Personalized Risk Assessment for Prevention and Early Detection of Breast Cancer: Integration and Implementation (PERSPECTIVE I&I)
Abstract
:1. Introduction
2. Overview of the PERSPECTIVE I&I Project
2.1. Identification and Validation of Novel Moderate- to High-Risk Breast Cancer Susceptibility Genes
Development of a Clinical Grade Genetic Test Including Validated Breast Cancer Susceptibility Genes and Polygenic Risk Score
2.2. Improvement, Validation and Adaptation of a Comprehensive Risk Prediction Web-Tool
2.3. Development of a Socio-Ethical Framework to Support Implementation of a Personalized Risk-Based Approach to Breast Cancer Screening
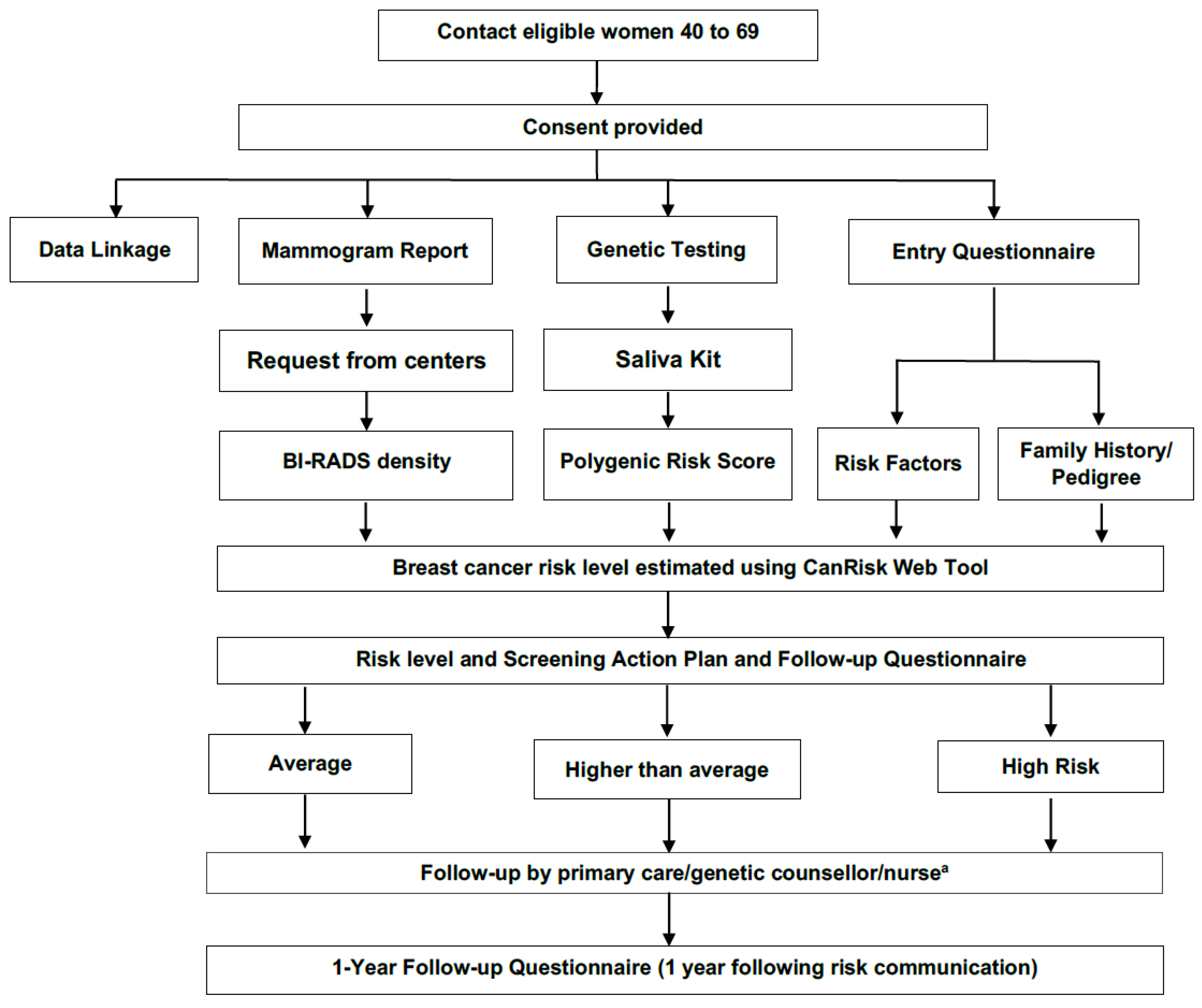
2.3.1. Screening Cohorts
2.3.2. Assessing Acceptability and Health Care System Readiness
2.3.3. Socio-Ethical and Legal Issues
2.4. Economic Analysis to Optimize Personalized Risk-Based Screening Implementation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadian Cancer Statistics Advisory Committee Canadian Cancer Statistics 2019. Available online: http://cancer.ca/Canadian-Cancer-Statistics-2019-EN (accessed on 6 February 2021).
- Guidelines for the Economic Evaluation of Health Technologies: Canada. Available online: https://www.cadth.ca/about-cadth/how-we-do-it/methods-and-guidelines/guidelines-for-the-economic-evaluation-of-health-technologies-canada (accessed on 28 April 2021).
- De Oliveira, C.; Pataky, R.; Bremner, K.E.; Rangrej, J.; Chan, K.K.; Cheung, W.Y.; Hoch, J.S.; Peacock, S.; Krahn, M.D. Phase-specific and lifetime costs of cancer care in Ontario, Canada. BMC Cancer 2016, 16, 809. [Google Scholar] [CrossRef]
- Patra, J.; Popova, S.; Rehm, J.; Bondy, S.; Flint, R.; Giesbrecht, N. Economic cost of chronic disease in Canada. Available online: https://www.ocdpa.ca/sites/default/files/publications/OCDPA_EconomicCosts.pdf (accessed on 28 April 2021).
- Will, B.; Berthelot, J.-M.; Le Petit, C.; Tomiak, E.; Verma, S.; Evans, W. Estimates of the lifetime costs of breast cancer treatment in Canada. Eur. J. Cancer 2000, 36, 724–735. [Google Scholar] [CrossRef]
- Klarenbach, S.; Sims-Jones, N.; Lewin, G.; Singh, H.; Thériault, G.; Tonelli, M.; Doull, M.; Courage, S.; Garcia, A.J.; Thombs, B.D. Recommendations on screening for breast cancer in women aged 40–74 years who are not at increased risk for breast cancer. Can. Med. Assoc. J. 2018, 190, E1441–E1451. [Google Scholar] [CrossRef]
- Korenstein, D. Wise guidance and its challenges: The new Canadian recommendations on breast cancer screening. Can. Med. Assoc. J. 2018, 190, E1432–E1433. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Benbrahim-Tallaa, L.; Bouvard, V.; Bianchini, F.; Straif, K. Breast-Cancer Screening—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2015, 372, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Sutton, S.; Saidi, G.; Bickler, G.; Hunter, J. Does routine screening for breast cancer raise anxiety? Results from a three wave prospective study in England. J. Epidemiol. Community Health 1995, 49, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Puliti, D.; Miccinesi, G.; Paci, E. Overdiagnosis in breast cancer: Design and methods of estimation in observational studies. Prev. Med. 2011, 53, 131–133. [Google Scholar] [CrossRef]
- Pharoah, P.D.; Antoniou, A.; Bobrow, M.; Zimmern, R.L.; Easton, D.F.; Ponder, B.A. Polygenic susceptibility to breast cancer and implications for prevention. Nat. Genet. 2002, 31, 33–36. [Google Scholar] [CrossRef]
- Pharoah, P.D.; Antoniou, A.C.; Easton, D.F.; Ponder, B.A. Polygenes, risk prediction, and targeted prevention of breast cancer. N. Engl. J. Med. 2008, 358, 2796–2803. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Prummel, M.V.; Muradali, D.; Majpruz, V.; Horgan, M.; Carroll, J.C.; Eisen, A.; Meschino, W.S.; Shumak, R.S.; Warner, E.; et al. Effectiveness of screening with annual magnetic resonance imaging and mammography: Results of the initial screen from the ontario high risk breast screening program. J. Clin. Oncol. 2014, 32, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Canadian Partnership Against Cancer. Breast Cancer Screening in Canada: Monitoring and Evaluation of Quality Indicator—Results Report, January 2011 to December 2012; Canadian Partnership Against Cancer: Toronto, ON, Canada, 2017. [Google Scholar]
- Chiarelli, A.M.; Blackmore, K.M.; Muradali, D.; Done, S.J.; Majpruz, V.; Weerasinghe, A.; Mirea, L.; Eisen, A.; Rabeneck, L.; Warner, E. Performance Measures of Magnetic Resonance Imaging Plus Mammography in the High Risk Ontario Breast Screening Program. J. Natl. Cancer Inst. 2019, 112, 136–144. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). NCCN Guidelines Version 1.2019 Breast Cancer Screening and Diagnosis. Available online: www.nccn.org (accessed on 2 June 2021).
- National Institute of Health and Care Excellence. Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer; National Collaborating Centre for Cancer: Cardiff, UK, 2013. [Google Scholar]
- Saslow, D.; Boetes, C.; Burke, W.; Harms, S.; Leach, M.O.; Lehman, C.D.; Morris, E.; Pisano, E.; Schnall, M.; Sener, S.; et al. American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. CA Cancer J. Clin. 2007, 57, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Easton, D.F.; Pharoah, P.D.P.; Antoniou, A.C.; Tischkowitz, M.; Tavtigian, S.V.; Nathanson, K.L.; Devilee, P.; Meindl, A.; Couch, F.J.; Southey, M.; et al. Gene-Panel Sequencing and the Prediction of Breast-Cancer Risk. N. Engl. J. Med. 2015, 372, 2243–2257. [Google Scholar] [CrossRef]
- Dorling, L.; Carvalho, S.; Allen, J.; González-Neira, A.; Luccarini, C.; Wahlström, C.; Pooley, K.A.; Parsons, M.T.; Fortuno, C.; Wang, Q.; et al. Breast Cancer Risk Gene—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021, 384, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hart, S.N.; Gnanaolivu, R.; Huang, H.; Lee, K.Y.; Na, J.; Gao, C.; Lilyquist, J.; Yadav, S.; Boddicker, N.J.; et al. A Population-Based Study of Genes Previously Implicated in Breast Cancer. N. Engl. J. Med. 2021, 384, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, K.; Lindström, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemaçon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017, 551, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Mavaddat, N.; Michailidou, K.; Dennis, J.; Lush, M.; Fachal, L.; Lee, A.; Tyrer, J.P.; Chen, T.-H.; Wang, Q.; Bolla, M.K. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am. J. Hum. Genet. 2019, 104, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.; Brook, M.N.; Hurson, A.N.; Lee, A.; Mulder, C.V.; Coulson, P.; Schoemaker, M.J.; Jones, M.E.; Swerdlow, A.J.; Chatterjee, N.; et al. Comparative validation of the BOADICEA and Tyrer-Cuzick breast cancer risk models incorporating classical risk factors and polygenic risk in a population-based prospective cohort of women of European ancestry. Breast Cancer Res. 2021, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Lakeman, I.M.M.; Rodríguez-Girondo, M.; Lee, A.; Ruiter, R.; Stricker, B.H.; Wijnant, S.R.A.; Kavousi, M.; Antoniou, A.C.; Schmidt, M.K.; Uitterlinden, A.G.; et al. Validation of the BOADICEA model and a 313-variant polygenic risk score for breast cancer risk prediction in a Dutch prospective cohort. Genet. Med. 2020, 22, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Mavaddat, N.; Wilcox, A.N.; Cunningham, A.P.; Carver, T.; Hartley, S.; Babb de Villiers, C.; Izquierdo, A.; Simard, J.; Schmidt, M.K.; et al. BOADICEA: A comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet. Med. 2019, 21, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.B.; Liao, Y.; Whittemore, A.S.; Leoce, N.; Buchsbaum, R.; Zeinomar, N.; Dite, G.S.; Chung, W.K.; Knight, J.A.; Southey, M.C. 10-year performance of four models of breast cancer risk: A validation study. Lancet Oncol. 2019, 20, 504–517. [Google Scholar] [CrossRef]
- Pashayan, N.; Antoniou, A.C.; Ivanus, U.; Esserman, L.J.; Easton, D.F.; French, D.; Sroczynski, G.; Hall, P.; Cuzick, J.; Evans, D.G.; et al. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat. Rev. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Nepomuceno, T.C.; Carvalho, M.A.; Rodrigue, A.; Simard, J.; Masson, J.Y.; Monteiro, A.N.A. PALB2 Variants: Protein Domains and Cancer Susceptibility. Trends Cancer 2020. [Google Scholar] [CrossRef] [PubMed]
- Rodrigue, A.; Margaillan, G.; Torres Gomes, T.; Coulombe, Y.; Montalban, G.; da Costa, E.S.C.S.; Milano, L.; Ducy, M.; De-Gregoriis, G.; Dellaire, G.; et al. A global functional analysis of missense mutations reveals two major hotspots in the PALB2 tumor suppressor. Nucleic Acids Res. 2019, 47, 10662–10677. [Google Scholar] [CrossRef]
- Wiltshire, T.; Ducy, M.; Foo, T.K.; Hu, C.; Lee, K.Y.; Belur Nagaraj, A.; Rodrigue, A.; Gomes, T.T.; Simard, J.; Monteiro, A.N.A.; et al. Functional characterization of 84 PALB2 variants of uncertain significance. Genet. Med. 2020, 22, 622–632. [Google Scholar] [CrossRef]
- Boonen, R.A.C.M.; Rodrigue, A.; Stoepker, C.; Wiegant, W.W.; Vroling, B.; Sharma, M.; Rother, M.B.; Celosse, N.; Vreeswijk, M.P.G.; Couch, F.; et al. Functional analysis of genetic variants in the high-risk breast cancer susceptibility gene PALB2. Nat. Commun. 2019, 10, 5296. [Google Scholar] [CrossRef] [PubMed]
- Findlay, G.M.; Boyle, E.A.; Hause, R.J.; Klein, J.C.; Shendure, J. Saturation editing of genomic regions by multiplex homology-directed repair. Nature 2014, 513, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Fachal, L.; Aschard, H.; Beesley, J.; Barnes, D.R.; Allen, J.; Kar, S.; Pooley, K.A.; Dennis, J.; Michailidou, K.; Turman, C.; et al. Fine-mapping of 150 breast cancer risk regions identifies 191 likely target genes. Nat. Genet. 2020, 52, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.L.; Kuchenbaecker, K.B.; Michailidou, K.; Beesley, J.; Kar, S.; Lindström, S.; Hui, S.; Lemaçon, A.; Soucy, P.; Dennis, J.; et al. Identification of ten variants associated with risk of estrogen-receptor-negative breast cancer. Nat. Genet. 2017, 49, 1767–1778. [Google Scholar] [CrossRef]
- Zhang, H.; Ahearn, T.U.; Lecarpentier, J.; Barnes, D.; Beesley, J.; Qi, G.; Jiang, X.; O’Mara, T.A.; Zhao, N.; Bolla, M.K.; et al. Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat. Genet. 2020, 52, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.R.; Rookus, M.A.; McGuffog, L.; Leslie, G.; Mooij, T.M.; Dennis, J.; Mavaddat, N.; Adlard, J.; Ahmed, M.; Aittomäki, K.; et al. Polygenic risk scores and breast and epithelial ovarian cancer risks for carriers of BRCA1 and BRCA2 pathogenic variants. Genet. Med. 2020, 22, 1653–1666. [Google Scholar] [CrossRef] [PubMed]
- Maas, P.; Barrdahl, M.; Joshi, A.D.; Auer, P.L.; Gaudet, M.M.; Milne, R.L.; Schumacher, F.R.; Anderson, W.F.; Check, D.; Chattopadhyay, S.; et al. Breast Cancer Risk From Modifiable and Nonmodifiable Risk Factors Among White Women in the United States. JAMA Oncol. 2016, 2, 1295–1302. [Google Scholar] [CrossRef]
- Kapoor, P.M.; Mavaddat, N.; Choudhury, P.P.; Wilcox, A.N.; Lindström, S.; Behrens, S.; Michailidou, K.; Dennis, J.; Bolla, M.K.; Wang, Q.; et al. Combined Associations of a Polygenic Risk Score and Classical Risk Factors With Breast Cancer Risk. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.C.; Cunningham, A.P.; Peto, J.; Evans, D.G.; Lalloo, F.; Narod, S.A.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Southey, M.C.; et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: Updates and extensions. Br. J. Cancer 2008, 98, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Cunningham, A.P.; Tischkowitz, M.; Simard, J.; Pharoah, P.D.; Easton, D.F.; Antoniou, A.C. Incorporating truncating variants in PALB2, CHEK2, and ATM into the BOADICEA breast cancer risk model. Genet. Med. 2016, 18, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, R.; Bickerstaffe, A.; Apicella, C.; Dite, G.S.; Dowty, J.G.; Aujard, K.; Phillips, K.; Weideman, P.; Lee, A.; Terry, M.B. Prospective validation of the breast cancer risk prediction model BOADICEA and a batch-mode version BOADICEACentre. Br. J. Cancer 2013, 109, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.-K.; Tan, M.-M.; Mavaddat, N.; Tai, M.-C.; Mariapun, S.; Li, J.; Ho, P.-J.; Dennis, J.; Tyrer, J.P.; Bolla, M.K.; et al. European polygenic risk score for prediction of breast cancer shows similar performance in Asian women. Nat. Commun. 2020, 11, 3833. [Google Scholar] [CrossRef]
- Fortier, I.; Dragieva, N.; Saliba, M.; Craig, C.; Robson, P.J. Harmonization of the Health and Risk Factor Questionnaire Data of the Canadian Partnership for Tomorrow Project: A descriptive analysis. CMAJ Open 2019, 7, E272–E282. [Google Scholar] [CrossRef]
- Carver, T.; Hartley, S.; Lee, A.; Cunningham, A.P.; Archer, S.; Babb de Villiers, C.; Roberts, J.; Ruston, R.; Walter, F.M.; Tischkowitz, M.; et al. CanRisk Tool—A Web Interface for the Prediction of Breast and Ovarian Cancer Risk and the Likelihood of Carrying Genetic Pathogenic Variants. Cancer Epidemiol. Biomark. Prev. 2020. [Google Scholar] [CrossRef]
- Archer, S.; Babb de Villiers, C.; Scheibl, F.; Carver, T.; Hartley, S.; Lee, A.; Cunningham, A.P.; Easton, D.F.; McIntosh, J.G.; Emery, J.; et al. Evaluating clinician acceptability of the prototype CanRisk tool for predicting risk of breast and ovarian cancer: A multi-methods study. PLoS ONE 2020, 15, e0229999. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2017/745 of the European Parliament and of the Council on medical devices. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0745 (accessed on 28 April 2021).
- Regulation (EU) 2017/746 of the European Parliament and of the Council on in vitro Diagnostic Medical Devices. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0746 (accessed on 28 April 2021).
- Medical Devices Regulations, SOR/98-282. Available online: https://laws-lois.justice.gc.ca/eng/regulations/sor-98-282/ (accessed on 28 April 2021).
- European Commission Guidelines on the Qualification and Classification of Stand Alone Software Used in Healthcare within the Regulatory Framework of Medical Devices. Available online: https://ec.europa.eu/docsroom/documents/17921 (accessed on 28 April 2021).
- Health Canada. Guidance Document Software as Medical Device (SaMD). Definition and Classification. (Canada: Health Canada, 2019). Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/application-information/guidance-documents/software-medical-device-guidance-document.html (accessed on 28 April 2021).
- Thorogood, A.; Touré, S.B.; Ordish, J.; Hall, A.; Knoppers, B. Genetic database software as medical devices. Hum. Mutat. 2018, 39, 1702–1712. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Blackmore, K.M.; Mirea, L.; Done, S.J.; Majpruz, V.; Weerasinghe, A.; Rabeneck, L.; Muradali, D. Annual vs Biennial Screening: Diagnostic Accuracy Among Concurrent Cohorts Within the Ontario Breast Screening Program. J. Natl. Cancer Inst. 2020, 112, 400–409. [Google Scholar] [CrossRef]
- Perron, L.; Chang, S.L.; Daigle, J.M.; Vandal, N.; Theberge, I.; Diorio, C.; Lemieux, J.; Pelletier, E.; Brisson, J. Breast cancer subtype and screening sensitivity in the Quebec Mammography Screening Program. J. Med. Screen. 2019, 26, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Green, L.; Kreuter, M. Health Program Planning: An Educational and Ecological Approach; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Dent, T.; Jbilou, J.; Rafi, I.; Segnan, N.; Törnberg, S.; Chowdhury, S.; Hall, A.; Lyratzopoulos, G.; Eeles, R.; Eccles, D. Stratified cancer screening: The practicalities of implementation. Public Health Genom. 2013, 16, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.S. The complexity of achieving the promise of precision breast cancer screening. J. Natl. Cancer Inst. 2017, 109, djw301. [Google Scholar] [CrossRef] [PubMed]
- Marcus, P.M.; Pashayan, N.; Church, T.R.; Doria-Rose, V.P.; Gould, M.K.; Hubbard, R.A.; Marrone, M.; Miglioretti, D.L.; Pharoah, P.D.; Pinsky, P.F. Population-based precision cancer screening: A symposium on evidence, epidemiology, and next steps. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Mbuya Bienge, C.; Pashayan, N.; Brooks, J.D.; Dorval, M.; Chiquette, J.; Eloy, L.; Turgeon, A.; Lambert-Côté, L.; Paquette, J.S.; Lévesque, E.; et al. Women’s Views on Multifactorial Breast Cancer Risk Assessment and Risk-Stratified Screening: A Population-Based Survey from Four Provinces in Canada. J. Pers. Med. 2021, 11, 95. [Google Scholar] [CrossRef]
- Hagan, J.; Lévesque, E.; Knoppers, B.M. Influence of organizational factors on implementation of a personalized approach to breast cancer screening. Sante Publique 2016, 28, 353–361. [Google Scholar] [CrossRef]
- Lévesque, E.; Hagan, J.; Knoppers, B.M.; Simard, J. Organizational challenges to equity in the delivery of services within a new personalized risk-based approach to breast cancer screening. New Genet. Soc. 2019, 38, 38–59. [Google Scholar] [CrossRef]
- Esquivel-Sada, D.; Lévesque, E.; Hagan, J.; Knoppers, B.; Simard, J. Envisioning Implementation of a Personalized Approach in Breast Cancer Screening Programs: Stakeholder Perspectives. Healthc. Policy 2019, 15, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Dalpé, G.; Ngueng Feze, I.; Salman, S.; Joly, Y.; Hagan, J.; Lévesque, E.; Dorval, V.; Blouin-Bougie, J.; Amara, N.; Dorval, M. Breast cancer risk estimation and personal insurance: A qualitative study presenting perspectives from canadian patients and decision makers. Front. Genet. 2017, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Joly, Y.; Koutrikas, G.; Tasse, A.-M.; Issa, A. Regulatory approval for new pharmacogenomic tests: A comparative overview. Food Drug Law J. 2011, 66, 1. [Google Scholar]
- Lévesque, E.; Knoppers, B.M. Faire Jouer un Rôle Élargi aux Infirmières Dans une Approche Individualisée de Dépistage du Cancer du Sein: Analyse des Options Juridiques; Revue de droit et santé de McGill; Faculty of Law, McGill University: Montreal, Canada, 2021; in press. [Google Scholar]
- Schwartz, M.D.; Valdimarsdottir, H.B.; Peshkin, B.N.; Mandelblatt, J.; Nusbaum, R.; Huang, A.-T.; Chang, Y.; Graves, K.; Isaacs, C.; Wood, M. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J. Clin. Oncol. 2014, 32, 618. [Google Scholar] [CrossRef] [PubMed]
- Kinney, A.Y.; Steffen, L.E.; Brumbach, B.H.; Kohlmann, W.; Du, R.; Lee, J.H.; Gammon, A.; Butler, K.; Buys, S.S.; Stroup, A.M.; et al. Randomized Noninferiority Trial of Telephone Delivery of BRCA1/2 Genetic Counseling Compared With In-Person Counseling: 1-Year Follow-Up. J. Clin. Oncol. 2016, 34, 2914–2924. [Google Scholar] [CrossRef] [PubMed]
- Peshkin, B.N.; Kelly, S.; Nusbaum, R.H.; Similuk, M.; DeMarco, T.A.; Hooker, G.W.; Valdimarsdottir, H.B.; Forman, A.D.; Joines, J.R.; Davis, C. Patient perceptions of telephone vs. in-person BRCA1/BRCA2 genetic counseling. J. Genet. Couns. 2016, 25, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, E.; Knoppers, B.M. La Télésanté́ au Québec: Quel Encadrement Pour la Consultation Vidéo? Revue de Droit de l’Université́ de Sherbrooke; Éditions RDUS: Sherbrooke, Canada, 2021; in press. [Google Scholar]
- Joly, Y.; Dalpé, G.; Dupras, C.; Bévière-Boyer, B.; de Paor, A.; Dove, E.S.; Granados Moreno, P.; Ho, C.W.L.; Ho, C.H.; Cathaoir, K.Ó.; et al. Establishing the International Genetic Discrimination Observatory. Nat. Genet. 2020, 52, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Joly, Y.; Dalpé, G.; Pinkesz, M. Is Genetic Discrimination Back on the Radar? A Commentary on the Recent Court of Appeal Reference Decision on the Genetic Non-Discrimination Act (GNDA). Can. J. Bioeth./Revue Can. Bioéth. 2019, 2, 94–96. [Google Scholar] [CrossRef]
- Gauvreau, C.L.; Fitzgerald, N.R.; Memon, S.; Flanagan, W.M.; Nadeau, C.; Asakawa, K.; Garner, R.; Miller, A.B.; Evans, W.K.; Popadiuk, C.M.; et al. The OncoSim model: Development and use for better decision-making in Canadian cancer control. Curr. Oncol. 2017, 24, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, M.; Gribble, S.; Pashayan, N.; Easton, D.F.; Antoniou, A.C.; Lee, A.; van Katwyk, S.; Simard, J. Potential of Polygenic Risk Scores for Improving Population Estimates of Women’s Breast Cancer Genetic Risks. Genet. Med. accepted.
- Mittmann, N.; Stout, N.K.; Lee, P.; Tosteson, A.N.; Trentham-Dietz, A.; Alagoz, O.; Yaffe, M.J. Total cost-effectiveness of mammography screening strategies. Health Rep. 2015, 26, 16. [Google Scholar]
- Mittmann, N.; Stout, N.K.; Tosteson, A.N.; Trentham-Dietz, A.; Alagoz, O.; Yaffe, M.J. Cost-effectiveness of mammography from a publicly funded health care system perspective. CMAJ Open 2018, 6, E77. [Google Scholar] [CrossRef]
- Shapiro, S. Evidence on screening for breast cancer from a randomized trial. Cancer 1977, 39, 2772–2782. [Google Scholar] [CrossRef]
- Coldman, A.; Phillips, N.; Wilson, C.; Decker, K.; Chiarelli, A.M.; Brisson, J.; Zhang, B.; Payne, J.; Doyle, G.; Ahmad, R. Pan-Canadian study of mammography screening and mortality from breast cancer. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef]
- Nelson, H.D.; Fu, R.; Cantor, A.; Pappas, M.; Daeges, M.; Humphrey, L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann. Intern. Med. 2016, 164, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Houssami, N. Discussing the benefits and harms of screening mammography. Maturitas 2016, 92, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Gøtzsche, P.C.; Jørgensen, K.J. Screening for breast cancer with mammography. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.R.; Moorman, P.; Gierisch, J.M.; Havrilesky, L.J.; Grimm, L.J.; Ghate, S.; Davidson, B.; Mongtomery, R.C.; Crowley, M.J.; McCrory, D.C.; et al. Benefits and Harms of Breast Cancer Screening: A Systematic Review. JAMA 2015, 314, 1615–1634. [Google Scholar] [CrossRef]
- Ma, L.; Fishell, E.; Wright, B.; Hanna, W.; Allan, S.; Boyd, N.F. Case-Control Study of Factors Associated With Failure to Detect Breast Cancer by Mammography. J. Natl. Cancer Inst. 1992, 84, 781–785. [Google Scholar] [CrossRef]
- Mandelson, M.T.; Oestreicher, N.; Porter, P.L.; White, D.; Finder, C.A.; Taplin, S.H.; White, E. Breast Density as a Predictor of Mammographic Detection: Comparison of Interval- and Screen-Detected Cancers. J. Natl. Cancer Inst. 2000, 92, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Esserman, L.J. The WISDOM Study: Breaking the deadlock in the breast cancer screening debate. NPJ Breast Cancer 2017, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Shieh, Y.; Eklund, M.; Madlensky, L.; Sawyer, S.D.; Thompson, C.K.; Stover Fiscalini, A.; Ziv, E.; Van’t Veer, L.J.; Esserman, L.J.; Tice, J.A. Breast Cancer Screening in the Precision Medicine Era: Risk-Based Screening in a Population-Based Trial. J. Natl. Cancer Inst. 2017, 109, djw290. [Google Scholar] [CrossRef] [PubMed]
- The Project—MyPeBS. Available online: https://mypebs.eu/the-project/ (accessed on 6 February 2021).
- Evans, D.G.; Astley, S.; Stavrinos, P.; Harkness, E.; Donnelly, L.S.; Dawe, S.; Jacob, I.; Harvie, M.; Cuzick, J.; Brentnall, A.; et al. Programme Grants for Applied Research. In Improvement in Risk prediction, Early Detection and Prevention of Breast Cancer in the NHS Breast Screening Programme and Family History Clinics: A Dual Cohort Study; NIHR Journals Library: Southampton, UK, 2016. [Google Scholar]
- Pashayan, N.; Morris, S.; Gilbert, F.J.; Pharoah, P.D.P. Cost-effectiveness and Benefit-to-Harm Ratio of Risk-Stratified Screening for Breast Cancer: A Life-Table Model. JAMA Oncol. 2018, 4, 1504–1510. [Google Scholar] [CrossRef] [PubMed]


| Risk Level | Risk Communication a | Screening Action Plan | Follow-Up |
|---|---|---|---|
| Average 10-year absolute risk equivalent to lifetime risk of <15% | Risk Letter: includes risk level and range | 40–49 years: No regular screening with a mammogram | Phone call with study genetic counsellor or nurse available upon request |
| Average risk: “In this risk level, up to <number> out of 1000 women your age may get breast cancer over the next 10 years”. | 50–69 years: Screened every 2 years with a mammogram | Follow-up with questionnaire at time of risk communication and 1 year later. | |
| Higher than Average 10-year absolute risk equivalent to lifetime risk of 15 to <25% | Risk Letter: includes risk level and range | Ontario 40–49 years: Talk to primary care provider about screening with a mammogram every year. | Phone call with study genetic counsellor or nurse available upon request |
| Higher than Average risk: “In this risk level, about <number> to <number> out of 1000 women your age may get breast cancer over the next 10 years”. | 50–69 years: Screened every year with a mammogram | Follow-up with questionnaire at time of risk communication and 1 year later. | |
| Québec Screened every 1–2 years with a mammogram, ultrasound considered if breast density is >75% | |||
| High 10-year absolute risk equivalent to lifetime risk of ≥25% | Risk Letter: includes risk level and range | 40–69 years: Screened every year with a mammogram and magnetic resonance imaging (MRI) | Phone call with study genetic counsellor or nurse |
| High risk: “In this risk level, <number> or more out of 1000 women your age may get breast cancer over the next 10 years”. | Follow-up with questionnaire at time of risk communication and 1 year later. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brooks, J.D.; Nabi, H.; Andrulis, I.L.; Antoniou, A.C.; Chiquette, J.; Després, P.; Devilee, P.; Dorval, M.; Droit, A.; Easton, D.F.; et al. Personalized Risk Assessment for Prevention and Early Detection of Breast Cancer: Integration and Implementation (PERSPECTIVE I&I). J. Pers. Med. 2021, 11, 511. https://doi.org/10.3390/jpm11060511
Brooks JD, Nabi H, Andrulis IL, Antoniou AC, Chiquette J, Després P, Devilee P, Dorval M, Droit A, Easton DF, et al. Personalized Risk Assessment for Prevention and Early Detection of Breast Cancer: Integration and Implementation (PERSPECTIVE I&I). Journal of Personalized Medicine. 2021; 11(6):511. https://doi.org/10.3390/jpm11060511
Chicago/Turabian StyleBrooks, Jennifer D., Hermann Nabi, Irene L. Andrulis, Antonis C. Antoniou, Jocelyne Chiquette, Philippe Després, Peter Devilee, Michel Dorval, Arnaud Droit, Douglas F. Easton, and et al. 2021. "Personalized Risk Assessment for Prevention and Early Detection of Breast Cancer: Integration and Implementation (PERSPECTIVE I&I)" Journal of Personalized Medicine 11, no. 6: 511. https://doi.org/10.3390/jpm11060511
APA StyleBrooks, J. D., Nabi, H., Andrulis, I. L., Antoniou, A. C., Chiquette, J., Després, P., Devilee, P., Dorval, M., Droit, A., Easton, D. F., Eisen, A., Eloy, L., Fienberg, S., Goldgar, D., Hahnen, E., Joly, Y., Knoppers, B. M., Lofters, A., Masson, J.-Y., ... Simard, J. (2021). Personalized Risk Assessment for Prevention and Early Detection of Breast Cancer: Integration and Implementation (PERSPECTIVE I&I). Journal of Personalized Medicine, 11(6), 511. https://doi.org/10.3390/jpm11060511









