Investigational Drug Treatments for Triple-Negative Breast Cancer
Abstract
:1. Introduction
2. Material and Methods
3. Results
3.1. PI3K/AKT/mTOR Pathway
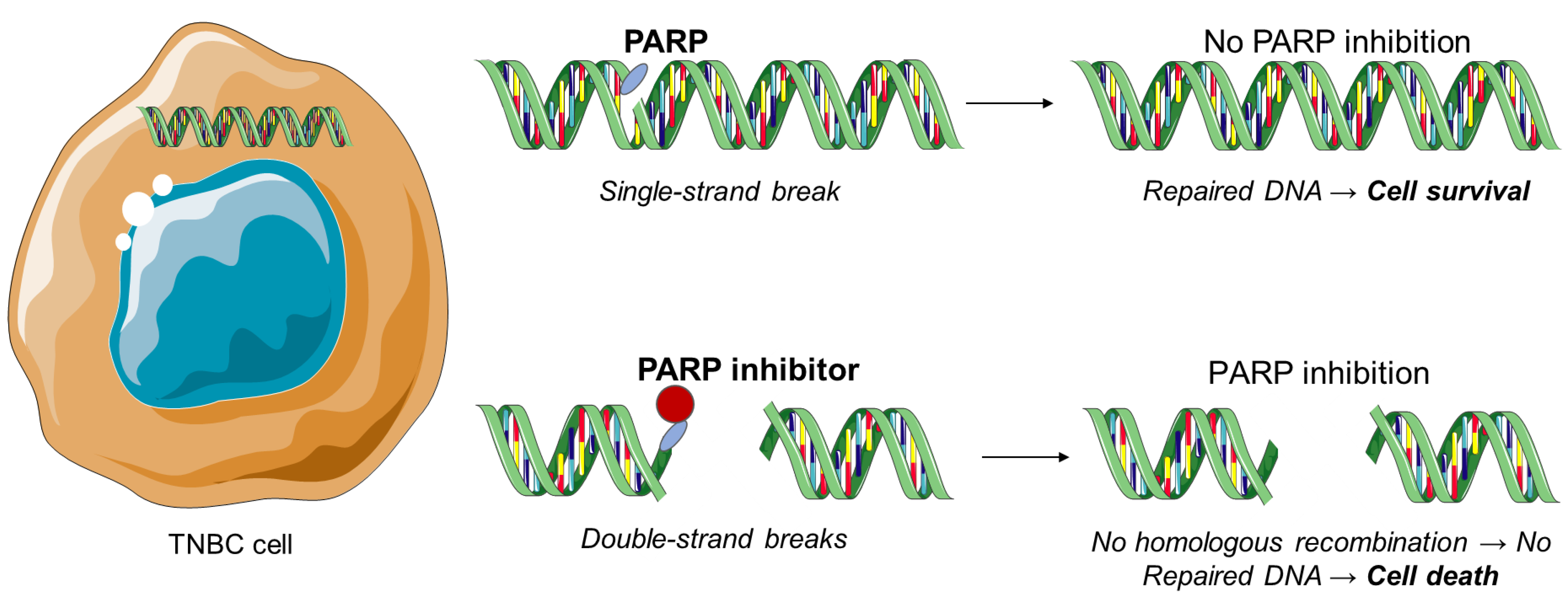
3.2. PARP Inhibitors
3.3. Aurora Kinase Inhibitors
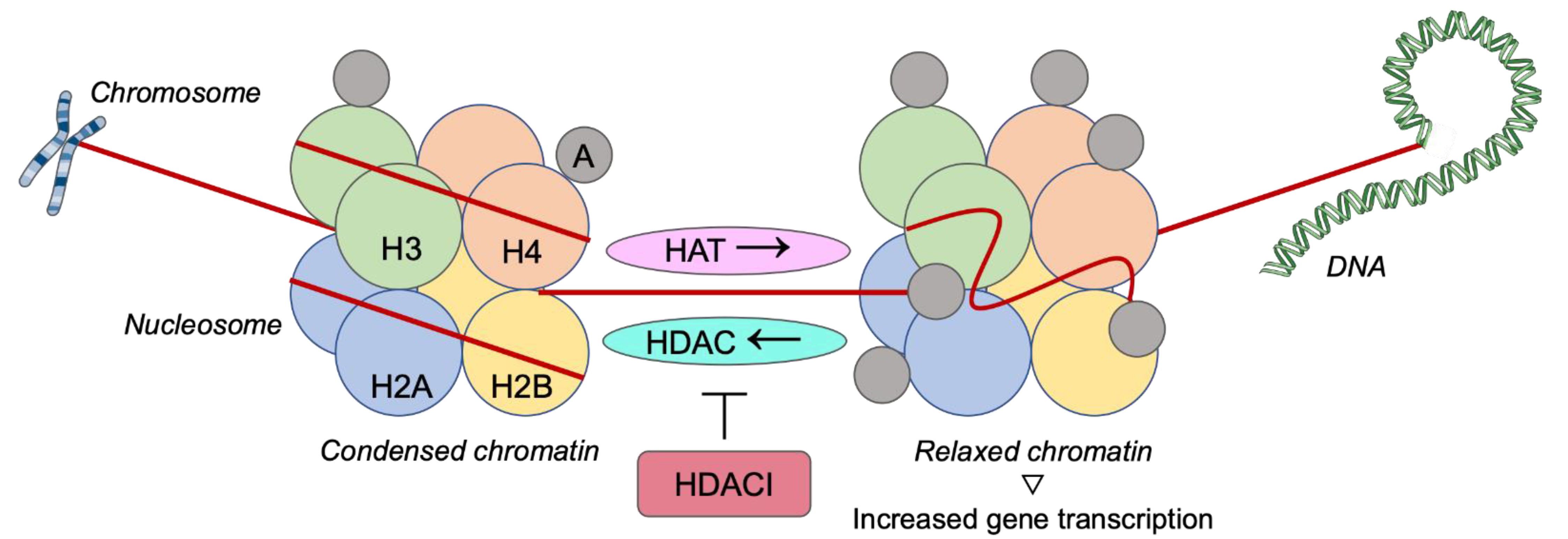
3.4. Histone Deacetylase Inhibitors
3.5. Other Inhibitors
3.6. Immunotherapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 43–46. [Google Scholar] [CrossRef] [Green Version]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniou, A.C.; Easton, D.F. Models of genetic susceptibility to breast cancer. Oncogene 2006, 25, 5898–5905. [Google Scholar] [CrossRef] [Green Version]
- Rivenbark, A.G.; O’Connor, S.M.; Coleman, W.B. Molecular and cellular heterogeneity in breast cancer: Challenges for personalized medicine. Am. J. Pathol. 2013, 183, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyante, S.J.; Lee, S.S.; Benefield, T.S.; Hoots, T.N.; Henderson, L.M. The association between mammographic calcifications and breast cancer prognostic factors in a population-based registry cohort. Cancer 2017, 123, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSantis, C.E.; Bray, F.; Ferlay, J.; Lortet-Tieulent, J.; Anderson, B.O.; Jemal, A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1495–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damaskos, C.; Garmpi, A.; Nikolettos, K.; Vavourakis, M.; Diamantis, E.; Patsouras, A.; Farmaki, P.; Nonni, A.; Dimitroulis, D.; Mantas, D.; et al. Triple-negative breast cancer: The progress of targeted therapies and future tendencies. Anticancer Res. 2019, 39, 5285–5296. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [Green Version]
- Tai, Y.C.; Domchek, S.; Parmigiani, G.; Chen, S. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J. Natl. Cancer Inst. 2007, 99, 1811–1814. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Fedele, P.; Orlando, L.; Cinieri, S. Targeting triple-negative breast cancer with histone deacetylase inhibitors. Expert Opin. Investig. Drugs 2017, 26, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Saloustros, E.; Nikolaou, M.; Kalbakis, K.; Polyzos, A.; Christofillakis, C.; Kentepozidis, N.; Pistamaltzian, N.; Kourousis, C.; Vamvakas, L.; Georgoulias, V.; et al. Weekly paclitaxel and carboplatin plus bevacizumab as first-line treatment of metastatic triple-negative breast cancer. A multicenter phase II trial by the Hellenic oncology research group. Clin. Breast Cancer 2018, 18, 88–94. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.E.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, A.; Karamouzis, M.V.; Stavrinides, H.; Ardavanis, A.; Kandilis, K.; Stavrakakis, J.; Georganta, C.; Rigatos, G. Phase II study of pegylated liposomal doxorubicin (Caelyx) and docetaxel as first-line treatment in metastatic breast cancer. Ann. Oncol. 2004, 15, 891–895. [Google Scholar] [CrossRef]
- Grimaldi, A.M.; Salvatore, M.; Incoronato, M. miRNA-based therapeutics in breast cancer: A systematic review. Front. Oncol. 2021, 11, 668464. [Google Scholar] [CrossRef]
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Nikolettos, K.; Dimitroulis, D.; Diamantis, E.; Farmaki, P.; Patsouras, A.; Voutyritsa, E.; Syllaios, A.; et al. Molecular classification and future therapeutic challenges of triple-negative breast cancer. In Vivo 2020, 34, 1715–1727. [Google Scholar] [CrossRef]
- Damaskos, C.; Garmpis, N.; Valsami, S.; Kontos, M.; Spartalis, E.; Kalampokas, T.; Kalampokas, E.; Athanasiou, A.; Moris, D.; Daskalopoulou, A.; et al. Histone deacetylase inhibitors: An attractive therapeutic strategy against breast cancer. Anticancer Res. 2017, 37, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Kalampokas, E.; Kalampokas, T.; Spartalis, E.; Daskalopoulou, A.; Valsami, S.; Kontos, M.; Nonni, A.; et al. Histone deacetylases as new therapeutic targets in triple-negative breast cancer: Progress and promises. Cancer Genom. Proteom. 2017, 14, 299–313. [Google Scholar]
- Fruman, D.A.; Romme, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Genome Atlas Network; Collaborators. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Zaytseva, Y.Y.; Valentino, J.D.; Gulhati, P.; Evers, B.M. mTOR inhibitors in cancer therapy. Cancer Lett. 2012, 319, 1–7. [Google Scholar] [CrossRef]
- Liu, T.; Yacoub, R.; Taliaferro-Smith, L.D.; Sun, S.Y.; Graham, T.R.; Dolan, R.; Lobo, C.; Tighiouart, M.; Yang, L.; Adams, A.; et al. Combinatorial effects of lapatinib and rapamycin in triple negative breast cancer cells. Mol. Cancer Ther. 2011, 10, 1460–1469. [Google Scholar] [CrossRef] [Green Version]
- Cossu-Rocca, P.; Orru, S.; Muroni, M.R.; Sanges, F.; Sotgiu, G.; Ena, S.; Pira, G.; Murgia, L.; Manca, A.; Uras, M.G.; et al. Analysis of PIK3CA mutations and activation pathways in triple negative breast cancer. PLoS ONE 2015, 10, e0141763. [Google Scholar] [CrossRef] [Green Version]
- Ooms, L.M.; Binge, L.C.; Davies, E.M.; Rahman, P.; Conway, J.R.; Gurung, R.; Ferguson, D.T.; Papa, A.; Fedele, C.G.; Vieusseux, J.L.; et al. The inositol polyphosphate 5-phosphatase PIPP regulates AKT1-dependent breast cancer growth and metastasis. Cancer Cell 2015, 28, 155–169. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, P.; Moulder, S.; Lee, J.J.; Janku, F.; Valero, V.; Zinner, R.G.; Naing, A.; Fu, S.; Tsimberidou, A.M.; Hong, D.; et al. Triple-negative breast cancer patients treated at MD Anderson cancer center in phase I trials: Improved outcomes with combination chemotherapy and targeted agents. Mol. Cancer Ther. 2014, 13, 3175–3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basho, R.K.; Gilcrease, M.; Murthy, R.K.; Helgason, T.; Karp, D.D.; Meric-Bernstam, F.; Hess, K.R.; Herbrich, S.M.; Valero, V.; Albarracin, C.; et al. Targeting the PI3K/AKT/mTOR pathway for the treatment of mesenchymal triple-negative breast cancer evidence from a phase 1 trial of mTOR inhibition in combination with liposomal doxorubicin and bevacizumab. JAMA Oncol. 2016, 3, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Basho, R.K.; Yam, C.; Gilcrease, M.; Murthy, R.K.; Helgason, T.; Karp, D.D.; Meric-Bernstam, F.; Hess, K.R.; Valero, V.; Albarracin, C.; et al. Comparative effectiveness of an mTOR-based systemic therapy regimen in advanced, metaplastic and nonmetaplastic triple-negative breast cancer. Oncologist 2018, 23, 1300–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Yost, S.E.; Blanchard, S.; Schmolze, D.; Yin, H.H.; Pillai, R.; Robinson, K.; Tang, A.; Martinez, N.; Portnow, J.; et al. Phase I clinical trial of the combination of eribulin and everolimus in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2019, 21, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owusu-Brackett, N.; Zhao, M.; Akcakanat, A.; Evans, K.W.; Yuca, E.; Dumbrava, E.I.; Janku, F.; Meric-Bernstam, F. Targeting PI3Kβ alone and in combination with chemotherapy or immunotherapy in tumors with PTEN loss. Oncotarget 2020, 11, 969–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Yang, X.; Han, H.; Wen, Z.; Yang, M.; Zhang, Y.; Fu, J.; Wang, X.; Yin, T.; Lu, G.; et al. Design, synthesis and biological evaluation of anilide (dicarboxylic acid) shikonin esters as antitumor agents through targeting PI3K/Akt/mTOR signaling pathway. Bioorg. Chem. 2021, 111, 104872. [Google Scholar] [CrossRef] [PubMed]
- Park, S.R.; Chen, A. Poly(Adenosine diphosphate-ribose) polymerase inhibitors in cancer treatment. Hematol. Oncol. Clin. N. Am. 2012, 26, 649–670. [Google Scholar] [CrossRef] [Green Version]
- De Vos, M.; Schreiber, V.; Dantzer, F. The diverse roles and clinical relevance of PARPs in DNA damage repair: Current state of the art. Biochem. Pharmacol. 2012, 84, 137–146. [Google Scholar] [CrossRef]
- Krishnakumar, R.; Kraus, W.L. The PARP side of the nucleus: Molecular actions, physiological outcomes, and clinical targets. Mol. Cell 2010, 39, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Eustermann, S.; Wu, W.F.; Langelier, M.F.; Yang, J.C.; Easton, L.E.; Riccio, A.A.; Pascal, J.M.; Neuhaus, D. Structural basis of detection and signaling of DNA single-strand breaks by human PARP-1. Mol. Cell 2015, 60, 742–754. [Google Scholar] [CrossRef] [Green Version]
- Dawicki-McKenna, J.M.; Langelier, M.F.; DeNizio, J.E.; Riccio, A.A.; Cao, C.D.; Karch, K.R.; McCauley, M.; Steffen, J.D.; Black, B.E.; Pascal, J.M. PARP-1 activation requires local unfolding of an autoinhibitory domain. Mol. Cell 2015, 60, 755–768. [Google Scholar] [CrossRef] [Green Version]
- Satoh, M.S.; Lindahl, T. Role of poly(ADP-ribose) formation in DNA repair. Nature 1992, 356, 356–358. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Jovanović, B.; Mayer, I.A.; Mayer, E.L.; Abramson, V.G.; Bardia, A.; Sanders, M.E.; Kuba, M.G.; Estrada, M.V.; Beeler, J.S.; Shaver, T.M.; et al. A randomized phase II neoadjuvant study of cisplatin, paclitaxel with or without everolimus in patients with stage II/III triple negative breast cancer (TNBC): Responses and long-term outcome correlated with increased frequency of DNA damage response gene mutations, TNBC subtype, AR status, and Ki67. Clin. Cancer Res. 2017, 23, 4035–4045. [Google Scholar]
- Llombart-Cussac, A.; Bermejo, B.; Villanueva, C.; Delaloge, S.; Morales, S.; Balmaña, J.; Amillano, K.; Bonnefoi, H.; Casas, A.; Manso, L.; et al. SOLTI NeoPARP: A phase II randomized study of two schedules of iniparib plus paclitaxel versus paclitaxel alone as neoadjuvant therapy in patients with triple-negative breast cancer. Breast Cancer Res. Treat. 2015, 154, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.G.; De Lorenzo, S.B.; Flatten, K.S.; Poirier, G.G.; Kaufmann, S.H. Failure of iniparib to inhibit poly(ADP-Ribose) polymerase in vitro. Clin. Cancer Res. 2012, 18, 1655–1662. [Google Scholar] [CrossRef] [Green Version]
- Kummar, S.; Wade, J.L.; Oza, A.M.; Sullivan, D.; Chen, A.P.; Gandara, D.R.; Ji, J.; Kinders, R.J.; Wang, L.; Allen, D.; et al. Randomized phase II trial of cyclophosphamide and the oral poly (ADP-ribose) polymerase inhibitor veliparib in patients with recurrent, advanced triple-negative breast cancer. Investig. New Drugs 2016, 34, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Evans, K.W.; Yuca, E.; Akcakanat, A.; Scott, S.M.; Arango, N.P.; Zheng, X.; Chen, K.; Tapia, C.; Tarco, E.; Eterovic, A.K.; et al. A population of heterogeneous breast cancer patient-derived xenografts demonstrate broad activity of PARP inhibitor in BRCA1/2 wild-type tumors. Clin. Cancer Res. 2017, 23, 6468–6477. [Google Scholar] [CrossRef] [Green Version]
- Pothuri, B.; Brodsky, A.L.; Sparano, J.A.; Blank, S.V.; Kim, M.; Hershman, D.L.; Tiersten, A.; Kiesel, B.F.; Beumer, J.H.; Liebes, L.; et al. Phase I and pharmacokinetic study of veliparib, a PARP inhibitor, and pegylated liposomal doxorubicin (PLD) in recurrent gynecologic cancer and triple negative breast cancer with long-term follow-up. Cancer Chemother. Pharmacol. 2020, 85, 741–751. [Google Scholar] [CrossRef]
- Eikesdal, H.P.; Yndestad, S.; Elzawahry, A.; Llop-Guevara, A.; Gilje, B.; Blix, E.S.; Espelid, H.; Lundgren, S.; Geisler, J.; Vagstad, G.; et al. Olaparib monotherapy as primary treatment in unselected triple negative breast cancer. Ann. Oncol. 2021, 32, 240–249. [Google Scholar] [CrossRef]
- Bischoff, J.R.; Anderson, L.; Zhu, Y.; Mossie, K.; Ng, L.; Souza, B.; Schryver, B.; Flanagan, P.; Clairvoyant, F.; Ginther, C.; et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998, 17, 3052–3065. [Google Scholar] [CrossRef]
- Carmena, M.; Earnshaw, W.C. The cellular geography of aurora kinases. Nat. Rev. Mol. Cell Biol. 2003, 4, 842–854. [Google Scholar] [CrossRef]
- Giet, R.; Prigent, C. Aurora/Ipl1p-related kinases, a new oncogenic family of mitotic serine-threonine kinases. J. Cell Sci. 1999, 112, 3591–3601. [Google Scholar] [CrossRef]
- Tayyar, Y.; Jubair, L.; Fallaha, S.; McMillan, N.A.J. Critical risk-benefit assessment of the novel anti-cancer aurora a kinase inhibitor alisertib (MLN8237): A comprehensive review of the clinical data. Crit. Rev. Oncol. Hematol. 2017, 119, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Girdler, F.; Gascoigne, K.E.; Eyers, P.A.; Hartmuth, S.; Crafter, C.; Foote, K.M.; Keen, N.J.; Taylor, S.S. Validating aurora B as an anti-cancer drug target. J. Cell Sci. 2006, 119, 3664–3675. [Google Scholar] [CrossRef] [Green Version]
- Carpinelli, P.; Moll, J. Aurora kinase inhibitors: Identification and preclinical validation of their biomarkers. Expert Opin. Ther. Targets 2008, 12, 69–80. [Google Scholar] [CrossRef]
- Gautschi, O.; Heighway, J.; Mack, P.C.; Purnell, P.R.; Lara, P.N., Jr.; Gandara, D.R. Aurora kinases as anticancer drug targets. Clin. Cancer Res. 2008, 14, 1639–1648. [Google Scholar] [CrossRef] [Green Version]
- Vader, G.; Medema, R.H.; Lens, S.M. The chromosomal passenger complex: Guiding aurora-B through mitosis. J. Cell Biol. 2006, 173, 833–837. [Google Scholar] [CrossRef] [Green Version]
- Ulisse, S.; Delcros, J.G.; Baldini, E.; Toller, M.; Curcio, F.; Giacomelli, L.; Prigent, C.; Ambesi-Impiombato, F.S.; D’Armiento, M.; Arlot-Bonnemains, Y. Expression of aurora kinases in human thyroid carcinoma cell lines and tissues. Int. J. Cancer 2006, 119, 275–282. [Google Scholar] [CrossRef]
- Bernard, M.; Sanseau, P.; Henry, C.; Couturier, A.; Prigent, C. Cloning of STK13, a third human protein kinase related to Drosophila aurora and budding yeast Ipl1 that maps on chromosome 19q13.3-ter. Genomics 1998, 53, 406–409. [Google Scholar] [CrossRef]
- Kimura, M.; Matsuda, Y.; Yoshioka, T.; Okano, Y. Cell cycle-dependent expression and centrosome localization of a third human aurora/Ipl1-related protein kinase, AIK3. J. Biol. Chem. 1999, 274, 7334–7340. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.T.; Li, S.K.; Chang, C.C.; Tang, C.J.; Lin, Y.N.; Lee, S.C.; Tang, T.K. Aurora-C kinase deficiency causes cytokinesis failure in meiosis-I and production of large polyploid oocytes in mouse. Mol. Biol. Cell 2010, 21, 2371–2383. [Google Scholar] [CrossRef] [Green Version]
- Huck, J.J.; Zhang, M.; Jerome Mettetal, J.; Chakravarty, A.; Venkatakrishnan, K.; Zhou, X.; Kleinfield, R.; Hyer, M.L.; Kannan, K.; Shinde, V.; et al. Translational exposure-efficacy modeling to optimize the dose and schedule of taxanes combined with the investigational aurora A kinase inhibitor MLN8237 (Alisertib). Mol. Cancer Ther. 2014, 139, 2170–2183. [Google Scholar] [CrossRef] [Green Version]
- Carducci, M.; Shaheen, M.; Markman, B.; Hurvitz, S.; Mahadevan, D.; Kotasek, D.; Goodman, O.B., Jr.; Rasmussen, E.; Chow, V.; Juan, G.; et al. A phase 1, first-in-human study of AMG 900, an orally administered pan-Aurora kinase inhibitor, in adult patients with advanced solid tumors. Investig. New Drugs 2018, 36, 1060–1071. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Kurzrock, R.; Valero, V.; Gonzalez, R.; Heist, R.S.; Tan, A.R.; Means-Powell, J.; Werner, T.L.; Becerra, C.; Wang, C.; et al. Phase I dose-escalation trial of the oral AKT inhibitor uprosertib in combination with the oral MEK1/MEK2 inhibitor trametinib in patients with solid tumors. Cancer Chemother. Pharmacol. 2020, 85, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Grant, S.; Dai, Y. Histone deacetylase inhibitors and rational combination therapies. Adv. Cancer Res. 2012, 116, 199–237. [Google Scholar]
- Deroanne, C.F.; Bonjean, K.; Servotte, S.; Devy, L.; Colige, A.; Clausse, N.; Blacher, S.; Verdin, E.; Foidart, J.M.; Nusgens, B.V.; et al. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene 2002, 21, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.A.; Bass, K.E.; Balasubramaniam, S.; Liu, L.; Schultz, B.; Verner, E.; Dai, Y.; Molina, R.A.; Davis, J.R.; Misialek, S.; et al. CRA-026440: A potent, broad spectrum, hydroxamic histone deacetylase inhibitor with antiproliferative and antiangiogenic activity in vitro and in vivo. Mol. Cancer Ther. 2006, 5, 1693–1701. [Google Scholar] [CrossRef] [Green Version]
- Garmpi, A.; Garmpis, N.; Damaskos, C.; Valsami, S.; Spartalis, E.; Lavaris, A.; Patelis, N.; Margonis, G.A.; Apostolou, K.G.; Spartalis, M.; et al. Histone deacetylase inhibitors as a new anticancer option: How far can we go with expectations? J. BUON 2018, 23, 846–861. [Google Scholar]
- Leoni, F.; Fossati, G.; Lewis, E.C.; Lee, J.K.; Porro, G.; Pagani, P.; Modena, D.; Moras, M.L.; Pozzi, P.; Reznikov, L.L.; et al. The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Mol. Med. 2005, 11, 1–15. [Google Scholar] [CrossRef]
- Min, A.; Im, S.A.; Kim, D.K.; Song, S.H.; Kim, H.J.; Lee, K.H.; Kim, T.Y.; Han, S.W.; Oh, D.Y.; Kim, T.Y.; et al. Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA), enhances anti-tumor effects of the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib in triple-negative breast cancer cells. Breast Cancer Res. 2015, 17, 33. [Google Scholar] [CrossRef] [Green Version]
- Ono, H.; Sowa, Y.; Horinaka, M.; Iizumi, Y.; Watanabe, M.; Morita, M.; Nishimoto, E.; Taguchi, T.; Sakai, T. The histone deacetylase inhibitor OBP-801 and eribulin synergistically inhibit the growth of triple-negative breast cancer cells with the suppression of survivin, Bcl-xL, and the MAPK pathway. Breast Cancer Res. Treat. 2018, 171, 43–52. [Google Scholar] [CrossRef]
- Song, X.; Wu, J.Q.; Yu, X.F.; Yang, X.S.; Yang, Y. Trichostatin A inhibits proliferation of triple negative breast cancer cells by inducing cell cycle arrest and apoptosis. Neoplasma 2018, 65, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Maiti, A.; Qi, Q.; Peng, X.; Yan, L.; Takabe, K.; Hait, N.C. Class I histone deacetylase inhibitor suppresses vasculogenic mimicry by enhancing the expression of tumor suppressor and anti-angiogenesis genes in aggressive human TNBC cells. Int. J. Oncol. 2019, 55, 116–130. [Google Scholar] [CrossRef]
- Milazzo, F.M.; Vesci, L.; Anastasi, A.M.; Chiapparino, C.; Rosi, A.; Giannini, G.; Taddei, M.; Cini, E.; Faltoni, V.; Petricci, E.; et al. ErbB2 targeted epigenetic modulation: Anti-tumor efficacy of the ADC trastuzumab-HDACi ST8176AA1. Front. Oncol. 2020, 9, 1534. [Google Scholar] [CrossRef]
- Schmidt, H.B.; Görlich, D. Transport selectivity of nuclear pores, phase separation, and membraneless organelles. Trends Biochem. Sci. 2016, 41, 46–61. [Google Scholar] [CrossRef]
- Görlich, D.; Mattaj, I.W. Nucleocytoplasmic transport. Science 1996, 271, 1513–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamali, T.; Jamali, Y.; Mehrbod, M.; Mofrad, M.R.K. Nuclear pore complex: Biochemistry and biophysics of nucleocytoplasmic transport in health and disease. Int. Rev. Cell Mol. Biol. 2011, 287, 233–286. [Google Scholar] [PubMed]
- Sun, Q.; Chen, X.; Zhou, Q.; Burstein, E.; Yang, S.; Jia, D. Inhibiting cancer cell hallmark features through nuclear export inhibition. Signal Transduct. Target.Ther. 2016, 1, 16010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Wei, G.; Parikh, K.; Liu, D. Selective inhibitors of nuclear export (SINE) in hematological malignancies. Exp. Hematol. Oncol. 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arango, N.P.; Yuca, E.; Zhao, M.; Evans, K.W.; Scott, S.; Kim, C.; Gonzalez-Angulo, A.M.; Janku, F.; Ueno, N.T.; Tripathy, D.; et al. Selinexor (KPT-330) demonstrates antitumor efficacy in preclinical models of triple-negative breast cancer. Breast Cancer Res. 2017, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Porter, P.L.; Malone, K.E.; Heagerty, P.J.; Alexander, G.M.; Firpo, E.J.; Daling, J.R.; Roberts, J.M. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat. Med. 1997, 3, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Keyomarsi, K.; Tucker, S.L.; Buchholz, T.A.; Callister, M.; Ding, Y.; Hortobagyi, G.N.; Bedrosian, I.; Knickerbocker, C.; Toyofuku, W.; Lowe, M.; et al. Cyclin E and survival in patients with breast cancer. N. Engl. J. Med. 2002, 347, 1566–1575. [Google Scholar] [CrossRef]
- Kallakury, B.V.; Sheehan, C.E.; Ambros, R.A.; Fisher, H.A.; Kaufman, R.P.; Ross, J.S. The prognostic significance of p34cdc2 and cyclin D1 protein expression in prostate adenocarcinoma. Cancer 1997, 80, 753–763. [Google Scholar] [CrossRef]
- Soria, J.C.; Jang, S.J.; Khuri, F.R.; Hassan, K.; Liu, D.; Hong, W.K.; Mao, L. Overexpression of cyclin B1 in early-stage non-small lung cancer and its clinical implications. Cancer Res. 2000, 60, 4000–4004. [Google Scholar]
- Takerno, S.; Noguchi, T.; Kikuchi, R.; Uchida, Y.; Yokoyama, S.; Muller, W. Prognostic value of cyclin B1 in patients with esophageal squamous cell carcinoma. Cancer 2002, 94, 2874–2881. [Google Scholar] [CrossRef]
- Mitri, Z.; Karakas, C.; Wei, C.; Briones, B.; Simmons, H.; Ibrahim, N.; Alvarez, R.; Murray, J.L.; Keyomarsi, K.; Moulder, S. A phase 1 study with dose expansion of the CDK inhibitor dinaciclib (SCH 727965) in combination with epirubicin in patients with metastatic triple negative breast cancer. Investig. New Drugs 2015, 33, 890–894. [Google Scholar] [CrossRef]
- Krause, D.S.; Van Etten, R.A. Tyrosine kinases as targets for cancer therapy. N. Engl. J. Med. 2005, 353, 172–187. [Google Scholar] [CrossRef] [Green Version]
- Tolaney, S.M.; Tan, S.; Guo, H.; Barry, W.; Van Allen, E.; Wagle, N.; Brock, J.; Larrabee, K.; Paweletz, C.; Ivanova, E.; et al. Phase II study of tivantinib (ARQ 197) in patients with metastatic triple-negative breast cancer. Investig. New Drugs 2015, 33, 1108–1114. [Google Scholar] [CrossRef]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2006, 23, 1011–1027. [Google Scholar] [CrossRef]
- Pham, E.; Yin, M.; Peters, C.G.; Lee, C.R.; Brown, D.; Xu, P.; Man, S.; Jayaraman, L.; Rohde, E.; Chow, A.; et al. Preclinical efficacy of bevacizumab with CRLX101, an investigational nanoparticle-drug conjugate, in treatment of metastatic triple-negative breast cancer. Cancer Res. 2016, 76, 4493–4503. [Google Scholar] [CrossRef] [Green Version]
- Herbst, R.S.; Shin, D.M. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: A new paradigm for cancer therapy. Cancer 2002, 94, 1593–1611. [Google Scholar] [CrossRef]
- Brabender, J.; Danenberg, K.D.; Metzger, R.; Schneider, P.M.; Park, J.; Salonga, D.; Hölscher, A.H.; Danenberg, P.V. Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer is correlated with survival. Clin. Cancer Res. 2001, 7, 1850–1855. [Google Scholar]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signaling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Mendelsohn, J. The epidermal growth factor as a target for cancer therapy. Endocr. Relat. Cancer 2001, 8, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Brinkman, A.M.; Chen, G.; Wang, Y.; Hedman, C.J.; Sherer, N.M.; Havighurst, T.C.; Gong, S.; Xu, W. Aminoflavone-loaded EGFR-targeted unimolecular micelle nanoparticles exhibit anti-cancer effects in triple negative breast cancer. Biomaterials 2016, 101, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Wali, V.B.; Langdon, C.G.; Held, M.A.; Platt, J.T.; Patwardhan, G.A.; Safonov, A.; Aktas, B.; Pusztai, L.; Stern, D.F.; Hatzis, C. Systematic drug screening identifies tractable targeted combination therapies in triple-negative breast cancer. Cancer Res. 2017, 77, 566–578. [Google Scholar] [CrossRef] [Green Version]
- Kubiczkova, L.; Pour, L.; Sedlarikova, L.; Hajek, R.; Sevcikova, S. Proteasome inhibitors-molecular basis and current perspectives in multiple myeloma. J. Cell Mol. Med. 2014, 18, 947–961. [Google Scholar] [CrossRef]
- Rinnerthaler, G.; Gampenrieder, S.P.; Petzer, A.; Burgstaller, S.; Fuchs, D.; Rossmann, D.; Balic, M.; Egle, D.; Rumpold, H.; Singer, C.F.; et al. Ixazomib in combination with carboplatin in pretreated women with advanced triple-negative breast cancer, a phase I/II trial of the AGMT (AGMT MBC-10 Trial). BMC Cancer 2018, 18, 1074. [Google Scholar] [CrossRef]
- Belkina, A.C.; Denis, G.V. BET domain co-regulators in obesity, inflammation and cancer. Nat. Rev. Cancer 2012, 12, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Choi, G.; Lee, K. BET inhibitors as anticancer agents: A patent review. Recent Pat. Anticancer Drug Discov. 2017, 12, 340–364. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, P.; Chen, H.; World, E.A.; Tian, B.; Brasier, A.R.; Zhou, J. Drug discovery targeting bromodomain-containing protein 4. J. Med. Chem. 2017, 60, 4533–4558. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Yang, H.N.; Jeon, S.Y.; Hwang, J.A.; Kim, M.K.; Kong, S.Y.; Shim, S.H.; Lee, K.S. Anti-tumor activity of BET inhibitors in androgen-receptor-expressing triple-negative breast cancer. Sci. Rep. 2019, 9, 13305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Strooper, B.; Vassar, R.; Golde, T. The secretases: Enzymes with therapeutic potential in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetrivel, K.S.; Cheng, H.; Kim, S.H.; Chen, Y.; Barnes, N.Y.; Parent, A.T.; Sisodia, S.S.; Thinakaran, G. Spatial segregation of gamma-secretase and substrates in distinct membrane domains. J. Biol. Chem. 2005, 280, 25892–25900. [Google Scholar] [CrossRef] [Green Version]
- Golde, T.E.; Petrucelli, L.; Lewis, J. Targeting Abeta and tau in Alzheimer’s disease, an early interim report. Exp. Neurol. 2010, 223, 252–266. [Google Scholar] [CrossRef] [Green Version]
- Sardesai, S.; Badawi, M.; Mrozek, E.; Morgan, E.; Phelps, M.; Stephens, J.; Wei, L.; Kassem, M.; Ling, Y.; Lustberg, M.; et al. A phase I study of an oral selective gamma secretase (GS) inhibitor RO4929097 in combination with neoadjuvant paclitaxel and carboplatin in triple negative breast cancer. Investig. New Drugs 2020, 38, 1400–1410. [Google Scholar] [CrossRef]
- Brufsky, A.; Kim, S.B.; Zvirbule, Ž.; Eniu, A.; Mebis, J.; Sohn, J.H.; Wongchenko, M.; Chohan, S.; Amin, R.; Yan, Y.; et al. A phase II randomized trial of cobimetinib plus chemotherapy, with or without atezolizumab, as first-line treatment for patients with locally advanced or metastatic triple-negative breast cancer (COLET): Primary analysis. Ann. Oncol. 2021, 32, 652–660. [Google Scholar] [CrossRef]
- Salimi, M. Future of triple negative breast cancer: Can immunotherapy treat this deadly subtype of breast cancer? Iran. Biomed. J. 2018, 22, 76–77. [Google Scholar]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [Green Version]
- Nanda, R.; Chow, L.Q.M.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Ziehr, D.R.; Guo, H.; Ng, M.R.; Barry, W.T.; Higgins, M.J.; Isakoff, S.J.; Brock, J.E.; Ivanova, E.V.; Paweletz, C.P.; et al. Phase II and biomarker study of cabozantinib in metastatic triple-negative breast cancer patients. Oncologist 2017, 22, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Bernier, C.; Soliman, A.; Gravel, M.; Dankner, M.; Savage, P.; Petrecca, K.; Park, M.; Siegel, P.M.; Shore, G.C.; Roulston, A. DZ-2384 has a superior preclinical profile to taxanes for the treatment of triple-negative breast cancer and is synergistic with anti-CTLA-4 immunotherapy. Anticancer Drugs 2018, 29, 774–785. [Google Scholar] [CrossRef]
- Santa-Maria, C.A.; Kato, T.; Park, J.H.; Kiyotani, K.; Rademaker, A.; Shah, A.N.; Gross, L.; Blanco, L.Z.; Jain, S.; Flaum, L.; et al. A pilot study of durvalumab and tremelimumab and immunogenomic dynamics in metastatic breast cancer. Oncotarget 2018, 9, 18985–18996. [Google Scholar] [CrossRef] [Green Version]
- Cortés, J.; André, F.; Gonçalves, A.; Kümmel, S.; Martín, M.; Schmid, P.; Schuetz, F.; Swain, S.M.; Easton, V.; Pollex, E.; et al. IMpassion132 phase III trial: Atezolizumab and chemotherapy in early relapsing metastatic triple-negative breast cancer. Future Oncol. 2019, 15, 1951–1961. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef]
- Winer, E.P.; Lipatov, O.; Im, S.A.; Goncalves, A.; Muñoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 499–511. [Google Scholar] [CrossRef]
- Simone, B.A.; Dan, T.; Palagani, A.; Jin, L.; Han, S.Y.; Wright, C.; Savage, J.E.; Gitman, R.; Lim, M.K.; Palazzo, J.; et al. Caloric restriction coupled with radiation decreases metastatic burden in triple negative breast cancer. Cell Cycle 2016, 15, 2265–2274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: Clinicaltrials.gov (accessed on 18 January 2016).




| Study | Sample Source | Therapy | Pathway/Mechanism of Action | Results | ||
|---|---|---|---|---|---|---|
| 1 | Ganesan et al., 2014 [25] | Phase I trial. | 106 (98 evaluated) consecutive patients with advanced or metastatic TNBC. | Chemotherapy only (n = 8), combination chemotherapy and targeted therapy (n = 62), single-agent targeted therapy (n = 16), and targeted therapy with 2 or more agents (n = 20). | PI3K/AKT/mTOR. | Treatment with anti-angiogenic factors and/or PI3K/AKT/mTOR inhibitors demonstrated prolonged free survival in patients with metastatic TNBC respectively (p = 0.023 and p = 0.018). |
| 2 | Huck et al., 2014 [57] | In vivo study in immunocompromised mice-followed by clinical study. | In vivo models of TNBC grown in immune compromised mice. | 60 and 80 mg/m2 of paclitaxel (every week), MLN8237 twice a day. | Aurora kinase inhibitor. | The highest dose of MLN8237 and paclitaxel offer the best efficacy. |
| 3 | Llombart-Cussacet al., 2015 [39] | Phase II trial. | 141 patients with TNBC Stage II-IIIa. | Praclitaxel (80 mg/m2, n = 47) alone or in combination with iniparib, either once weekly (11.2 mg/kg, n = 46) or twice weekly (5.6 mg/kg, n = 48) for 12 weeks. | PARP inhibitor. | Best overall response in the breast (60, 61 and 63%) and breast conservation rate (53, 54 and 50%). Addition of iniparib to weekly praclitaxel did not add relevant antitumor activity or toxicity. |
| 4 | Min et al., 2015 [65] | In vitro and in vivo studies. | TNBC cell lines, xenografts models. | SAHA in combination with olaparib. | HDACIs and PARP inhibitors. | Down-regulation of the proliferative signaling pathway, increased apoptotic and autophagic cell death, and accumulation of DNA damage. |
| 5 | Arango et al., 2015 [75] | In vitro and in vivo cell lines. | 26 TNBC patient-derived xenografts (PDXs). | Selinexor was combined with paclitaxel, carboplatin, eribulin, gemcitabine and doxorubicin. | Nucleo-cytoplasmatic transport inhibitor. | Selinexor as a single agent reduced tumor growth in vivo in 4 of 5 different TNBCPDX models, with a median tumor growth inhibition ratio of 42% and demonstrated greater antitumor efficacy in combination with paclitaxel or eribulin. |
| 6 | Mitri et al., 2015 [81] | Phase I study. | 9 patients with TNBC. | Escalating doses of dinaciclib given on day 1 followed by standard dose of epirubicin given on day 2 of a 21-day cycle. | Cyclin dependent kinase inhibitor. | Dose escalation did not proceed past the second cohort due to toxicity. The first dose level was also found to be too toxic. No treatment responses were noted, median time to progression was 5.5 weeks. |
| 7 | Tolaney et al., 2015 [83] | Phase II study. | 22 patients with TNBC. | Twice daily oral dosing of tivantinib (360 mg po bid) during a 21-day cycle. | Tyrosine kinase inhibitor. | The overall response rate was 5% (95% CI 0–25%) and the 6-month PFS was 5% (95% CI 0–25%), with 1 patient achieving a partial response. |
| 8 | Basho et al., 2016 [26] | Phase I trial. | 52 women with metaplastic TNBC. | Liposomal doxorubicin, bevacizumab and temsirolimus (DAT) (n = 39) or liposomal doxorubicin, bevacizumab, and everolimus (DAE) (n = 13). | PI3K/AKT/mTOR. | The response rate was 21% (complete response = 4, 8%, partial response = 7, 13%) and 19% of patients had stable disease for at least 6 months, for a clinical benefit rate of 40%. |
| 9 | Kummar et al., 2016 [41] | Phase II study. | 45 adult patients with TNBC. | Oral cyclophosphamide 50 mg once daily with or without oral veliparib at 60 mg daily in 21-day cycles. | PARP inhibitors. | Response rates and median PFS did not significantly differ between the 2 groups. The addition of veliparib to cyclophosphamide, did not improve the response rate. |
| 10 | Pham et al., 2016 [86] | In vivo preclinical study with xenografts. | Preclinical mouse models of orthotopic primary TNBC xenografts. | Bevacizumab and CRLX101. | Anti-VEGF. | CRLX101 showed antitumor efficacy, reduced metastasis, and prolonged survival. |
| 11 | Brinkman et al., 2016 [91] | In vivo study in mice. | Human TNBC cell lines. | Aminoflavone 7 mg/kg intravenously every 4 days. | Anti-EGFR. | Aminoflavone demonstrated antitumor efficacy against EGFR- over-expressing TNBC. |
| 12 | Nanda et al., 2016 [108] | Phase I clinical trial. | 111 patients with TNBC. | Pembrolizumab given intravenously at 10 mg/kg every 2 weeks. | Anti-PD-1. | The overall response rate was 18.5%, the median time to response was 17.9 weeks and the median duration of response was not reached. |
| 13 | Evans et al., 2017 [42] | Normal and tumor DNA sequencing, RNASeq, and reverse phase protein arrays (RPPA), immunohistochemistry and in vivo treatment in BC patient derived xenografts. | 26 patient-derived xenografts, obtained from surgical samples of recurrent tumors from 25 patients. | Use of chemotherapy with trametinib, buparlisib and/or talazoparib. | PARP inhibitor. | Talazoparib caused dramatic regression in 5 of 12 PDXs. 4 of 5 talazoparib-sensitive models did not harbor germline BRCA1/mutations, but several had somatic alterations in homologous repair pathways, including ATM deletion and BRCA2 alterations. |
| 14 | Wali et al., 2017 [92] | Clinical study. | TNBC cell lines. | 128 investigational drugs as either single agents or in 768 pairwise drug combinations. | ROS1 inhibitor. | The ABT-263/crizotinib combination offers a rapid path to clinic demonstrated RTK blockade, inhibition of mitogenic signaling and pro-apoptotic signal induction in basal and mesenchymal stem-like TNBC. |
| 15 | Tolaney et al., 2017 [109] | Phase II study. | 35 patients with TNBC. | Cabozantinib (60 mg daily) on a 3-week cycle and were restaged after 6 weeks and then every 9 weeks. | Tyrosine kinase inhibitor. | 3 patients achieved a partial response, 9 patients achieved stable disease for at least 15 weeks, and thus the clinical benefit rate was 34%/Median PFS was 2 months. 2 patients had TNBC with MET amplification. |
| 16 | Basho et al., 2018 [27] | Phase I trial. | 43 patients with non-metaplastic TNBC and 59 patients with advanced metaplastic BC. | mTOR inhibition weekly (temsirolimus or everolimus) with liposomal doxorubicin and bevacizumab every 3 weeks (DAT/DAE). | PI3K/AKT/mTOR inhibition and anti-VEGF. | Median PFS for the non-metaplastic TNBC and MpBC patients was 2.5 months and 4.8 months, respectively. Median OS for the non-metaplastic TNBC and MpBC patients was 3.7 months and 10 months, respectively. DAT/DAE appeared to be more effective in MpBC compared with non-metaplastic TNBC. |
| 17 | Carducci et al., 2018 [58] | In-human trial included dose-escalation and dose-expansion phases. | Patients with 3 tumor types: taxane- and platinum-resistant ovarian cancer, taxane-resistant TNBC, and castration-resistant and taxane- or cisplatin/etoposide resistant prostate cancer. | AMG 900 for 4 days on/10 days off at 1–50 mg/day. | Aurora kinase inhibitors. | 3 of 29 (10.3%, 95% CI:2.0–28.0%) patients with ovarian cancer showed partial response. median duration of response was 24.1 weeks (95% CI: 16.1–34.1). 7 patients (24.1%, 95% CI:10.3–43.5%) experienced partial response. 5/9 patients positive for p53 expression responded to treatment. No objective responses were observed in patients with TNBC or CRPC. |
| 18 | Ono et al., 2018 [66] | Flow cytometry analysis. | TNBC cell lines. | OBP-801 or OBP-801 in combination with eribulin. | HDACIs. | Suppression of Bcl-xL and the MAPK pathway. |
| 19 | Song et al., 2018 [67] | MTT dye reduction method. | TNBC cell lines HCC1806 and HCC38. | Trichostatin A (TSA) or TSA in combination with doxorubicin. | HDACIs. | Decreased expression of CYCLIN D1, CDK4, CDK6 and BCL-XL, but increased P21 expression and inhibition of the proliferation of HCC1806 and HCC38 cells. |
| 20 | Rinnerthaler et al., 2018 [94] | Phase I and II clinical trials. | Patients with metastatic TNBC, already treated with at least 1 prior line of chemotherapy. | Ixazomib in combination with carboplatin on days 1, 8, and 15 in a 28-day cycle. The phase I part of this study utilizes an alternate dose escalation accelerated titration design. After establishing the maximum tolerated dose, the combination will be further evaluated (phase II, including 41 evaluable patients). | Proteasome inhibitor. | The results will be recorded in the future. |
| 21 | Schmid et al., 2018 [110] | Phase III trial. | 451 patients with untreated metastatic TNBC. | Atezolizumab plus nab-paclitaxel or placebo plus nab-paclitaxel. | Anti-PD-L1. | The median overall survival was 21.3 months with atezolizumab plus nab-paclitaxel and 17.6 months with placebo plus nab-paclitaxel. Among patients with PD-L1-positive tumors, the median overall survival was 25 months and 15.5 months, respectively. |
| 22 | Bernier et al., 2018 [111] | In vivo study. | Mice with TNBC | CTLA-4 inhibitor and DZ- 2384 co-administration. | CTLA-4 inhibition. | CTLA-4 immunotherapy exerted synergistic action with DZ- 2384. In preclinical models, this combination was superior and with less side-effects, comparing to CTLA-4 immunotherapy and taxanes. |
| 23 | Santa-maria et al., 2018 [112] | Pilot study | 18 patients with advanced estrogen receptor positive BC or TNBC | Durvalumab and tremelimumab. | PD-1/PD-L1/CTLA-4 inhibition. | This combination was more effective in patients with TNBC, as it increased cytotoxicity of T-cells and lead to clonal T-cell expression. Responses were made only in patients with TNBC (ORR = 43%), who had higher mutational gene expression and up-regulation of perforin 1 and CD8. |
| 24 | Lee et al., 2019 [28] | Phase I trial. | Patients with metastatic TNBC. | Everolimus and eribulin in different dosages combination in 25 patients. | PI3K/AKT/mTOR inhibition. | Among the 25 patients, 9 were stable, 9 reported partial response and 7 had progressive disease. Toxicity due to chemotherapy included hematological disorders, fatigue, stomatitis and hyperglycemia. |
| 25 | Maiti et al., 2019 [68] | Sphere formation assay. | TNBC cell lines. | Entinostat. | HDACIs. | Re-expression of the anti-angiogenic genes, serpin family F member 1 (SERPINF1) and thrombospondin 2 (THBS2), and to that of the tumor suppressor genes, phosphatase and tensin homolog (PTEN) and p21, and reduced VM structures. Down-regulation of the expression of vascular endothelial growth factor A (VEGF-A), and that of the epithelial-mesenchymal transition (EMT)-related genes, Vimentin and β-catenin. |
| 26 | Park et al., 2019 [98] | In vivo study. | A xenograft model of AR expressing TNBC in mouse models. | BET inhibitor JQ1. | BET inhibitor. | JQ1 showed significant anti-tumor activity in vivo in TNBC xenograft mouse models as a monotherapy and in combination with anti-AR therapy. |
| 27 | Cortés et al., 2019 [113] | Phase III trial. | Patients with PD-L1-positive tumors. | Atezolizumab 1200 mg or placebo every 3 weeks with the chosen chemotherapy. | Anti-PD-L1. | Unacceptable toxicity or withdrawal. |
| 28 | Voorwerk et al., 2019 [114] | Phase II trial. | 67 patients with TNBC. | Nivolumab only or radiation or cyclphosphamide or cisplatin or doxorubicin all followed by nivolumab. | Anti-PD-1. | The most effective responses were done in the doxorubicin and cisplatin groups with ORR 35% and 23% respectively. After the use of this chemotherapeutic regimens, up-regulation of PD-L1 pathway and increase in inflammation and T-cell cytotoxicity occurred. Thus, the administration of these drugs before immunotherapy might enhance its action. |
| 29 | Owusu-Brackett et al., 2020 [29] | In vitro cell viability assay. | TNBC cell lines. | AZD8186 in combination with paclitaxel, eribulin. | PI3K/AKT/mTOR inhibition. | AZD8186 had single agent efficacy in PTEN-deficient TNBC cell lines in vitro but had limited single agent efficacy in vivo. AZD8186 had enhanced efficacy when combined with paclitaxel and anti-PD1 in vivo. |
| 30 | Pothuri et al.,2020 [43] | Clinical trial. | 44 patients with ovarian or TNBC. | Veliparib and doxorubicin in various dosages. | PARP inhibitor. | Although complete clinical response was observed in two cases, and the anti-tumor efficacy was generally acceptable, complications such as oral squamous cell carcinomas appeared. |
| 31 | Tolcher et al.,2020 [59] | Clinical trial. | 126 patients with TNBC or melanoma. | Trametinib and uprosertib in various dosages. | Aurora kinase inhibitors. | The anti-tumor efficacy was minimal, whereas adverse effects such as severe diarrheas or rashes appeared. |
| 32 | Milazzo et al., 2020 [69] | In vitro and in vivo studies. | TNBC cell lines, xenografts models. | ST8176AA1 (ADC). | HDACIs. | Higher anti-tumor activity of ST8176AA1 compared to trastuzumab, increased expression of ErbB2 and estrogen receptor in TNBC cells, lower expression of the proliferation marker Ki67 and higher expression of cleaved caspase-3 in mice treated with the ADC compared to those treated with trastuzumab. |
| 33 | Sardesai et al., 2020 [102] | Phase I study. | Patients with TNBC. | Carboplatin on day 1, weekly paclitaxel at 80 mg and RO4929097 10 mg daily given orally on days 1–3, 8–10 and 15–17 for 6 21-day cycles. RO4929097 was escalated in 10 mg using the 3 + 3 dose escalation design. | γ-secretase inhibitor. | RO4929097 at 10 mg would have been the likely dose level for further development. |
| 34 | Ma et al., 2021 [30] | In vitro study. | MDA-MB-231, A549 and HeLa cell lines. | Anilide. | PI3K/AKT/mTOR inhibition. | Anilide enhance apoptosis and inhibit the migration and the proliferation of TNBC cells. |
| 35 | Eikesdal et al., 2021 [44] | Clinical trial. | 32 patients with TNBC, who have not received previously chemotherapy. | Olaparib. | PARP inhibitor. | Olaparib is effective against treatment-naïve TNBC cells with HR deficiency. |
| 36 | Brufsky et al.,2021 [103] | Phase II clinical trial. | Patients with locally advanced or metastatic TNBC. | Cobimetinib plus chemotherapy, with or without atezolizumab. | MAPK inhibition. | No increase in survival was noticed in any regimen. |
| 37 | Winer et al., 2021 [115] | Clinical trial. | 1098 patients with metastatic TNBC. | Pembrolizumab versus chemotherapy. | Anti-PD-1. | Pembrolizumab did not increase survival rates. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damaskos, C.; Garmpis, N.; Garmpi, A.; Nikolettos, K.; Sarantis, P.; Georgakopoulou, V.E.; Nonni, A.; Schizas, D.; Antoniou, E.A.; Karamouzis, M.V.; et al. Investigational Drug Treatments for Triple-Negative Breast Cancer. J. Pers. Med. 2021, 11, 652. https://doi.org/10.3390/jpm11070652
Damaskos C, Garmpis N, Garmpi A, Nikolettos K, Sarantis P, Georgakopoulou VE, Nonni A, Schizas D, Antoniou EA, Karamouzis MV, et al. Investigational Drug Treatments for Triple-Negative Breast Cancer. Journal of Personalized Medicine. 2021; 11(7):652. https://doi.org/10.3390/jpm11070652
Chicago/Turabian StyleDamaskos, Christos, Nikolaos Garmpis, Anna Garmpi, Konstantinos Nikolettos, Panagiotis Sarantis, Vasiliki E. Georgakopoulou, Afroditi Nonni, Dimitrios Schizas, Efstathios A. Antoniou, Michalis V. Karamouzis, and et al. 2021. "Investigational Drug Treatments for Triple-Negative Breast Cancer" Journal of Personalized Medicine 11, no. 7: 652. https://doi.org/10.3390/jpm11070652
APA StyleDamaskos, C., Garmpis, N., Garmpi, A., Nikolettos, K., Sarantis, P., Georgakopoulou, V. E., Nonni, A., Schizas, D., Antoniou, E. A., Karamouzis, M. V., Nikolettos, N., Kontzoglou, K., Patsouras, A., Voutyritsa, E., Syllaios, A., Koustas, E., Trakas, N., & Dimitroulis, D. (2021). Investigational Drug Treatments for Triple-Negative Breast Cancer. Journal of Personalized Medicine, 11(7), 652. https://doi.org/10.3390/jpm11070652













