In Situ Delivery and Production System (iDPS) of Anti-Cancer Molecules with Gene-Engineered Bifidobacterium
Abstract
:1. Introduction
1.1. Molecular Target Cancer Therapy and its Limitations
1.2. The Enhanced Permeability and Retention (EPR) Effect
1.3. Our Approach Targeting the Low pO2 in Solid Cancers with Bifidobacterium
1.4. Hypoxia and Immature Blood Vasculature in Malignant Tumors
1.5. Bacterial Therapy for Cancer
The EPR Effect’s Importance in Bacterial Therapy
2. Our Trials for Bacterial Therapy
2.1. Selective Localization of i.v. Injected Bifidobacterium in Tumors
2.2. Transformation of Bifidobacterium with an Expression Vector for Cytosine Deaminase (CD)
2.3. Therapy Experiments on Solid Cancers Using Transformed Bifidobacterium with 5FC
2.4. Immunological Safety
2.5. Translational Research
2.6. Combination Therapy of APS001F Plus 5FC (APS001F/5FC) in Combination with the Immune Checkpoint Inhibitor (ICPI) Anti-PD-1
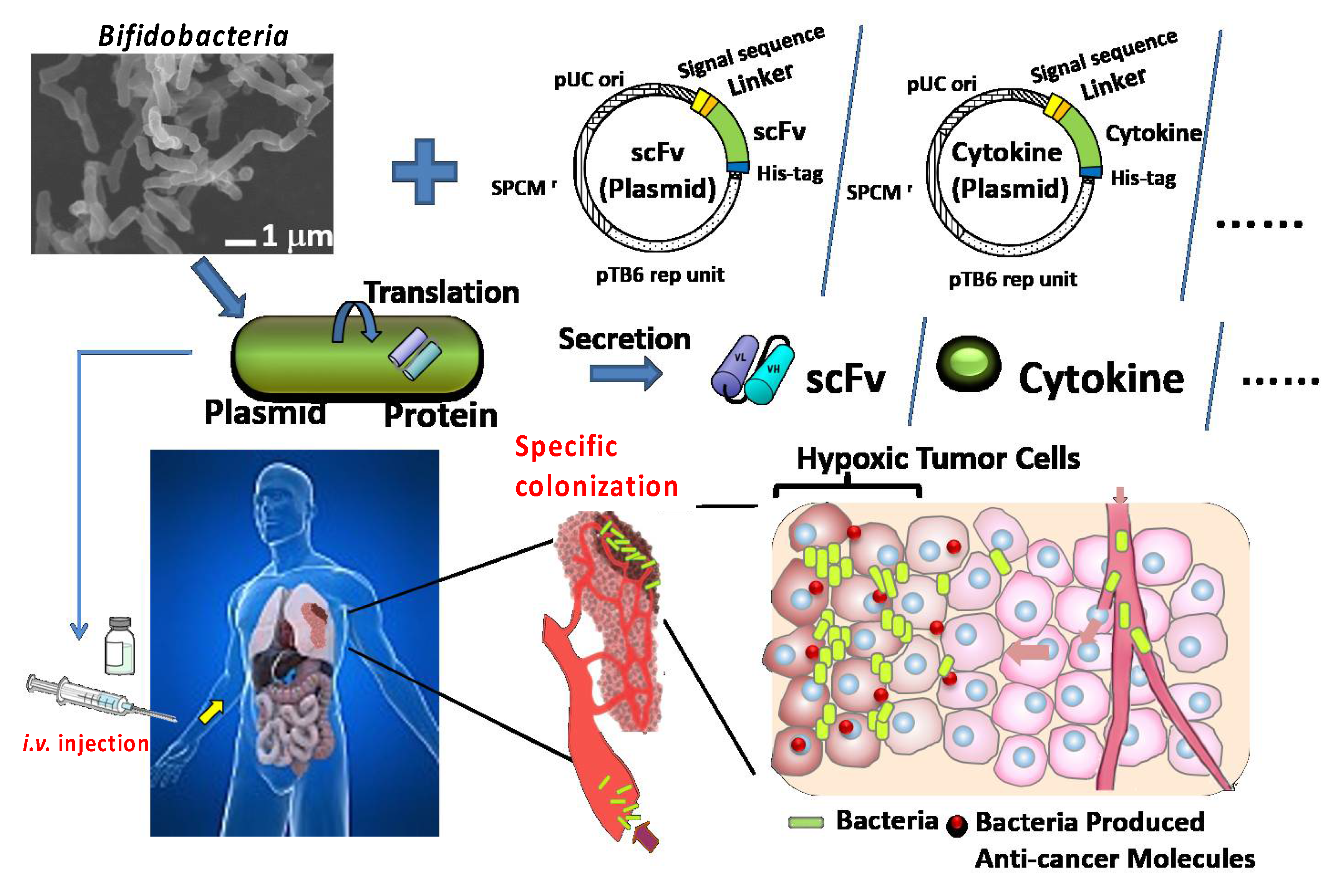
2.7. Establishment of a Protein-Secreting System
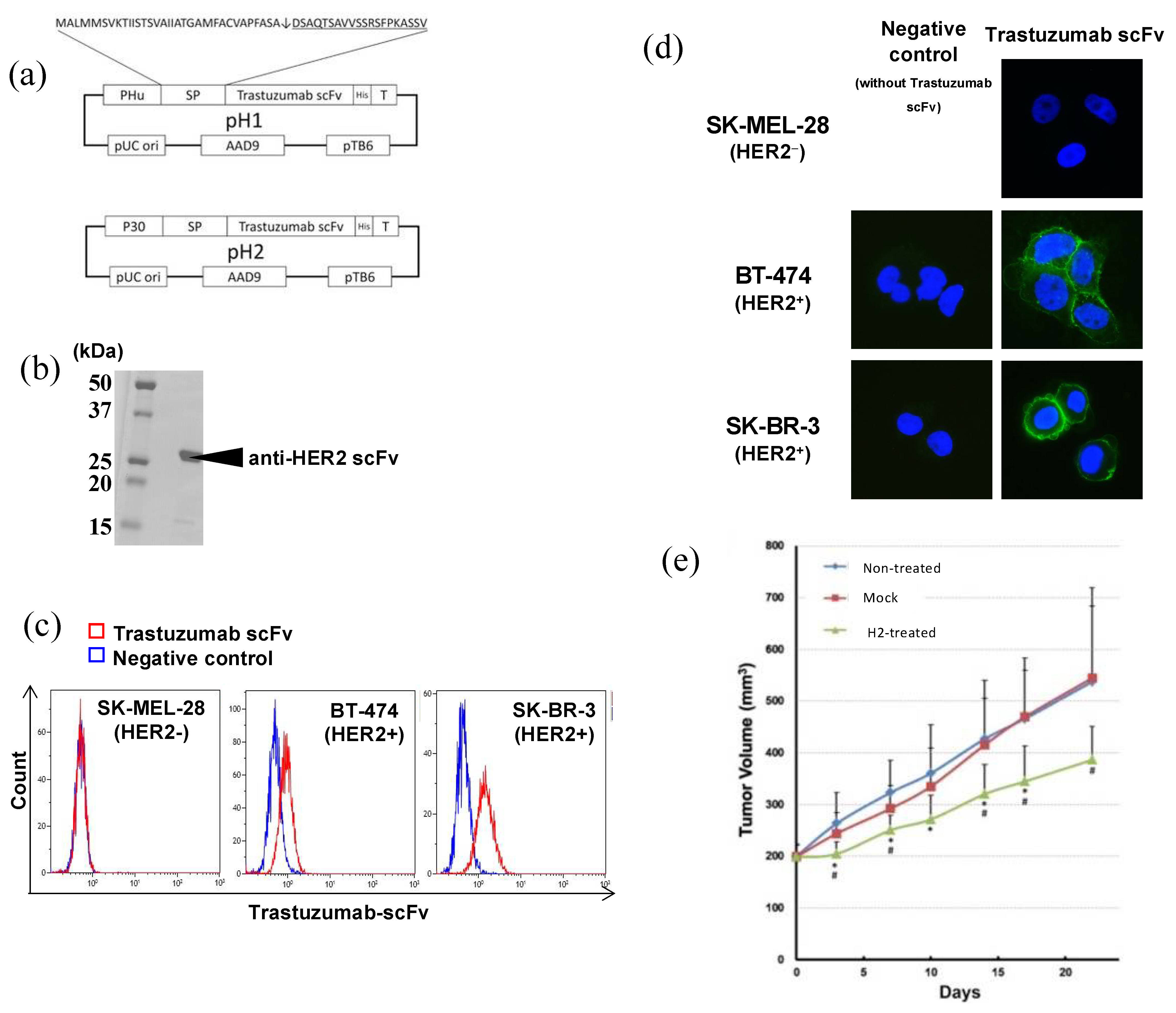
2.7.1. Anti-HER2 scFv
2.7.2. scFvs for ICP Antagonistic or Agonistic Antibodies, including Anti-PD-1, Anti-CTLA4, Anti-41BB, and Anti-Tumor Cytokines
3. For Further Improvement of the iDPS
3.1. Notes for the Presnt iDPS
3.1.1. Animal Experiments
3.1.2. As for Safety of iDPS with Bifidobacterium
3.1.3. The Specific Advantages of Using Bifidobacterium for Our iDPS
3.2. Seeking an Ideal Micro-Factory with Guaranteed Safety
3.2.1. Modification of Tumor Hemodynamics with Vasodilators to Enhance the EPR Effect
3.2.2. Transient Evasion from Bacteria Trapping by the Reticuloendothelial System (RES) and/or Neutrophils
3.2.3. Other Factors for Improving the iDPS
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, B.; Singh, S.; Skvortsova, I.; Kumar, V. Promising Targets in Anti-cancer Drug Development: Recent Updates. Curr. Med. Chem. 2017, 24, 4729–4752. [Google Scholar] [CrossRef] [PubMed]
- Olgen, S. Overview on Anticancer Drug Design and Development. Curr. Med. Chem. 2018, 25, 1704–1719. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.J.; Lei, K.F.; Han, F. Tumor microenvironment: Recent advances in various cancer treatments. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3855–3864. [Google Scholar]
- McGranahan, N.; Swanton, C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 2015, 27, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Martin, J.D.; Snuderl, M.; Mpekris, F.; Jain, S.R.; Jain, R.K. Coevolution of solid stress and interstitial fluid pressure in tumors during progression: Implications for vascular collapse. Cancer Res. 2013, 73, 3833–3841. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, S.; Takeoka, M.; Ehara, T.; Hashimoto, S.; Shibuki, H.; Yoshimura, N.; Shigematsu, H.; Takahashi, K.; Katsuki, M. Structural fragility of blood vessels and peritoneum in calponin h1-deficient mice, resulting in an increase in hematogenous metastasis and peritoneal dissemination of malignant tumor cells. Cancer Res. 2001, 61, 7627–7634. [Google Scholar] [PubMed]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Taniguchi, S.; Fujimori, M.; Sasaki, T.; Tsutsui, H.; Shimatani, Y.; Seki, K.; Amano, J. Targeting solid tumors with non-pathogenic obligate anaerobic bacteria. Cancer Sci. 2010, 101, 1925–1932. [Google Scholar] [CrossRef]
- Taniguchi, S.; Shimatani, Y.; Fujimori, M. Tumor-Targeting Therapy Using Gene-Engineered Anaerobic-Nonpathogenic Bifidobacterium longum. Methods Mol. Biol. 2016, 1409, 49–60. [Google Scholar] [PubMed]
- Kimura, N.T.; Taniguchi, S.; Aoki, K.; Baba, T. Selective localization and growth of Bifidobacterium bifidum in mouse tumors following intravenous administration. Cancer Res. 1980, 40, 2061–2068. [Google Scholar] [PubMed]
- Brown, J.M. The hypoxic cell: A target for selective cancer therapy—Eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1999, 59, 5863–5870. [Google Scholar]
- Vaupel, P.; Höckel, M.; Mayer, A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal 2007, 122, 1–35. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.B.; Robinson, S.P.; Waterton, J.C. Imaging tumor hypoxia with oxygen-enhanced MRI and BOLD MRI. Br. J. Radiol. 2019, 92, 20180642. [Google Scholar] [CrossRef] [PubMed]
- Pries, A.R.; Höpfner, M.; le Noble, F.; Dewhirst, M.W.; Secomb, T.W. The shunt problem: Control of functional shunting in normal and tumor vasculature. Nat. Rev. Cancer 2010, 10, 587–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmgren, R.A.; Flanigan, C.C. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 1955, 15, 473–478. [Google Scholar]
- Mowday, A.M.; Guise, C.P.; Ackerley, D.F.; Minton, N.P.; Lambin, P.; Dubois, L.J.; Theys, J.; Smaill, J.B.; Patterson, A.V. Advancing Clostridia to Clinical Trial: Past Lessons and Recent Progress. Cancers 2016, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Nauts, H.C.; Swift, W.E.; Coley, B.L. The treatment of malignant tumors by bacterial toxins as developed by the late William B. Coley, M.D., reviewed in the light of modern research. Cancer Res. 1946, 6, 205–216. [Google Scholar]
- Forbes, N.S. Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer 2010, 10, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Sarotra, P.; Medhi, B. Use of Bacteria in Cancer Therapy. Recent Results Cancer Res. 2016, 209, 111–121. [Google Scholar]
- Hoffman, R.M. Future of Bacterial Therapy of Cancer. Methods Mol. Biol. 2016, 1409, 177–184. [Google Scholar]
- Zhou, S.; Gravekamp, C.; Bermudes, D.; Liu, K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 2018, 18, 727–743. [Google Scholar] [CrossRef]
- Toso, J.F.; Sznol, M.; Rosenberg, S.A. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. 2002, 20, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.J.; Zhang, L.; Janku, F.; Collins, A.; Bai, R.Y.; Staedtke, V.; Rusk, A.W.; Tung, D.; Miller, M.; Roix, J.; et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci. Transl. Med. 2014, 6, 249ra111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemunaitis, J.; Sznol, M. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003, 10, 737–744. [Google Scholar] [CrossRef]
- Thamm, D.H.; Kurzman, I.D.; King, I.; Li, Z.; Sznol, M.; Dubielzig, R.R.; Vail, D.M.; MacEwen, E.G. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: Phase I evaluation. Clin. Cancer Res. 2005, 11, 4827–4834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, S.E.; Henson, M.S.; Greengard, E.; Winter, A.L.; Stuebner, K.M.; Yoon, U.; Wilk, V.L.; Borgatti, A.; Augustin, L.B.; Modiano, J.F.; et al. A phase I clinical study to evaluate safety of orally administered, genetically engineered Salmonella enterica serovar Typhimurium for canine osteosarcoma. Vet. Med. Sci. 2016, 2, 179–190. [Google Scholar] [CrossRef]
- Fang, J.; Long, L.; Maeda, H. Enhancement of Tumor-Targeted Delivery of Bacteria with Nitroglycerin Involving Augmentation of the EPR Effect. Methods Mol. Biol. 2016, 1409, 9–23. [Google Scholar] [PubMed]
- Fang, J.; Liao, L.; Yin, H.; Nakamura, H.; Shin, T.; Maeda, H. Enhanced bacterial tumor delivery by modulating the EPR effect and therapeutic potential of Lactobacillus casei. J. Pharm. Sci. 2014, 103, 3235–3243. [Google Scholar] [CrossRef]
- Matsumura, H.; Takeuchi, A.; Kano, Y. Construction of Escherichia coli-Bifidobacterium longum shuttle vector transforming B. longum 105-A and 108-A. Biosci. Biotechnol. Biochem. 1997, 61, 1211–1212. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, K.; Fujimori, M.; Amano, J.; Kano, Y.; Taniguchi, S. Bifidobacterium longum as a delivery system for cancer gene therapy: Selective localization and growth in hypoxic tumors. Cancer Gene Ther. 2000, 7, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Yazawa, K.; Fujimori, M.; Nakamura, T.; Sasaki, T.; Amano, J.; Kano, Y.; Taniguchi, S. Bifidobacterium longum as a delivery system for gene therapy of chemically induced rat mammary tumors. Breast Cancer Res. Treat. 2001, 66, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sasaki, T.; Fujimori, M.; Yazawa, K.; Kano, Y.; Amano, J.; Taniguchi, S. Cloned cytosine deaminase gene expression of Bifidobacterium longum and application to enzyme/pro-drug therapy of hypoxic solid tumors. Biosci. Biotechnol. Biochem. 2002, 66, 2362–2366. [Google Scholar] [CrossRef] [Green Version]
- Hidaka, A.; Hamaji, Y.; Sasaki, T.; Taniguchi, S.; Fujimori, M. Exogenous cytosine deaminase gene expression in Bifidobacterium breve I-53-8w for tumor-targeting enzyme/prodrug therapy. Biosci. Biotechnol. Biochem. 2007, 71, 2921–2926. [Google Scholar] [CrossRef]
- Sasaki, T.; Fujimori, M.; Hamaji, Y.; Hama, Y.; Ito, K.; Amano, J.; Taniguchi, S. Genetically engineered Bifidobacterium longum for tumor-targeting enzyme-prodrug therapy of autochthonous mammary tumors in rats. Cancer Sci. 2006, 97, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Mahan, S.D.; Ireton, G.C.; Knoeber, C.; Stoddard, B.L.; Black, M.E. Random mutagenesis and selection of Escherichia coli cytosine deaminase for cancer gene therapy. Protein. Eng. Des. Sel. 2004, 17, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. TLR signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef]
- Taniguchi, S.; Sagara, J. Regulatory molecules involved in inflammasome formation with special reference to a key mediator protein, ASC. Semin. Immunopathol. 2007, 29, 231–238. [Google Scholar] [CrossRef]
- Van Bergenhenegouwen, J.; Kraneveld, A.D.; Rutten, L.; Kettelarij, N.; Garssen, J.; Vos, A.P. Extracellular vesicles modulate host-microbe responses by altering TLR2 activity and phagocytosis. PLoS ONE 2014, 9, e89121. [Google Scholar]
- Kato, K.; Odamaki, T.; Mitsuyama, E.; Sugahara, H.; Xiao, J.Z.; Osawa, R. Age-Related Changes in the Composition of Gut Bifidobacterium Species. Curr. Microbiol. 2017, 74, 987–995. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L. CHILD Study Investigators. Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. CMAJ 2013, 185, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Phase I/II Study of APS001F With Flucytosine and Maltose in Solid Tumors, US National Library of Medicine, ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01562626 (accessed on 26 March 2012).
- Gotwals, P.; Cameron, S.; Cipolletta, D.; Cremasco, V.; Crystal, A.; Hewes, B.; Mueller, B.; Quaratino, S.; Sabatos-Peyton, C.; Petruzzelli, L.; et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer 2017, 17, 286–301. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef] [Green Version]
- Shioya, K.; Matsumura, T.; Seki, Y.; Shimizu, H.; Nakamura, T.; Taniguchi, S. Potentiated antitumor effects of APS001F/5-FC combined with anti-PD-1 antibody in a CT26 syngeneic mouse model. Biosci. Biotechnol. Biochem. 2021, 85, 324–331. [Google Scholar] [CrossRef]
- Vincent, J.; Mignot, G.; Chalmin, F.; Ladoire, S.; Bruchard, M.; Chevriaux, A.; Martin, F.; Apetoh, L.; Rébé, C.; Ghiringhelli, F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010, 70, 3052–3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zitvogel, L.; Galluzzi, L.; Smyth, M.J.; Kroemer, G. Mechanisms of action conventional and targeted anticancer therapies: Reinstating immunosurveillance. Immunity 2013, 39, 74–88. [Google Scholar] [CrossRef] [Green Version]
- Kadoyama, K.; Miki, I.; Tamura, T.; Brown, J.B.; Sakaeda, T.; Okuno, Y. Adverse event profiles of 5- fluorouracil and capecitabine: Data mining of the public version of the FDA adverse event reporting system, AERS, and reproducibility of clinical observations. Int. J. Med. Sci. 2012, 9, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Deng, Z.; Wang, H.; Ma, W.; Zhou, C.; Zhang, S. Repeated cycles of 5-fluorouracil chemotherapy impaired anti-tumor functions of cytotoxic T cells in a CT26 tumor-bearing mouse model. BMC Immunol. 2016, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, R.; Cheema, A.; Khan, T.; Amirpour, A.; Paul, A.; Chaughtai, S.; Patel, S.; Patel, T.; Bramson, J.; Gupta, V.; et al. Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J. Clin. Med. Res. 2019, 11, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Johncilla, M.; Grover, S.; Zhang, X.; Jain, D.; Srivastava, A. Morphological spectrum of immune check-point inhibitor therapy-associated gastritis. Histopathology 2020, 76, 531–539. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Bajaj, D.; Sankaramangalam, K.; Yoo, J.W.; Joshi, N.S.; Gettinger, S.; Price, C.; Farrell, J.J. Incidence of pancreatitis with the use of immune checkpoint inhibitors (ICI) in advanced cancers: A systematic review and meta-analysis. Pancreatology 2019, 19, 587–594. [Google Scholar] [CrossRef]
- Nemeth, B.T.; Varga, Z.V.; Wu, W.J.; Pacher, P. Trastuzumab cardiotoxicity: From clinical trials to experimental studies. Br. J. Pharmacol. 2017, 174, 3727–3748. [Google Scholar] [CrossRef] [Green Version]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G., Jr.; Untch, M.; Smith, I.; Gianni, L.; et al. Herceptin Adjuvant (HERA) Trial Study Team. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, T.; Shimizu, H.; Akiyama, Y.; Taniguchi, S. In situ delivery and production system of trastuzumab scFv with Bifidobacterium. Biochem. Biophys. Res. Commun. 2017, 493, 306–312. [Google Scholar] [CrossRef]
- Shioya, K.; Wang, L.; Matsumura, T.; Shimizu, H.; Kanari, Y.; Seki, Y.; Shimatani, Y.; Taniguchi, S. Anti-PD-1 antibody scFV producing recombinant Bifidobacterium exerts antitumor effect in a large fraction of the treated mice compared to full-length anti-PD-1 antibody. AACR Special Conference; 1–4 October 2017, Boston, USA. Cancer Immunol. Res. 2018, 6, A23. [Google Scholar]
- Shioya, K.; Kataoka, S.; Wang, L.; Matsumura, T.; Shimizu, H.; Kanari, Y.; Seki, Y.; Shimatani, Y.; Fujimori, M.; Taniguchi, S. anti-CTLA-4 antibody scFv producing recombinant Bifidobacterium secretes CTLA-4 blocker specifically inside hypoxic tumor and suppresses tumor growth in syngeneic mice model., AACR Special Conference on Tumor Immunology and Immunotherapy; 20–23 October 2016, Boston, USA. Cancer Immunol. Res. 2017, 5, A29. [Google Scholar]
- Matsumura, T.; Shioya, K.; Kanari, Y.; Shimatani, Y.; Kataoka, S.; Taniguchi, S.; Nakamura, T. Cancer immunotherapy with agonistic anti-4-1-BB scFv producing and secreting Bifidobacterium in syngeneic mouse model., AACR Annual Meeting; 14–18 April 2018, Chicago, IL, USA. Cancer Res. 2018, 78, A2735. [Google Scholar]
- Seki, Y.; Shioya, K.; Kobayashi, S.; Shimatani, Y.; Fujimori, M.; Taniguchi, S. Enhanced anti-tumor effects by a combination approach of interferon-producing recombinant Bifidobacterium and anti-mPD-1 antibody in syngeneic mouse model. AACR Annual Meeting, 1–5 April 2017, Washington, DC, USA. Cancer Res. 2017, 77, A2631. [Google Scholar]
- Patent Information. Available online: https://patents.google.com/patent/WO2011093465A1/ja (accessed on 17 June 2021).
- Van Horssen, R.; Ten Hagen, T.L.; Eggermont, A.M. TNF-alpha in cancer treatment: Molecular insights, antitumor effects, and clinical utility. Oncologist 2006, 11, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Shioya, K.; Yuji Seki, Y.; Matsumura, T.; Kanari, Y.; Shimatani, Y.; Nakazawa, H.; Umetsu, M.; Kataoka, S.; Nakamura, T. Anti-tumor activity of Bifidobacterium secreting dual specific T cell redirecting antibody against EGFR/HER3-expressing cancer. AACR Annual Meeting; 22–24 June 2020, Philadelphia, PA, USA. Cancer Res. 2020, 80, AS689. [Google Scholar]
- Wei, C.; Xun, A.Y.; Wei, X.X.; Yao, J.; Wang, J.Y.; Shi, R.Y.; Yang, G.H.; Li, Y.X.; Xu, Z.L.; Lai, M.G.; et al. Bifidobacteria expressing tumstatin protein for antitumor therapy in tumor-bearing mice. Technol. Cancer Res. Treat. 2015, 15, 498–508. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Vuletic, I.; Deng, D.; Crielaard, W.; Xie, Z.; Zhou, K.; Zhang, J.; Sun, H.; Ren, Q.; Gu, C. Bifidobacterium breve as a delivery vector of IL-24 gene therapy for bead and neck squamous cell carcinoma in vivo. Gene Ther. 2017, 24, 699–705. [Google Scholar] [CrossRef]
- Yasuda, H.; Yamaya, M.; Nakayama, K.; Sasaki, T.; Ebihara, S.; Kanda, A.; Asada, M.; Inoue, D.; Suzuki, T.; Okazaki, T.; et al. Randomized phase II trial comparing nitroglycerin plus vinorelbine and cisplatin with vinorelbine and cisplatin alone in previously untreated stage IIIB/IV non-small-cell lung cancer. J. Clin. Oncol. 2006, 24, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Nakayama, K.; Watanabe, M.; Suzuki, S.; Fuji, H.; Okinaga, S.; Kanda, A.; Zayasu, K.; Sasaki, T.; Asada, M.; et al. Nitroglycerin treatment may enhance chemosensitivity to docetaxel and carboplatin in patients with lung adenocarcinoma. Clin. Cancer Res. 2006, 12, 6748–6757. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, H.; Yanagihara, K.; Nakayama, K.; Mio, T.; Sasaki, T.; Asada, M.; Yamaya, M.; Fukushima, M. Therapeutic applications of nitric oxide for malignant tumor in animal models and human studies. In Nitric Oxide and Cancer; Bonavida, B., Ed.; Springer Science: New York, NY, USA, 2009. [Google Scholar]
- Siemens, D.R.; Heaton, J.P.; Adams, M.A.; Kawakami, J.; Graham, C.H. Phase II study of nitric oxide donor for men with increasing prostate-specific antigen level after surgery or radiotherapy for prostate cancer. Urology 2009, 74, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Tieken, C.; Versteeg, H.H. Anticoagulants versus cancer. Thromb Res. 2016, 140 (Suppl. 1), S148–S153. [Google Scholar] [CrossRef]
- Gadomska, G.; Ziołkowska, K.; Boinska, J.; Filipiak, J.; Rość, D. Activation of TF-Dependent Blood Coagulation Pathway and VEGF-A in Patients with Essential Thrombocythemia. Medicina 2019, 55, 54. [Google Scholar] [CrossRef] [Green Version]
- Dvorak, H.F. Tumors: Wounds that do not heal-redux. Cancer Immunol. Res. 2015, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Samoszuk, M.; Deng, T.; Hamamura, M.J.; Su, M.Y.; Asbrock, N.; Nalcioglu, O. Increased blood clotting, microvascular density, and inflammation in eotaxin-secreting tumors implanted into mice. Am. J. Pathol. 2004, 165, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S. Thrombolytic Therapy by Tissue Plasminogen Activator for Pulmonary Embolism. Adv. Exp. Med. Biol. 2017, 906, 67–74. [Google Scholar]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Cesar, S.; Huang, K.C. Thinking big: The tunability of bacterial cell size. FEMS Microbiol. Rev. 2017, 41, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Tocheva, E.I.; López-Garrido, J.; Hughes, H.V.; Fredlund, J.; Kuru, E.; Vannieuwenhze, M.S.; Brun, Y.V.; Pogliano, K.; Jensen, G.J. Peptidoglycan transformations during Bacillus subtilis sporulation. Mol. Microbiol. 2013, 88, 673–686. [Google Scholar] [CrossRef] [Green Version]
- Zahednezhad, F.; Saadat, M.; Valizadeh, H.; Zakeri-Milani, P.; Baradaran, B. Liposome and immune system interplay: Challenges and potentials. J. Control. Release 2019, 305, 194–209. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, M.D.; Chen, A.M. Beyond the red cell: Pegylation of other blood cells and tissues. Transfus. Clin. Biol. 2004, 11, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Scott, M.D. Current and future applications of immunological attenuation via pegylation of cells and tissue. BioDrugs 2001, 15, 833–847. [Google Scholar] [CrossRef]
- Zuo, F.; Zeng, Z.; Hammarström, L.; Marcotte, H. Inducible Plasmid Self-Destruction (IPSD) Assisted Genome Engineering in Lactobacilli and Bifidobacteria. ACS Synth. Biol. 2019, 8, 1723–1729. [Google Scholar] [CrossRef]
- Mruk, I.; Kobayashi, I. To be or not to be: Regulation of restriction-modification systems and other toxin-antitoxin systems. Nucleic Acids Res. 2014, 42, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Shirai, H.; Tsukada, K. Bacterial proteolytic activity improves drug delivery in tumors in a size, pharmacokinetic, and binding affinity dependent manner—A mechanistic understanding. J. Control. Release 2020, 321, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. Small Bioactive Peptides for Biomaterials Design and Therapeutics. Chem. Rev. 2017, 117, 14015–14041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
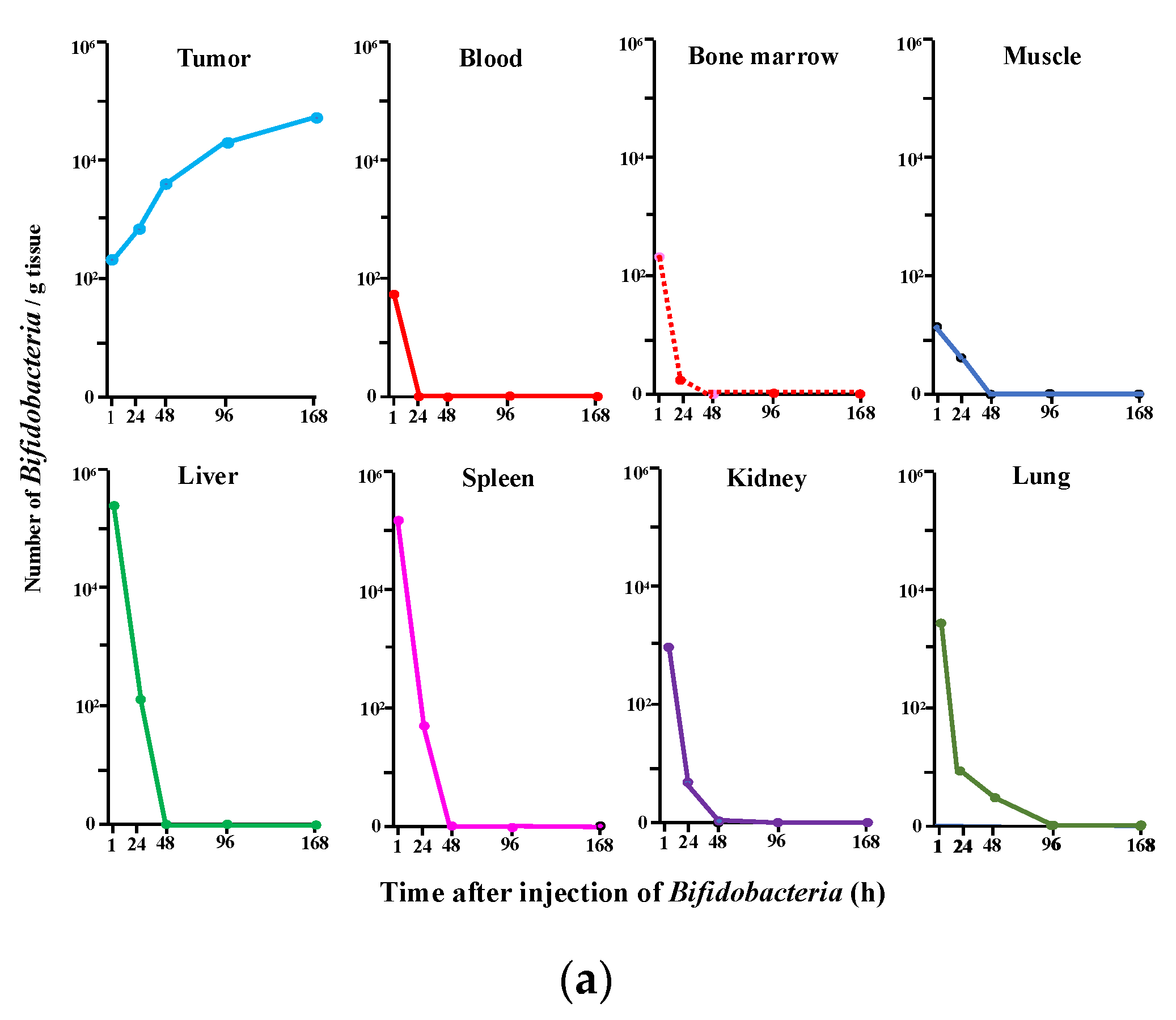
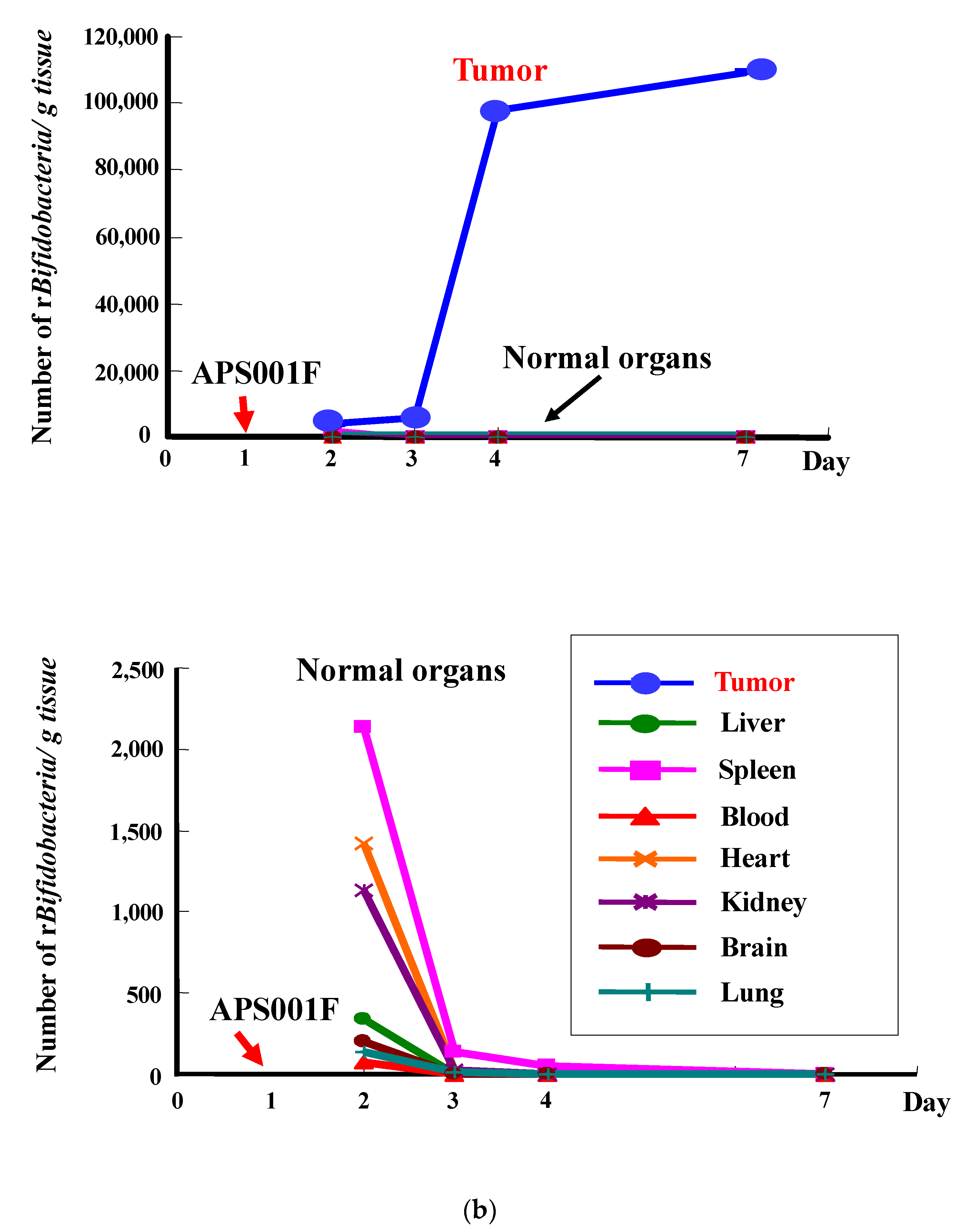

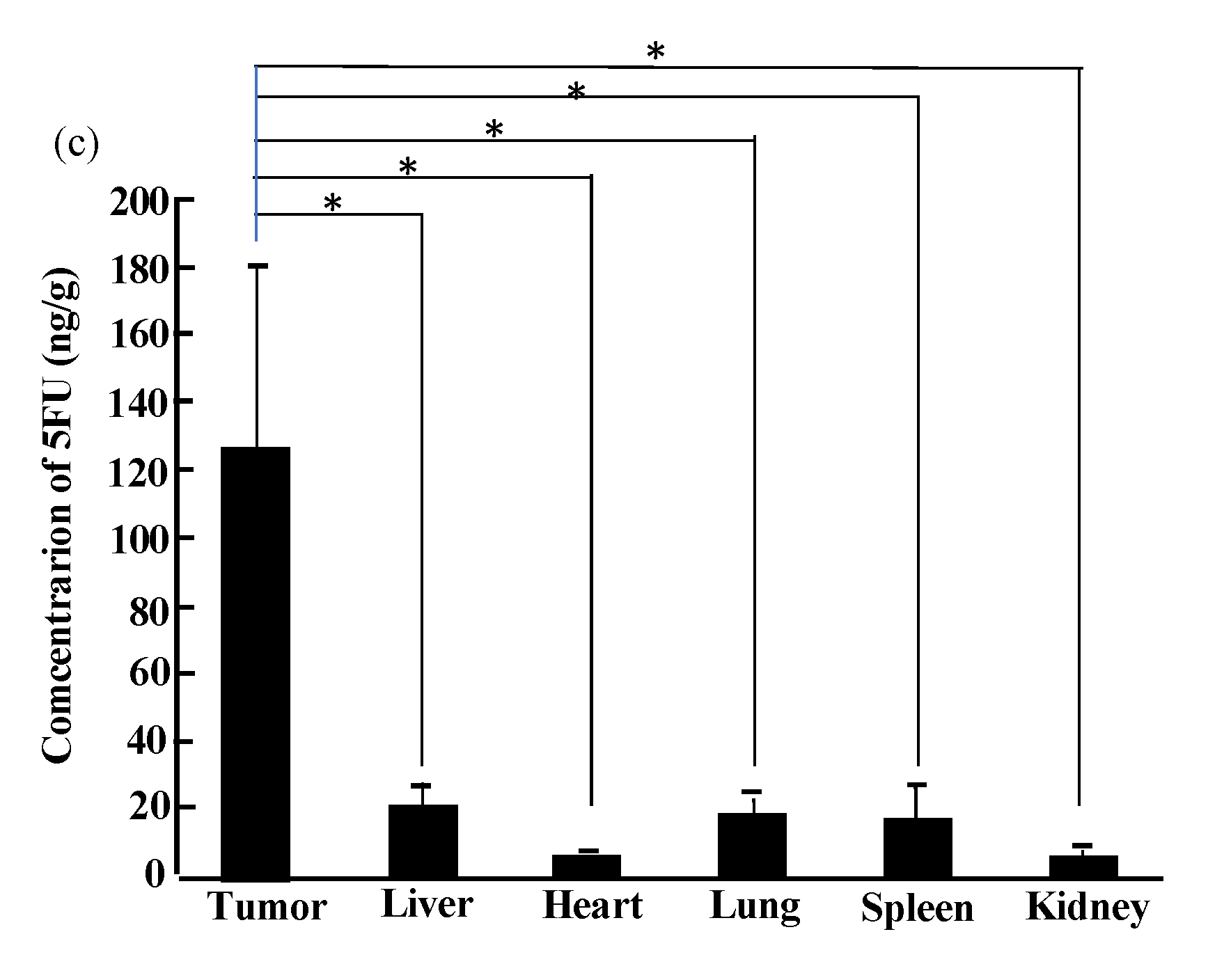
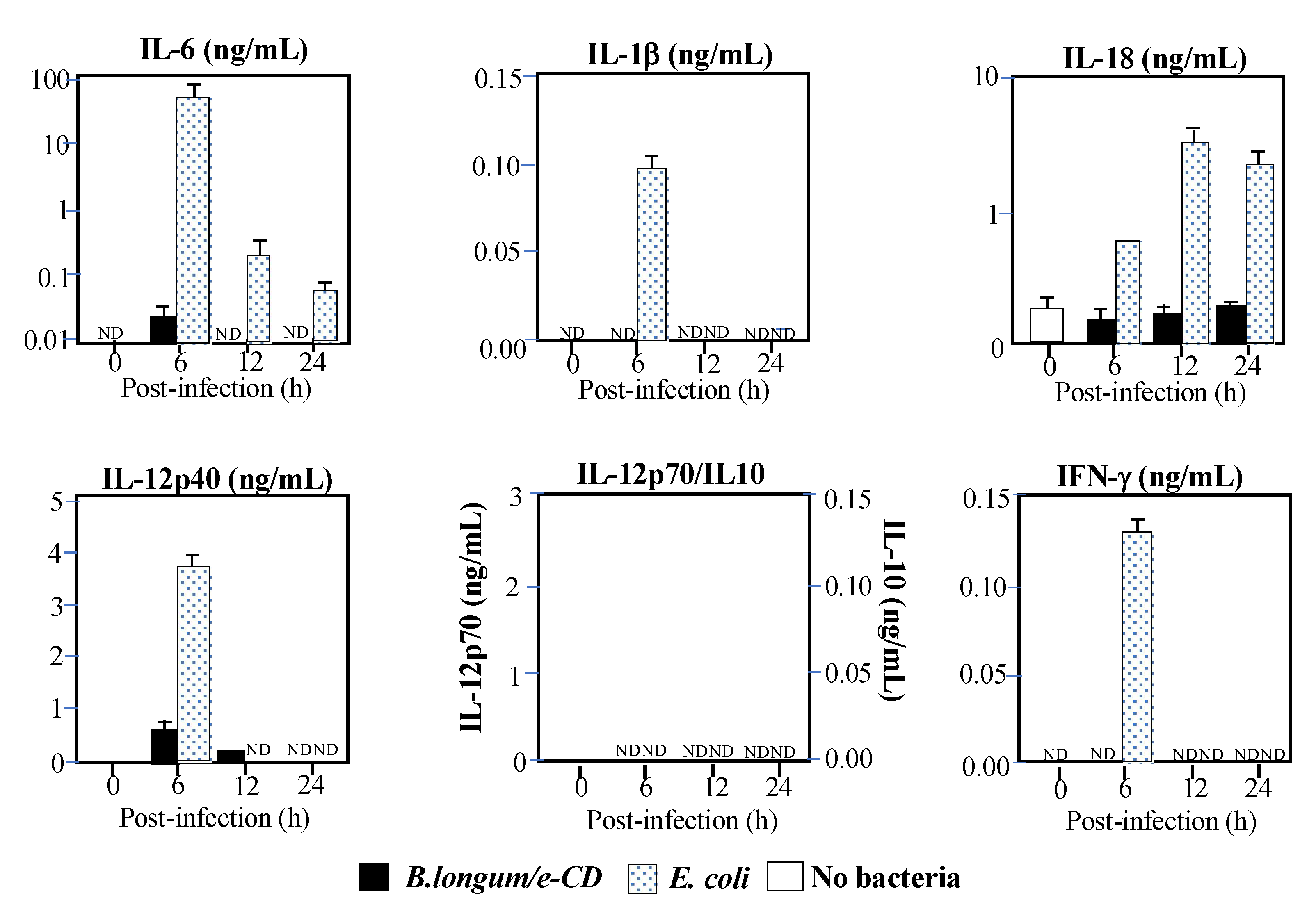

| (a) ASA Reaction | ||||||||
| Group | Sensitized Antigen | Cause Antigen | No.of Animals | Antigen Challenge Outcome | ||||
| (-) | (+/-) | (+) | (++) | (+++) | ||||
| A | B.longum/S-eCD | B.longum/S-eCD | 5 | 4 | 1 | 0 | 0 | 0 |
| B | B.longum/S-eCD + FCA | B.longum/S-eCD | 5 | 5 | 0 | 0 | 0 | 0 |
| C | OVA + FCA | OVA | 5 | 0 | 0 | 5 | 5 | 4 |
| D | Saline + FCA | B.longum/S-eCD | 5 | 5 | 0 | 0 | 0 | 0 |
| (b) PCA Reaction | ||||||||
| Group | Sensitized Antigen | Cause Antigen | No.of Animals | PCA Titer | ||||
| (×1) | (×4) | (×16) | (×64) | (×256) | ||||
| A | B.longum/S-eCD | B.longum/S-eCD | 5 | 0 | 0 | 0 | 0 | 0 |
| B | B.longum/S-eCD + FCA | B.longum/S-eCD | 5 | 3 | 2 | 2 | 2 | 2 |
| C | OVA + FCA | OVA | 5 | 5 | 5 | 5 | 5 | 4 |
| D | Saline + FCA | B.longum/S-eCD | 5 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taniguchi, S. In Situ Delivery and Production System (iDPS) of Anti-Cancer Molecules with Gene-Engineered Bifidobacterium. J. Pers. Med. 2021, 11, 566. https://doi.org/10.3390/jpm11060566
Taniguchi S. In Situ Delivery and Production System (iDPS) of Anti-Cancer Molecules with Gene-Engineered Bifidobacterium. Journal of Personalized Medicine. 2021; 11(6):566. https://doi.org/10.3390/jpm11060566
Chicago/Turabian StyleTaniguchi, Shun’ichiro. 2021. "In Situ Delivery and Production System (iDPS) of Anti-Cancer Molecules with Gene-Engineered Bifidobacterium" Journal of Personalized Medicine 11, no. 6: 566. https://doi.org/10.3390/jpm11060566
APA StyleTaniguchi, S. (2021). In Situ Delivery and Production System (iDPS) of Anti-Cancer Molecules with Gene-Engineered Bifidobacterium. Journal of Personalized Medicine, 11(6), 566. https://doi.org/10.3390/jpm11060566





