a Subset of Crizotinib-Responsive ALK-Rearranged Non-Small Cell Lung Cancer Not Detected by Fluorescence In Situ Hybridization. Oncologist 2016, 21, 762–770. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Masucci, G.V.; Cesano, A.; Hawtin, R.; Janetzki, S.; Zhang, J.; Kirsch, I.; Dobbin, K.K.; Alvarez, J.; Robbins, P.B.; Selvan, S.R.; et al. Validation of Biomarkers to Predict Response to Immunotherapy in Cancer: Volume I—Pre-Analytical and Analytical Validation. J. Immunother. Cancer 2016, 4, 76. [Google Scholar] [CrossRef]
- Grigg, C.; Rizvi, N.A. PD-L1 Biomarker Testing for Non-Small Cell Lung Cancer: Truth or Fiction? J. Immunother. Cancer 2016, 4, 48. [Google Scholar] [CrossRef]
- Madore, J.; Strbenac, D.; Vilain, R.; Menzies, A.M.; Yang, J.Y.H.; Thompson, J.F.; Long, G.V.; Mann, G.J.; Scolyer, R.A.; Wilmott, J.S. PD-L1 Negative Status Is Associated with Lower Mutation Burden, Differential Expression of Immune-Related Genes, and Worse Survival in Stage III Melanoma. Clin. Cancer Res. 2016, 22, 3915–3923. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Fernández, P.; Pose, P.; Dolz-Gaitón, R.; García, A.; Trigo-Sánchez, I.; Rodríguez-Zarco, E.; Garcia-Ruiz, M.; Barba, I.; Izquierdo-García, M.; Valero-Garcia, J.; et al. Comprehensive NGS Panel Validation for the Identification of Actionable Alterations in Adult Solid Tumors. J. Pers. Med. 2021, 11, 360. https://doi.org/10.3390/jpm11050360
Martínez-Fernández P, Pose P, Dolz-Gaitón R, García A, Trigo-Sánchez I, Rodríguez-Zarco E, Garcia-Ruiz M, Barba I, Izquierdo-García M, Valero-Garcia J, et al. Comprehensive NGS Panel Validation for the Identification of Actionable Alterations in Adult Solid Tumors. Journal of Personalized Medicine. 2021; 11(5):360. https://doi.org/10.3390/jpm11050360
Chicago/Turabian StyleMartínez-Fernández, Paula, Patricia Pose, Raquel Dolz-Gaitón, Arantxa García, Inmaculada Trigo-Sánchez, Enrique Rodríguez-Zarco, MJose Garcia-Ruiz, Ibon Barba, Marta Izquierdo-García, Jennifer Valero-Garcia, and et al. 2021. "Comprehensive NGS Panel Validation for the Identification of Actionable Alterations in Adult Solid Tumors" Journal of Personalized Medicine 11, no. 5: 360. https://doi.org/10.3390/jpm11050360
APA StyleMartínez-Fernández, P., Pose, P., Dolz-Gaitón, R., García, A., Trigo-Sánchez, I., Rodríguez-Zarco, E., Garcia-Ruiz, M., Barba, I., Izquierdo-García, M., Valero-Garcia, J., Ruiz, C., Lázaro, M., Carbonell, P., Gargallo, P., Méndez, C., Ríos-Martín, J. J., Palmeiro-Uriach, A., Camarasa-Lillo, N., Forteza-Vila, J., & Calabria, I. (2021). Comprehensive NGS Panel Validation for the Identification of Actionable Alterations in Adult Solid Tumors. Journal of Personalized Medicine, 11(5), 360. https://doi.org/10.3390/jpm11050360





