COVID-19 Infection during Pregnancy: Risk of Vertical Transmission, Fetal, and Neonatal Outcomes
Abstract
1. Introduction
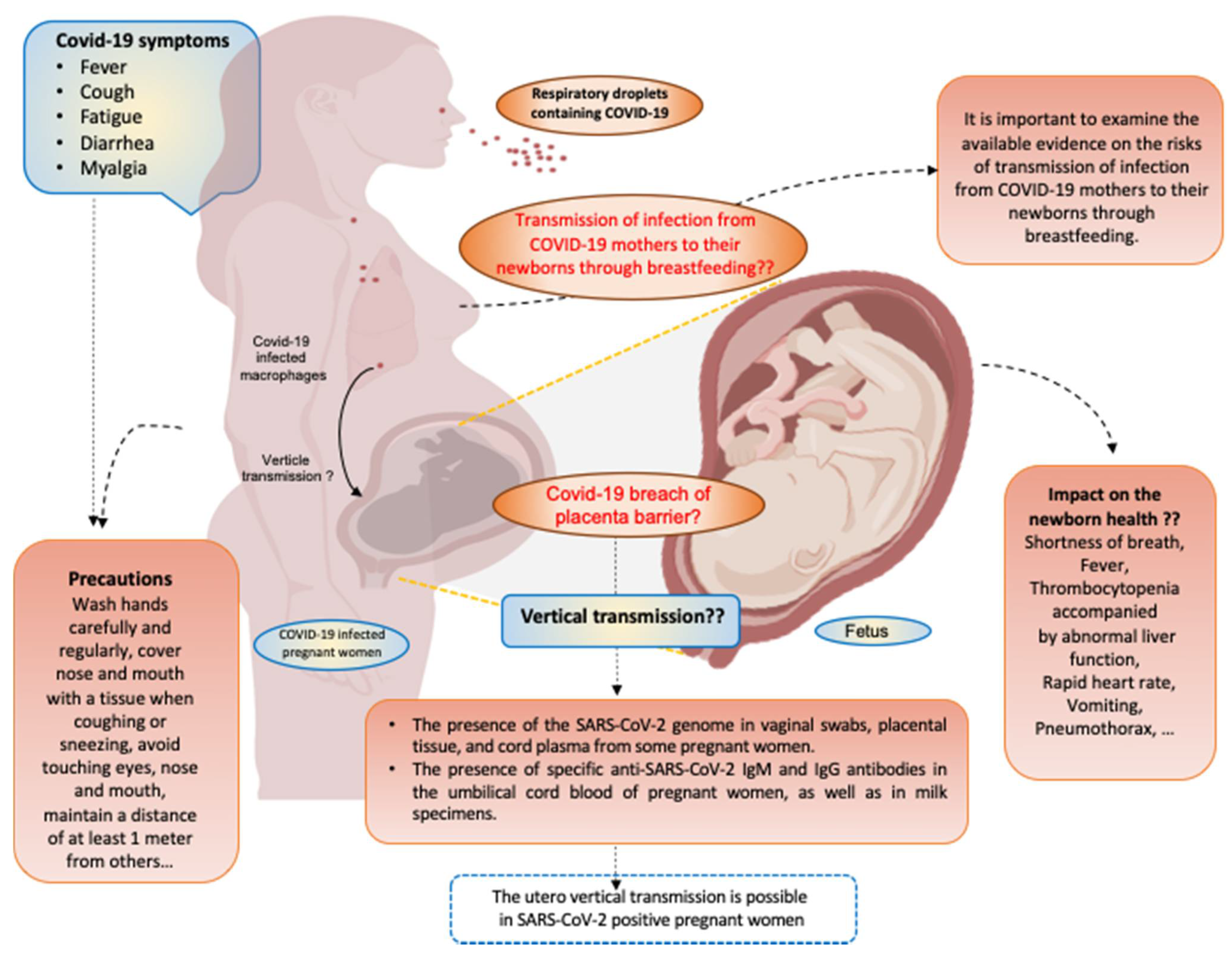
2. Symptoms of COVID-19 Infection during Pregnancy
3. Immune Response to COVID-19 Infection in Pregnancy
4. Vertical Transmission of SARS-CoV-2 and the Role of ACE-2 Receptor
5. COVID-19 Infection and Transplacental Antibody Transfer
6. Role of COVID-19 Infection in Fetal and Neonatal Outcomes
7. Risks of Transmission of SARS-CoV-2 via Breastfeeding
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, P.; Magon, N. Hormones in pregnancy. Niger. Med. J. 2012, 53, 179–183. [Google Scholar] [CrossRef]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef]
- Regal, J.F.; Gilbert, J.S.; Burwick, R.M. The complement system and adverse pregnancy outcomes. Mol. Immunol. 2015, 67, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.L.; Roberts, J.M.; Marsland, A.L.; Hall, M. How disturbed sleep may be a risk factor for adverse pregnancy outcomes. Obstet. Gynecol. Surv. 2009, 64, 273–280. [Google Scholar] [CrossRef]
- Kumar, M.; Murugesan, S.; Singh, P.; Saadaoui, M.; Elhag, D.A.; Terranegra, A.; Kabeer, B.S.A.; Marr, A.K.; Kino, T.; Brummaier, T.; et al. Vaginal Microbiota and Cytokine Levels Predict Preterm Delivery in Asian Women. Front. Cell Infect. Microbiol. 2021, 11, 639665. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. Surviving Birth: Every 11 Seconds, A Pregnant Woman or Newborn Dies Somewhere Around the World; UNICEF: Causeway Bay, Hong Kong, 2019. [Google Scholar]
- Watson, C. Stillbirth rate rises dramatically during pandemic. Nature 2020, 585, 490–491. [Google Scholar] [CrossRef] [PubMed]
- Fenizia, C.; Biasin, M.; Cetin, I.; Vergani, P.; Mileto, D.; Spinillo, A.; Gismondo, M.R.; Perotti, F.; Callegari, C.; Mancon, A.; et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020, 11, 5128. [Google Scholar] [CrossRef] [PubMed]
- Kotlyar, A.M.; Grechukhina, O.; Chen, A.; Popkhadze, S.; Grimshaw, A.; Tal, O.; Taylor, H.S.; Tal, R. Vertical transmission of coronavirus disease 2019: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021, 224, 35–53. [Google Scholar] [CrossRef]
- Parker, E.L.; Silverstein, R.B.; Verma, S.; Mysorekar, I.U. Viral-Immune Cell Interactions at the Maternal-Fetal Interface in Human Pregnancy. Front. Immunol. 2020, 11, 522047. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Kumar, M.; Al Khodor, S. Pathophysiology and treatment strategies for COVID-19. J. Transl. Med. 2020, 18, 353. [Google Scholar] [CrossRef]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Woolf, S.H.; Chapman, D.A.; Sabo, R.T.; Weinberger, D.M.; Hill, L.; Taylor, D.D.H. Excess Deaths from COVID-19 and Other Causes, March–July 2020. JAMA 2020, 324, 1562–1564. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. Pregnant Mothers and Babies Born during COVID-19 Pandemic Threatened by Strained Health Systems and Disruptions in Services; UNICEF: Causeway Bay, Hong Kong, 2020. [Google Scholar]
- Saito, S.; Asai, Y.; Matsunaga, N.; Hayakawa, K.; Terada, M.; Ohtsu, H.; Tsuzuki, S.; Ohmagari, N. First and second COVID-19 waves in Japan: A comparison of disease severity and characteristics. J. Infect. 2020. [Google Scholar] [CrossRef]
- Vahidy, F.S.; Drews, A.L.; Masud, F.N.; Schwartz, R.L.; Askary, B.B.; Boom, M.L.; Phillips, R.A. Characteristics and Outcomes of COVID-19 Patients during Initial Peak and Resurgence in the Houston Metropolitan Area. JAMA 2020, 324, 998–1000. [Google Scholar] [CrossRef]
- Fan, G.; Yang, Z.; Lin, Q.; Zhao, S.; Yang, L.; He, D. Decreased Case Fatality Rate of COVID-19 in the Second Wave: A study in 53 countries or regions. Transbound. Emerg. Dis. 2020. [Google Scholar] [CrossRef]
- Iftimie, S.; López-Azcona, A.F.; Vallverdú, I.; Hernàndez-Flix, S.; de Febrer, G.; Parra, S.; Hernández-Aguilera, A.; Riu, F.; Joven, J.; Camps, J.; et al. First and second waves of coronavirus disease-19: A comparative study in hospitalized patients in Reus, Spain. medRxiv 2020. [Google Scholar] [CrossRef]
- Available online: https://www.worldometers.info/coronavirus/#countries (accessed on 25 May 2020).
- van Oosterhout, C.; Hall, N.; Ly, H.; Tyler, K.M. COVID-19 evolution during the pandemic—Implications of new SARS-CoV-2 variants on disease control and public health policies. Virulence 2021, 12, 507–508. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.A.; Khan, M.N.; Mustagir, M.G.; Rana, J.; Haque, M.R.; Rahman, M.M. COVID-19 infection during pregnancy: A systematic review to summarize possible symptoms, treatments, and pregnancy outcomes. medRxiv 2020. [Google Scholar] [CrossRef]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef]
- Berghella, V.; Hughes, B. Coronavirus disease 2019 (COVID-19): Pregnancy issues and antenatal care. Walth. UpToDate 2020. [Google Scholar]
- Hassanipour, S.; Faradonbeh, S.B.; Momeni, K.; Heidarifard, Z.; Khosousi, M.J.; Khosousi, L.; Ameri, H.; Arab-Zozani, M. A systematic review and meta-analysis of pregnancy and COVID-19: Signs and symptoms, laboratory tests, and perinatal outcomes. Int. J. Reprod. Biomed. 2020, 18, 1005–1018. [Google Scholar] [CrossRef]
- Breslin, N.; Baptiste, C.; Gyamfi-Bannerman, C.; Miller, R.; Martinez, R.; Bernstein, K.; Ring, L.; Landau, R.; Purisch, S.; Friedman, A.M.; et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am. J. Obstet. Gynecol. MFM 2020, 2, 100118. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Q.; Zheng, D.; Jiang, H.; Wei, Y.; Zou, L.; Feng, L.; Xiong, G.; Sun, G.; Wang, H.; et al. Clinical Characteristics of Pregnant Women with Covid-19 in Wuhan, China. N. Engl. J. Med. 2020, 382, e100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Wei, M.; Cheng, B.H.; Zhou, X.C.; Li, J.; Tian, J.H.; Dong, L.; Hu, R.H. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province. Zhonghua Fu Chan Ke Za Zhi 2020, 55, 166–171. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Tang, K.; Guo, Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J. Infect. 2020. [Google Scholar] [CrossRef]
- Juusela, A.; Nazir, M.; Gimovsky, M. Two cases of coronavirus 2019-related cardiomyopathy in pregnancy. Am. J. Obstet. Gynecol. MFM 2020, 2, 100113. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Portilla, R.J.; Sotiriadis, A.; Chatzakis, C.; Torres-Torres, J.; Espino, Y.S.S.; Sandoval-Mandujano, K.; Castro-Bernabe, D.A.; Medina-Jimenez, V.; Monarrez-Martin, J.C.; Figueras, F.; et al. Pregnant women with SARS-CoV-2 infection are at higher risk of death and pneumonia: Propensity score matched analysis of a nationwide prospective cohort (COV19Mx). Ultrasound Obstet. Gynecol. 2021, 57, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Oakes, M.C.; Kernberg, A.S.; Carter, E.B.; Foeller, M.E.; Palanisamy, A.; Raghuraman, N.; Kelly, J.C. Pregnancy as a risk factor for severe coronavirus disease 2019 using standardized clinical criteria. Am. J. Obstet. Gynecol. MFM 2021, 3, 100319. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Pregnant women with virus are more likely to need intensive care, study finds. BMJ 2020, 370, m3391. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Chen, S.; Huang, B.; Luo, D.J.; Li, X.; Yang, F.; Zhao, Y.; Nie, X.; Huang, B.X. Pregnancy with new coronavirus infection: Clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi 2020, 49, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Sherer, M.L.; Lei, J.; Creisher, P.; Jang, M.; Reddy, R.; Voegtline, K.; Olson, S.; Littlefield, K.; Park, H.S.; Ursin, R.L.; et al. Dysregulated immunity in SARS-CoV-2 infected pregnant women. medRxiv 2020. [Google Scholar] [CrossRef]
- Bouchghoul, H.; Vigoureux, S. Do pregnant women have protective immunity against COVID-19? BJOG 2020, 127, 1298–1299. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, L.L.; Zhao, S.J.; Kwak-Kim, J.; Mor, G.; Liao, A.H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J. Reprod. Immunol. 2020, 139, 103122. [Google Scholar] [CrossRef] [PubMed]
- Ashokka, B.; Loh, M.H.; Tan, C.H.; Su, L.L.; Young, B.E.; Lye, D.C.; Biswas, A.; Illanes, S.E.; Choolani, M. Care of the pregnant woman with coronavirus disease 2019 in labor and delivery: Anesthesia, emergency cesarean delivery, differential diagnosis in the acutely ill parturient, care of the newborn, and protection of the healthcare personnel. Am. J. Obstet. Gynecol. 2020, 223, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Dashraath, P.; Wong, J.L.J.; Lim, M.X.K.; Lim, L.M.; Li, S.; Biswas, A.; Choolani, M.; Mattar, C.; Su, L.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 521–531. [Google Scholar] [CrossRef]
- Aghaeepour, N.; Ganio, E.A.; McIlwain, D.; Tsai, A.S.; Tingle, M.; Van Gassen, S.; Gaudilliere, D.K.; Baca, Q.; McNeil, L.; Okada, R.; et al. An immune clock of human pregnancy. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef]
- Enninga, E.A.; Nevala, W.K.; Creedon, D.J.; Markovic, S.N.; Holtan, S.G. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am. J. Reprod. Immunol. 2015, 73, 251–262. [Google Scholar] [CrossRef]
- Guo, C.C.; Mi, J.Q.; Nie, H. Seropositivity rate and diagnostic accuracy of serological tests in 2019-nCoV cases: A pooled analysis of individual studies. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10208–10218. [Google Scholar] [CrossRef]
- Villalain, C.; Herraiz, I.; Luczkowiak, J.; Perez-Rivilla, A.; Folgueira, M.D.; Mejia, I.; Batllori, E.; Felipe, E.; Risco, B.; Galindo, A.; et al. Seroprevalence analysis of SARS-CoV-2 in pregnant women along the first pandemic outbreak and perinatal outcome. PLoS ONE 2020, 15, e0243029. [Google Scholar] [CrossRef]
- Crovetto, F.; Crispi, F.; Llurba, E.; Figueras, F.; Gomez-Roig, M.D.; Gratacos, E. Seroprevalence and presentation of SARS-CoV-2 in pregnancy. Lancet 2020, 396, 530–531. [Google Scholar] [CrossRef]
- Valdés, G.; Neves, L.A.; Anton, L.; Corthorn, J.; Chacón, C.; Germain, A.M.; Merrill, D.C.; Ferrario, C.M.; Sarao, R.; Penninger, J.; et al. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta 2006, 27, 200–207. [Google Scholar] [CrossRef]
- Yan, J.; Li, R.-Q.; Wang, H.-R.; Chen, H.-R.; Liu, Y.-B.; Gao, Y.; Chen, F. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol. Hum. Reprod. 2020, 26, 367–373. [Google Scholar] [CrossRef]
- Gengler, C.; Dubruc, E.; Favre, G.; Greub, G.; de Leval, L.; Baud, D. SARS-CoV-2 ACE-receptor detection in the placenta throughout pregnancy. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y.H.; Yang, H.X.; Poon, L.C. Intrauterine vertical transmission of SARS-CoV-2: What we know so far. Ultrasound Obstet. Gynecol. 2020, 55, 724–725. [Google Scholar] [CrossRef]
- Dong, L. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA 2020, 323, 1846–1848. [Google Scholar] [CrossRef]
- Alzamora, M.C.; Paredes, T.; Caceres, D.; Webb, C.M.; Valdez, L.M.; Rosa, M.L. Severe COVID-19 during Pregnancy and Possible Vertical Transmission. Am. J. Perinatol. 2020, 37, 861–865. [Google Scholar] [CrossRef]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Cao, J.D.; Benachi, A.; Luca, D.D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572. [Google Scholar] [CrossRef]
- Novazzi, F.; Cassaniti, I.; Piralla, A.; Sabatino, A.D.; Bruno, R.; Baldanti, F. SARS-CoV-2 positivity in rectal swabs: Implication for possible transmission. J. Glob. Antimicrob. Resist 2020, 22, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.A.; Puopolo, K.M. Infants Born to Mothers With COVID-19-Making Room for Rooming-in. JAMA Pediatr. 2021, 175, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Flannery, D.D.; Gouma, P.; Dhudasia, M.B.; Mukhopadhyay, S.; Pfeifer, M.R.; Woodford, E.C.; Gerber, J.S.; Arevalo, C.P.; Bolton, M.J.; Weirick, M.E. SARS-CoV-2 seroprevalence among parturient women in Philadelphia. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Edlow, A.G.; Li, J.Z.; Collier, A.-R.Y.; Atyeo, C.; James, K.E.; Boatin, A.A.; Gray, K.J.; Bordt, E.A.; Shook, L.L.; Yonker, L.M. Assessment of Maternal and Neonatal SARS-CoV-2 Viral Load, Transplacental Antibody Transfer, and Placental Pathology in Pregnancies During the COVID-19 Pandemic. JAMA Netw. Open 2020, 3, e2030455. [Google Scholar] [CrossRef]
- Atyeo, C.; Pullen, K.M.; Bordt, E.A.; Fischinger, S.; Burke, J.; Michell, A.; Slein, M.D.; Loos, C.; Shook, L.L.; Boatin, A.A. Compromised SARS-CoV-2-specific placental antibody transfer. Cell 2021, 184, 628–642. [Google Scholar] [CrossRef]
- Ackerman, M.E. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 2013, 123, 2183–2192. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, L.; Fang, C.; Peng, S.; Zhang, L.; Chang, G.; Xia, S.; Zhou, W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020, 9, 51–60. [Google Scholar] [CrossRef]
- Novoa, R.H.; Quintana, W.; Llancarí, P.; Urbina-Quispe, K.; Guevara-Ríos, E.; Ventura, W. Maternal clinical characteristics and perinatal outcomes among pregnant women with coronavirus disease 2019. A systematic review. Travel Med. Infect Dis. 2021, 39, 101919. [Google Scholar] [CrossRef]
- Yang, R.; Mei, H.; Zheng, T.Z.; Fu, Q.; Zhang, Y.M.; Buka, S.; Yao, X.; Tang, Z.Z.; Zhang, X.C.; Qiu, L. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: A population-based cohort study in Wuhan, China. BMC Med. 2020, 18, 330. [Google Scholar] [CrossRef]
- Wei, M.; Yuan, J.P.; Liu, Y.; Fu, T.; Yu, X.; Zhang, Z.-J. Novel Coronavirus Infection in Hospitalized Infants Under 1 Year of Age in China. JAMA 2020, 323, 1313–1314. [Google Scholar] [CrossRef]
- Hong, H.; Wang, Y.; Chung, H.T.; Chen, C.J. Clinical characteristics of novel coronavirus disease 2019 (COVID-19) in newborns, infants and children. Pediatr. Neonatol. 2020, 61, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, H.; Wang, L.; Zhao, Y.; Zeng, L.K.; Gao, H.; Liu, Y.L. Infants Born to Mothers With a New Coronavirus (COVID-19). Front. Pediatr. 2020, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.M.; Lawrence, R.A. Breast milk and infection. Clin. Perinatol. 2004, 31, 501–528. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, D.W.; Stagno, S. Can SARS-CoV-2 Infection Be Acquired In Utero? More Definitive Evidence Is Needed. JAMA 2020, 323, 1788–1789. [Google Scholar] [CrossRef] [PubMed]
- Dietary management of chronic kidney disease patients: Protein-restricted diets supplemented with keto/amino acids. Abstracts from the International Advisory Board Meetings 2003/2004. Am. J. Nephrol. 2005, 25 (Suppl. 1), 1–28.
- Aagaard, K.; Riehle, K.; Segata, J.; Ma, N.; Mistretta, T.-A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; Veyver, I.V.d.; Milosavljevic, A.; et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS ONE 2012, 7, e36466. [Google Scholar] [CrossRef]
- Martins-Filho, P.R.; Santos, V.S.; Santos, H.P., Jr. To breastfeed or not to breastfeed? Lack of evidence on the presence of SARS-CoV-2 in breastmilk of pregnant women with COVID-19. Rev. Panam. Salud Publica 2020, 44, e59. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.H.; Xu, J.; Lin, D.J.; Yang, Z.; Xu, L.; Qu, Z.H.; Zhang, Y.H.; Zhang, H.; Jia, R.; Liu, P.C. A Case Series of children with 2019 novel coronavirus infection: Clinical and epidemiological features. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Chambers, C.; Krogstad, P.; Bertrand, K.; Contreras, D.; Tobin, N.H.; Bode, L.; Aldrovandi, G. Evaluation for SARS-CoV-2 in Breast Milk from 18 Infected Women. JAMA 2020, 324, 1347–1348. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadaoui, M.; Kumar, M.; Al Khodor, S. COVID-19 Infection during Pregnancy: Risk of Vertical Transmission, Fetal, and Neonatal Outcomes. J. Pers. Med. 2021, 11, 483. https://doi.org/10.3390/jpm11060483
Saadaoui M, Kumar M, Al Khodor S. COVID-19 Infection during Pregnancy: Risk of Vertical Transmission, Fetal, and Neonatal Outcomes. Journal of Personalized Medicine. 2021; 11(6):483. https://doi.org/10.3390/jpm11060483
Chicago/Turabian StyleSaadaoui, Marwa, Manoj Kumar, and Souhaila Al Khodor. 2021. "COVID-19 Infection during Pregnancy: Risk of Vertical Transmission, Fetal, and Neonatal Outcomes" Journal of Personalized Medicine 11, no. 6: 483. https://doi.org/10.3390/jpm11060483
APA StyleSaadaoui, M., Kumar, M., & Al Khodor, S. (2021). COVID-19 Infection during Pregnancy: Risk of Vertical Transmission, Fetal, and Neonatal Outcomes. Journal of Personalized Medicine, 11(6), 483. https://doi.org/10.3390/jpm11060483





