MiR-126-3p and MiR-223-3p as Biomarkers for Prediction of Thrombotic Risk in Patients with Acute Myocardial Infarction and Primary Angioplasty
Abstract
:1. Introduction
1.1. Methods Section
1.2. Patients
1.3. Laboratory Analyses
1.4. RNA Isolation
1.5. MiREIA Assays
1.6. MiR-126-3p to miR-223-3p Ratio
1.7. Ischemic Risk Calculation
1.8. Statistical Analyses
2. Results
2.1. Patients
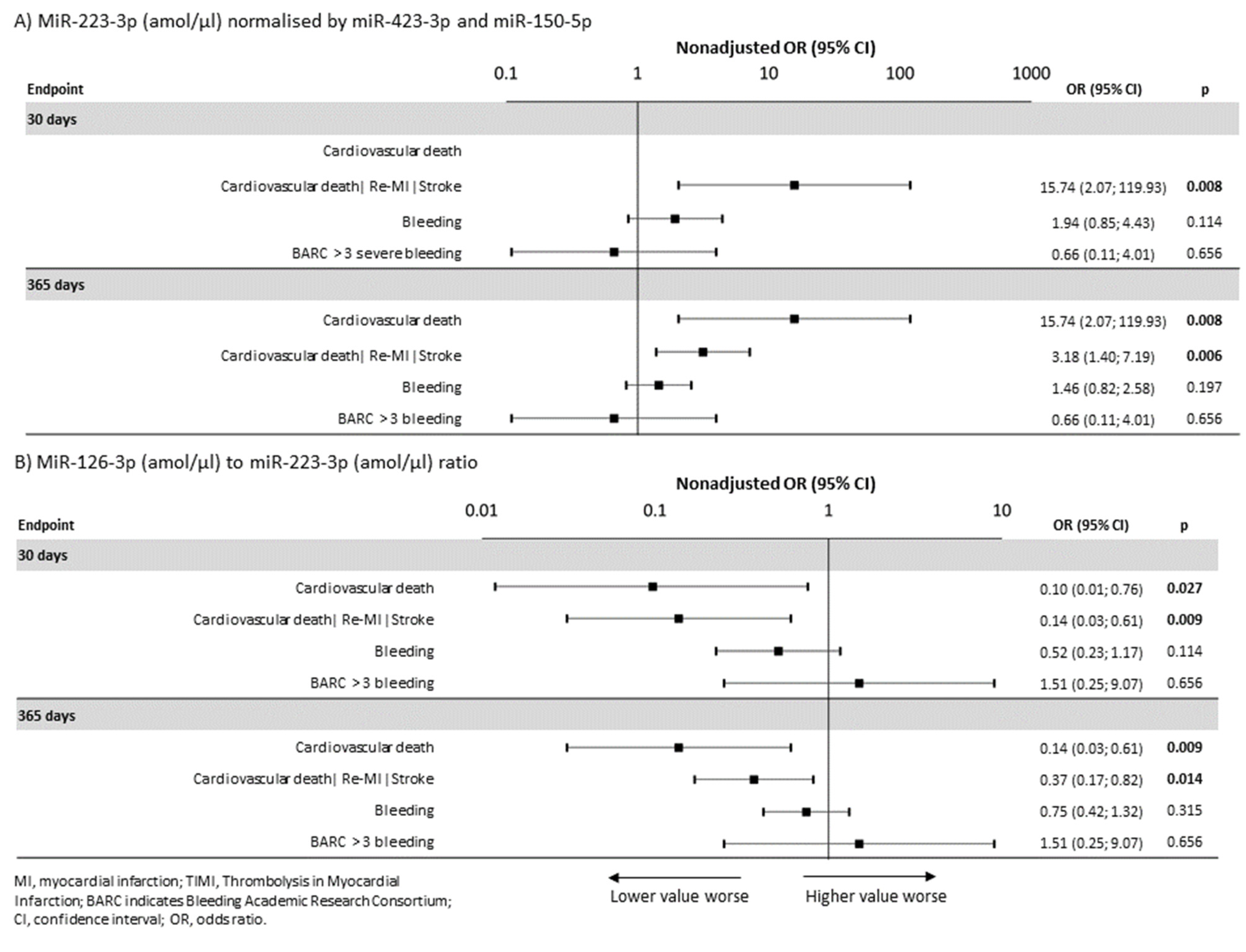
2.2. MiRNAs and Endpoints
2.3. MiRNAs and Bleeding
2.4. MiRNAs and Ischemic Risk Calculation
3. Discussions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pasquinelli, A.E. MicroRNAs and their targets: Recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 2012, 13, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Small, E.M.; Olson, E.N. Pervasive roles of microRNAs in cardiovascular biology. Nat. Cell Biol. 2011, 469, 336–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eitel, I.; Adams, V.; Dieterich, P.; Fuernau, G.; de Waha, S.; Desch, S.; Schuler, G.; Thiele, H. Relation of circulating MicroRNA-133a concentrations with myocardial damage and clinical prognosis in ST-elevation myocardial infarction. Am. Heart J. 2012, 164, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-K.; Zhu, J.-Q.; Zhang, J.-T.; Li, Q.; Li, Y.; He, J.; Qin, Y.-W.; Jing, Q. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010, 31, 659–666. [Google Scholar] [CrossRef]
- Jakob, P.; Kacprowski, T.; Briand-Schumacher, S.; Heg, D.; Klingenberg, R.; Stähli, B.E.; Jaguszewski, M.; Rodondi, N.; Nanchen, D.; Räber, L.; et al. Profiling and validation of circulating microRNAs for cardiovascular events in patients presenting with ST-segment elevation myocardial infarction. Eur. Heart J. 2016, 38, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Zampetaki, A.; Willeit, P.; Tilling, L.; Drozdov, I.; Prokopi, M.; Renard, J.-M.; Mayr, A.; Weger, S.; Schett, G.; Shah, A.; et al. Prospective Study on Circulating MicroRNAs and Risk of Myocardial Infarction. J. Am. Coll. Cardiol. 2012, 60, 290–299. [Google Scholar] [CrossRef] [Green Version]
- De Rosa, R.; De Rosa, S.; Leistner, D.; Boeckel, J.N.; Keller, T.; Fichtlscherer, S.; Dimmeler, S.; Zeiher, A.M. Transcoronary Concentration Gradient of microRNA-133a and Outcome in Patients With Coronary Artery Disease. Am. J. Cardiol. 2017, 120, 15–24. [Google Scholar] [CrossRef]
- Kaudewitz, D.; Skroblin, P.; Bender, L.H.; Barwari, T.; Willeit, P.; Pechlaner, R.; Sunderland, N.P.; Willeit, K.; Morton, A.C.; Armstrong, P.C.; et al. Association of MicroRNAs and YRNAs With Platelet Function. Circ. Res. 2016, 118, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Zampetaki, A.; Dudek, K.; Kaudewitz, D.; King, A.; Kirkby, N.S.; Crosby-Nwaobi, R.; Prokopi, M.; Drozdov, I.; Langley, S.R.; et al. Circulating MicroRNAs as Novel Biomarkers for Platelet Activation. Circ. Res. 2013, 112, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Ge, L.; Zhou, X.; Ji, W.-J.; Lu, R.-Y.; Zhang, Y.-Y.; Zeng, S.; Liu, X.; Zhao, J.-H.; Zhang, W.-C.; et al. Decreased platelet miR-223 expression is associated with high on-clopidogrel platelet reactivity. Thromb. Res. 2013, 131, 508–513. [Google Scholar] [CrossRef]
- Motovska, Z.; Hlinomaz, O.; Miklik, R.; Hromadka, M.; Varvarovsky, I.; Dusek, J.; Knot, J.; Jarkovsky, J.; Kala, P.; Rokyta, R.; et al. Prasugrel versus ticagrelor in patients with acute myocardial infarction treated with primary percutaneous coronary intervention: Multicentre randomized PRAGUE-18 study. Circulation 2016, 134, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Motovska, Z.; Hlinomaz, O.; Kala, P.; Hromadka, M.; Knot, J.; Varvarovsky, I.; Dušek, J.; Jarkovsky, J.; Miklik, R.; Rokyta, R.; et al. 1-Year Outcomes of Patients Undergoing Primary Angioplasty for Myocardial Infarction Treated with Prasugrel Versus Ticagrelor. J. Am. Coll. Cardiol. 2018, 71, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Krepelkova, I.; Mrackova, T.; Izakova, J.; Dvorakova, B.; Chalupova, L.; Mikulik, R.; Slaby, O.; Bartos, M.; Ruzicka, V. Evaluation of miRNA detection methods for the analytical characteristic necessary for clinical utilization. Biotechniques 2019, 66, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S.; et al. MicroRNA Expression in Circulating Microvesicles Predicts Cardiovascular Events in Patients with Coronary Artery Disease. J. Am. Heart Assoc. 2014, 3, e001249. [Google Scholar] [CrossRef] [Green Version]
- Schulte, C.; Molz, S.; Appelbaum, S.; Karakas, M.; Ojeda, F.; Lau, D.M.; Hartmann, T.; Lackner, K.J.; Westermann, D.; Schnabel, R.B.; et al. miRNA-197 and miRNA-223 Predict Cardiovascular Death in a Cohort of Patients with Symptomatic Coronary Artery Disease. PLoS ONE 2015, 10, e0145930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Sakata, Y.; Suna, S.; Nakatani, D.; Usami, M.; Hara, M.; Kitamura, T.; Hamasaki, T.; Nanto, S.; Kawahara, Y.; et al. Circulating p53-Responsive MicroRNAs Are Predictive Indicators of Heart Failure After Acute Myocardial Infarction. Circ. Res. 2013, 113, 322–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landry, P.; Plante, I.; Ouellet, D.L.; Perron, M.P.; Rousseau, G.; Provost, P. Existence of a microRNA pathway in anucleate platelets. Nat. Struct. Mol. Biol. 2009, 16, 961–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavarretta, E.; Chiariello, G.A.; Condorelli, G. Platelets, endothelium, and circulating microRNA-126 as a prognostic biomarker in cardiovascular diseases: Per aspirin ad astra. Eur. Heart J. 2013, 34, 3400–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-Y.; Hong, M.-K.; Palmerini, T.; Kim, H.-S.; Valgimigli, M.; Feres, F.; Colombo, A.; Gilard, M.; Shin, D.-H.; Kim, J.-S.; et al. Short-term versus long-term dual antiplatelet therapy after drug-eluting stent implantation in elderly patients: A meta-analysis of individual participant data from six randomized trials. J. Am. Coll. Cardiol. 2018, 71, A1323. [Google Scholar] [CrossRef]
- Chew, D.P.; Lincoff, A.M.; Gurm, H.; Wolski, K.; Cohen, D.J.; Henry, T.; Feit, F.; Topol, E.J. Bivalirudin versus heparin and glycoprotein IIb/IIIa inhibition among patients with renal impairment undergoing percutaneous coronary intervention (a subanalysis of the Replace-2 trial). Am. J. Cardiol. 2005, 95, 581–585. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Yeh, R.W.; Massaro, J.M.; Cutlip, D.E.; Steg, P.G.; Wiviott, S.D.; Mauri, L. DAPT Score Utility for Risk Prediction in Patients With or Without Previous Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 67, 2492–2502. [Google Scholar] [CrossRef]
- Costa, F.; van Klaveren, D.; James, S.; Heg, D.; Räber, L.; Feres, F.; Pilgrim, T.; Hong, M.-K.; Kim, H.-S.; Colombo, A.; et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (Precise-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet 2017, 389, 1025–1034. [Google Scholar] [CrossRef]
- Mehran, R.; Baber, U.; Sharma, S.K.; Cohen, D.J.; Angiolillo, D.J.; Briguori, C.; Cha, J.Y.; Collier, T.; Dangas, G.; Dudek, D.; et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N. Engl. J. Med. 2019, 381, 2032–2042. [Google Scholar] [CrossRef]
- Cesaro, A.; Moscarella, E.; Gragnano, F.; Perrotta, R.; Diana, V.; Pariggiano, I.; Concilio, C.; Alfieri, A.; Cesaro, F.; Mercone, G.; et al. Transradial access versus transfemoral access: A comparison of outcomes and efficacy in reducing hemorrhagic events. Expert Rev. Cardiovasc. Ther. 2019, 17, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.; Joshi, A.; Mayr, M. Response by Schulte et al to Letter Regarding Article, “Comparative Analysis of Circulating Noncoding RNAs Versus Protein Biomarkers in the Detection of Myocardial Injury”. Circ. Res. 2019, 125, e22–e23. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; Berger, P.B.; Teirstein, P.S.; Tanguay, J.F.; Angiolillo, D.J.; Spriggs, D.; Puri, S.; Robbins, M.; Garratt, K.N.; Bertrand, O.F.; et al. Gravitas Investigator. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: The GRAVITAS randomized trial. JAMA 2011, 305, 1097–1105. [Google Scholar] [CrossRef]
- Collet, J.-P.; Cuisset, T.; Rangé, G.; Cayla, G.; Elhadad, S.; Pouillot, C.; Henry, P.; Motreff, P.; Carrie, D.; Boueri, Z.; et al. Bedside Monitoring to Adjust Antiplatelet Therapy for Coronary Stenting. N. Engl. J. Med. 2012, 367, 2100–2109. [Google Scholar] [CrossRef] [Green Version]
- Mega, J.L.; Braunwald, E.; Wiviott, S.D.; Bassand, J.-P.; Bhatt, D.L.; Bode, C.; Burton, P.; Cohen, M.; Cook-Bruns, N.; Fox, K.A.; et al. Rivaroxaban in Patients with a Recent Acute Coronary Syndrome. N. Engl. J. Med. 2012, 366, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Connolly, S.J.; Eikelboom, J.W.; Bosch, J.; Dagenais, G.; Dyal, L.; Lanas, F.; Metsarinne, K.; O’Donnell, M.; Dans, A.L.; Ha, J.-W.; et al. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: An international, randomised, double-blind, placebo-controlled trial. Lancet 2018, 391, 205–218. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Steg, P.G.; Storey, R.; Cohen, M.; Im, K.; Ophuis, T.O.; Budaj, A.; Goto, S.; López-Sendón, J.; et al. Ischaemic risk and efficacy of ticagrelor in relation to time from P2Y12inhibitor withdrawal in patients with prior myocardial infarction: Insights from PEGASUS-TIMI 54. Eur. Heart J. 2016, 37, 1133–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesaro, A.; Taglialatela, V.; Gragnano, F.; Moscarella, E.; Fimiani, F.; Conte, M.; Barletta, V.; Monda, E.; Limongelli, G.; Severino, S.; et al. Low-Dose Ticagrelor in Patients With High Ischemic Risk and Previous Myocardial Infarction: A Multicenter Prospective Real-World Observational Study. J. Cardiovasc. Pharmacol. 2020, 76, 173–180. [Google Scholar] [CrossRef] [PubMed]


| Total | miR-223-3p (amol/µl) | miR-126-3P/miR-223-3p | |||||
|---|---|---|---|---|---|---|---|
| Characteristics 1 | N = 598 | Below Median (N = 299) | Above Median (N = 299) | p2 | Below Median (N = 299) | Above Median (N = 299) | p2 |
| Prasugrel | 294 (49.2%) | 155 (51.8%) | 139 (46.5%) | 0.220 | 149 (49.8%) | 145 (48.5%) | 0.806 |
| Ticagrelor | 304 (50.8%) | 144 (48.2%) | 160 (53.5%) | 0.220 | 150 (50.2%) | 154 (51.5%) | 0.806 |
| Switch to Clopidogrel | 243 (40.6%) | 134 (44.8%) | 109 (36.5%) | 0.046 | 117 (39.1%) | 126 (42.1%) | 0.505 |
| Age | 62.0 (42.7; 78.1%) | 61.9 (45; 75.9) | 62.1 (42; 79.2) | 0.897 | 62.8 (42; 78.7) | 61.2 (44; 77.5) | 0.105 |
| Age > 75 | 54 (9.0%) | 21 (7.0%) | 33 (11.0%) | 0.116 | 30 (10.0%) | 24 (8.0%) | 0.476 |
| Men | 465 (77.8%) | 237 (79.3%) | 228 (76.3%) | 0.432 | 218 (72.9%) | 247 (82.6%) | 0.006 |
| Hyperlipidemia | 226 (37.8%) | 122 (40.8%) | 104 (34.8%) | 0.152 | 122 (40.8%) | 104 (34.8%) | 0.152 |
| BMI > 25 | 473 (79.1%) | 236 (78.9%) | 237 (79.3%) | 1.000 | 225 (75.3%) | 248 (82.9%) | 0.027 |
| Hypertension | 294 (49.2%) | 144 (48.2%) | 150 (50.2%) | 0.683 | 142 (47.5%) | 152 (50.8%) | 0.462 |
| Current smoker | 320 (53.5%) | 172 (57.5%) | 148 (49.5%) | 0.059 | 153 (51.2%) | 167 (55.9%) | 0.286 |
| Diabetes mellitus | 124 (20.7%) | 52 (17.4%) | 72 (24.1%) | 0.055 | 67 (22.4%) | 57 (19.1%) | 0.364 |
| MI anamnesis | 41 (6.9%) | 25 (8.4%) | 16 (5.4%) | 0.195 | 21 (7.0%) | 20 (6.7%) | 1.000 |
| PCI anamnesis | 38 (6.4%) | 18 (6.0%) | 20 (6.7%) | 0.867 | 20 (6.7%) | 18 (6.0%) | 0.867 |
| CABG anamnesis | 6 (1.0%) | 2 (0.7%) | 4 (1.3%) | 0.686 | 4 (1.3%) | 2 (0.7%) | 0.686 |
| Chronic heart failure | 6 (1.0%) | 5 (1.7%) | 1 (0.3%) | 0.216 | 4 (1.3%) | 2 (0.7%) | 0.686 |
| Chronic renal failure | 7 (1.2%) | 5 (1.7%) | 2 (0.7%) | 0.450 | 3 (1.0%) | 4 (1.3%) | 1.000 |
| Peripheral arterial disease | 23 (3.8%) | 11 (3.7%) | 12 (4.0%) | 1.000 | 13 (4.3%) | 10 (3.3%) | 0.672 |
| History of bleeding | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 | 0 (0%) | 0 (0%) | 1.000 |
| STEMI | 567 (94.8%) | 278 (93.0%) | 289 (96.7%) | 0.064 | 283 (94.6%) | 284 (95.0%) | 1.000 |
| BBB | 16 (2.7%) | 9 (3.0%) | 7 (2.3%) | 0.801 | 7 (2.3%) | 9 (3.0%) | 0.801 |
| NSTEMI | 20 (3.3%) | 13 (4.3%) | 7 (2.3%) | 0.255 | 13 (4.3%) | 7 (2.3%) | 0.255 |
| Bare metal stent | 115 (19.2%) | 46 (15.4%) | 69 (23.1%) | 0.022 | 59 (19.7%) | 56 (18.7%) | 0.836 |
| Drug-eluting stent | 440 (73.6%) | 234 (78.3%) | 206 (68.9%) | 0.012 | 217 (72.6%) | 223 (74.6%) | 0.643 |
| Bioabsorbable vascular scaffold | 28 (4.7%) | 14 (4.7%) | 14 (4.7%) | 1.000 | 16 (5.4%) | 12 (4.0%) | 0.562 |
| TIMI after PCI < 3 | 28 (4.7%) | 7 (2.3%) | 21 (7.0%) | 0.011 | 16 (5.4%) | 12 (4.0%) | 0.562 |
| Number of diseased vessels >1 | 281 (47.0%) | 138 (46.2%) | 143 (47.8%) | 0.743 | 152 (50.8%) | 129 (43.1%) | 0.071 |
| Left main disease | 16 (2.7%) | 9 (3.0%) | 7 (2.3%) | 0.801 | 9 (3.0%) | 7 (2.3%) | 0.801 |
| Suboptimal or unsuccessful PCI | 25 (4.2%) | 4 (1.3%) | 21 (7.0%) | <0.001 | 16 (5.4%) | 9 (3.0%) | 0.220 |
| Time to hospital | 2.5 (0.8; 24.0%) | 2.8 (1; 23.0) | 2.4 (1; 24.0) | 0.098 | 2.7 (1; 28.0) | 2.5 (1; 16.4) | 0.561 |
| Time to hospital >3 h | 222 (41.3%) | 118 (44.4%) | 104 (38.4%) | 0.162 | 120 (44.3%) | 102 (38.3%) | 0.189 |
| Time to hospital >6 h | 109 (20.3%) | 56 (21.1%) | 53 (19.6%) | 0.670 | 63 (23.2%) | 46 (17.3%) | 0.107 |
| Discharge, n (%) | |||||||
| Aspirin | 578 (96.7%) | 294 (98.3%) | 284 (95.0%) | 0.038 | 286 (95.7%) | 292 (97.7%) | 0.255 |
| β-Blockers | 494 (82.6%) | 248 (82.9%) | 246 (82.3%) | 0.914 | 246 (82.3%) | 248 (82.9%) | 0.914 |
| ACE inhibitors | 463 (77.4%) | 243 (81.3%) | 220 (73.6%) | 0.031 | 221 (73.9%) | 242 (80.9%) | 0.050 |
| ARBs | 42 (7.0%) | 23 (7.7%) | 19 (6.4%) | 0.632 | 21 (7.0%) | 21 (7.0%) | 1.000 |
| Statins | 563 (94.1%) | 284 (95.0%) | 279 (93.3%) | 0.486 | 283 (94.6%) | 280 (93.6%) | 0.728 |
| Proton pump inhibitors | 374 (62.5%) | 197 (65.9%) | 177 (59.2%) | 0.108 | 181 (60.5%) | 193 (64.5%) | 0.353 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hromadka, M.; Motovska, Z.; Hlinomaz, O.; Kala, P.; Tousek, F.; Jarkovsky, J.; Beranova, M.; Jansky, P.; Svoboda, M.; Krepelkova, I.; et al. MiR-126-3p and MiR-223-3p as Biomarkers for Prediction of Thrombotic Risk in Patients with Acute Myocardial Infarction and Primary Angioplasty. J. Pers. Med. 2021, 11, 508. https://doi.org/10.3390/jpm11060508
Hromadka M, Motovska Z, Hlinomaz O, Kala P, Tousek F, Jarkovsky J, Beranova M, Jansky P, Svoboda M, Krepelkova I, et al. MiR-126-3p and MiR-223-3p as Biomarkers for Prediction of Thrombotic Risk in Patients with Acute Myocardial Infarction and Primary Angioplasty. Journal of Personalized Medicine. 2021; 11(6):508. https://doi.org/10.3390/jpm11060508
Chicago/Turabian StyleHromadka, Milan, Zuzana Motovska, Ota Hlinomaz, Petr Kala, Frantisek Tousek, Jiri Jarkovsky, Marketa Beranova, Pavel Jansky, Michal Svoboda, Iveta Krepelkova, and et al. 2021. "MiR-126-3p and MiR-223-3p as Biomarkers for Prediction of Thrombotic Risk in Patients with Acute Myocardial Infarction and Primary Angioplasty" Journal of Personalized Medicine 11, no. 6: 508. https://doi.org/10.3390/jpm11060508
APA StyleHromadka, M., Motovska, Z., Hlinomaz, O., Kala, P., Tousek, F., Jarkovsky, J., Beranova, M., Jansky, P., Svoboda, M., Krepelkova, I., Rokyta, R., Widimsky, P., & Karpisek, M. (2021). MiR-126-3p and MiR-223-3p as Biomarkers for Prediction of Thrombotic Risk in Patients with Acute Myocardial Infarction and Primary Angioplasty. Journal of Personalized Medicine, 11(6), 508. https://doi.org/10.3390/jpm11060508







