Figure 1.
Intravoxel incoherent motion (IVIM) diffusion weight (DW) images with b values of (a) 0, (b) 15, (c) 30, (d) 45, (e) 60, (f) 100, (g) 200, (h) 400, (i) 600, (j) 1000, (k) 1500, (l) 2000, and (m) 2500 s/mm2.
Figure 1.
Intravoxel incoherent motion (IVIM) diffusion weight (DW) images with b values of (a) 0, (b) 15, (c) 30, (d) 45, (e) 60, (f) 100, (g) 200, (h) 400, (i) 600, (j) 1000, (k) 1500, (l) 2000, and (m) 2500 s/mm2.
Figure 2.
Flow chart showing image pre-processing steps.
Figure 2.
Flow chart showing image pre-processing steps.
Figure 3.
Flowchart of tumor detection via iterative-constrained energy maximization (I-CEM).
Figure 3.
Flowchart of tumor detection via iterative-constrained energy maximization (I-CEM).
Figure 4.
Flowchart of tumor detection via K-CEM.
Figure 4.
Flowchart of tumor detection via K-CEM.
Figure 5.
Band expansion process (BEP).
Figure 5.
Band expansion process (BEP).
Figure 6.
Flowchart of tumor detection via K-means.
Figure 6.
Flowchart of tumor detection via K-means.
Figure 7.
Flowchart of tumor detection via Fuzzy C-means.
Figure 7.
Flowchart of tumor detection via Fuzzy C-means.
Figure 8.
K-means results when k = 2–6: (a) images without band expansion process; (b) images after band expansion process.
Figure 8.
K-means results when k = 2–6: (a) images without band expansion process; (b) images after band expansion process.
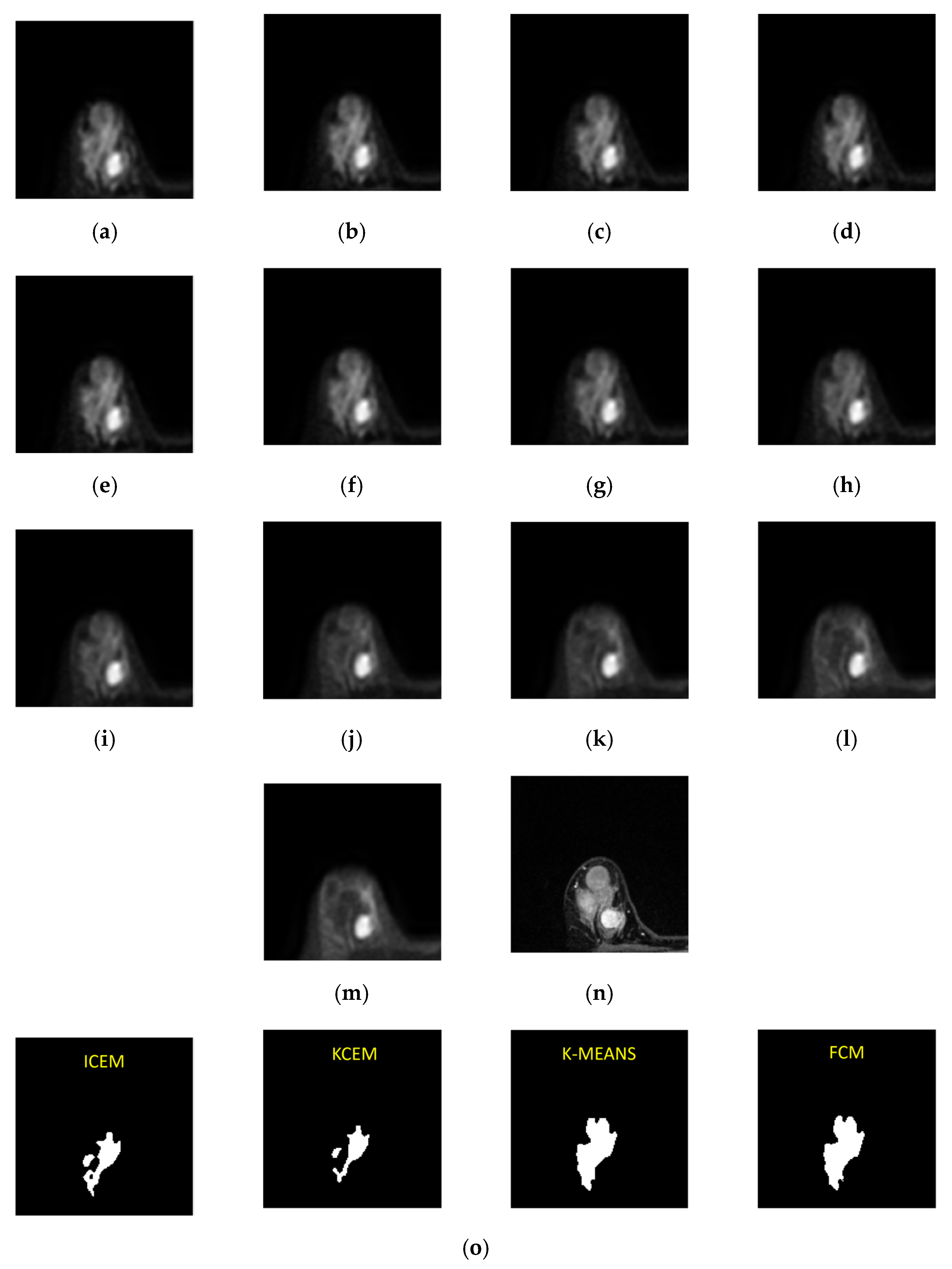
Figure 9.
Third-slice IVIM-DW images of a mass tumor with b values of (a) 0, (b) 15, (c) 30, (d) 45, (e) 60, (f) 100, (g) 200, (h) 400, (i) 600, (j) 1000, (k) 1500, (l) 2000, and (m) 2500 s/mm2. (n) Dynamic contrast-enhanced T1-MR imaging. (o) Tumor detection results obtained via I-CEM, K-CEM, K-means, and FCM. (p) Tumors detected via the four methods (left-to-right: I-CEM, K-CEM, K-means, and FCM) mapped onto a dynamic contrast-enhanced T1-MR image.
Figure 9.
Third-slice IVIM-DW images of a mass tumor with b values of (a) 0, (b) 15, (c) 30, (d) 45, (e) 60, (f) 100, (g) 200, (h) 400, (i) 600, (j) 1000, (k) 1500, (l) 2000, and (m) 2500 s/mm2. (n) Dynamic contrast-enhanced T1-MR imaging. (o) Tumor detection results obtained via I-CEM, K-CEM, K-means, and FCM. (p) Tumors detected via the four methods (left-to-right: I-CEM, K-CEM, K-means, and FCM) mapped onto a dynamic contrast-enhanced T1-MR image.
Figure 10.
Signal-intensity decay of a mass tumor detected by the four methods using different b values in the third slice.
Figure 10.
Signal-intensity decay of a mass tumor detected by the four methods using different b values in the third slice.
Figure 11.
Eighth-slice IVIM-DW images of a non-mass tumor with b-values of (a) 0, (b) 15, (c) 30, (d) 45, (e) 60, (f) 100, (g) 200, (h) 400, (i) 600, (j) 1000, (k) 1500, (l) 2000, and (m) 2500 s/mm2. (n) Dynamic contrast-enhanced T1-MR imaging. (o) Tumor detection results obtained via I-CEM, K-CEM, K-means, and FCM methods. (p) Tumors obtained via the four methods (left-to-right: I-CEM, K-CEM, K-means, and FCM) mapped onto a dynamic contrast-enhanced T1-MR image.
Figure 11.
Eighth-slice IVIM-DW images of a non-mass tumor with b-values of (a) 0, (b) 15, (c) 30, (d) 45, (e) 60, (f) 100, (g) 200, (h) 400, (i) 600, (j) 1000, (k) 1500, (l) 2000, and (m) 2500 s/mm2. (n) Dynamic contrast-enhanced T1-MR imaging. (o) Tumor detection results obtained via I-CEM, K-CEM, K-means, and FCM methods. (p) Tumors obtained via the four methods (left-to-right: I-CEM, K-CEM, K-means, and FCM) mapped onto a dynamic contrast-enhanced T1-MR image.
Figure 12.
Signal-intensity decay of a non-mass tumor detected by the four methods using different b values in the 8th slice.
Figure 12.
Signal-intensity decay of a non-mass tumor detected by the four methods using different b values in the 8th slice.
Figure 13.
Eighth-slice IVIM-DW images of a fibroadenoma with b values of (a) 0, (b) 15, (c) 30, (d) 45, (e) 60, (f) 100, (g) 200, (h) 400, (i) 600, (j) 1000, (k) 1500, (l) 2000, and (m) 2500 s/mm2. (n) Dynamic contrast-enhanced T1-MR image. (o) Tumor detection by I-CEM, K-CEM, K-means, and FCM.
Figure 13.
Eighth-slice IVIM-DW images of a fibroadenoma with b values of (a) 0, (b) 15, (c) 30, (d) 45, (e) 60, (f) 100, (g) 200, (h) 400, (i) 600, (j) 1000, (k) 1500, (l) 2000, and (m) 2500 s/mm2. (n) Dynamic contrast-enhanced T1-MR image. (o) Tumor detection by I-CEM, K-CEM, K-means, and FCM.
Figure 14.
Signal-intensity decay of a fibroadenoma detected by the four methods using different b values in the 8th slice.
Figure 14.
Signal-intensity decay of a fibroadenoma detected by the four methods using different b values in the 8th slice.
Figure 15.
Eighth-slice IVIM-DW images of a cyst with b values of (a) 0, (b) 15, (c) 30, (d) 45, (e) 60, (f) 100, (g) 200, (h) 400, (i) 600, (j) 1000, (k) 1500, (l) 2000, and (m) 2500 s/mm2. (n) Dynamic contrast-enhanced T1-MR imaging. (o) Tumor detection by I-CEM, K-CEM, K-means, and FCM.
Figure 15.
Eighth-slice IVIM-DW images of a cyst with b values of (a) 0, (b) 15, (c) 30, (d) 45, (e) 60, (f) 100, (g) 200, (h) 400, (i) 600, (j) 1000, (k) 1500, (l) 2000, and (m) 2500 s/mm2. (n) Dynamic contrast-enhanced T1-MR imaging. (o) Tumor detection by I-CEM, K-CEM, K-means, and FCM.
Figure 16.
Signal-intensity decay of a cyst detected by the four methods using different b values in the 8th slice.
Figure 16.
Signal-intensity decay of a cyst detected by the four methods using different b values in the 8th slice.
Figure 17.
Signal intensity decays using K-CEM.
Figure 17.
Signal intensity decays using K-CEM.
Table 1.
Results of applying different detection methods to a mass tumor.
Table 1.
Results of applying different detection methods to a mass tumor.
| Method Type | ADC | Signal Decay Slope | D* | D | PF |
|---|
| K-CEM | 1.20 × 10−3 | −1.60 × 10−4 | 5.53 × 10−3 | 7.59 × 10−4 | 21% |
| Fuzzy C-means | 1.21 × 10−3 | −1.68 × 10−4 | 5.86 × 10−3 | 7.43 × 10−4 | 21% |
| K-means | 1.22 × 10−3 | −1.65 × 10−4 | 6.03 × 10−3 | 7.93 × 10−4 | 22% |
| I-KCEM | 1.21 × 10−3 | −1.70 × 10−4 | 5.94 × 10−3 | 7.49 × 10−4 | 21% |
Table 2.
Results of applying different detection methods to a non-mass tumor.
Table 2.
Results of applying different detection methods to a non-mass tumor.
| Method Type | ADC | Signal Decay Slope | D* | D | PF |
|---|
| K-CEM | 1.50 × 10−3 | −2.16 × 10−4 | 7.58 × 10−3 | 1.07 × 10−3 | 30% |
| Fuzzy C-means | 1.51 × 10−3 | −2.07 × 10−4 | 7.50 × 10−3 | 0.99 × 10−3 | 32% |
| K-means | 1.51 × 10−3 | −2.19 × 10−4 | 7.64 × 10−3 | 1.12 × 10−3 | 31% |
| I-KCEM | 1.51 × 10−3 | −2.10 × 10−4 | 7.85 × 10−3 | 1.19 × 10−3 | 31% |
Table 3.
Results of applying different detection methods to a fibroadenoma tumor.
Table 3.
Results of applying different detection methods to a fibroadenoma tumor.
| Method Type | ADC | Signal Decay Slope | D* | D | PF |
|---|
| K-CEM | 2.08 × 10−3 | −3.14 × 10−4 | 4.09 × 10−3 | 1.26 × 10−3 | 45% |
| Fuzzy C-means | 2.10 × 10−3 | −3.12 × 10−4 | 4.14 × 10−3 | 1.27 × 10−3 | 45% |
| K-means | 2.09 × 10−3 | −3.15 × 10−4 | 4.44 × 10−3 | 1.26 × 10−3 | 45% |
| I-KCEM | 2.08 × 10−3 | −3.14 × 10−4 | 4.00 × 10−3 | 1.26 × 10−3 | 46% |
Table 4.
Results of applying different detection methods to a cyst tumor.
Table 4.
Results of applying different detection methods to a cyst tumor.
| Method Type | ADC | Signal Decay Slope | D* | D | PF |
|---|
| K-CEM | 2.64 × 10−3 | −3.71 × 10−4 | 4.38 × 10−3 | 1.36 × 10−3 | 52% |
| Fuzzy C-means | 2.80 × 10−3 | −3.96 × 10−4 | 4.46 × 10−3 | 1.23 × 10−3 | 49% |
| K-means | 2.78 × 10−3 | −3.88 × 10−4 | 5.33 × 10−3 | 1.02 × 10−3 | 63% |
| I-KCEM | 2.75 × 10−3 | −3.63 × 10−4 | 4.56 × 10−3 | 1.19 × 10−3 | 56% |
Table 5.
Mass tumor: Dice similarity coefficient and Jaccard similarity coefficient of each method.
Table 5.
Mass tumor: Dice similarity coefficient and Jaccard similarity coefficient of each method.
| | KCEM | K-Means | Fuzzy C-Means | ICEM |
|---|
| Dice (%) | 89.66% | 87.22% | 87.88% | 88.14% |
| Jaccard (%) | 81.25% | 77.33% | 78.38% | 78.79% |
Table 6.
Mass tumor: Confusion matrix.
Table 6.
Mass tumor: Confusion matrix.
| | KCEM | K-Means | Fuzzy C-Means | ICEM |
|---|
| Accuracy (%) | 99.54% | 99.35% | 99.39% | 99.47% |
| Precision (%) | 92.86% | 79.45% | 80.56% | 89.66% |
| Recall (%) | 86.67% | 96.67% | 96.67% | 86.67% |
Table 7.
Mass tumor: Average execution time of each method for 14 cases.
Table 7.
Mass tumor: Average execution time of each method for 14 cases.
| | KCEM | K-Means | Fuzzy C-Means | ICEM |
|---|
| Execution time (s) | 22 | 91.8 | 1.61 | 7.48 |
Table 8.
Non-mass tumor: Dice similarity coefficient and Jaccard similarity coefficient of each method.
Table 8.
Non-mass tumor: Dice similarity coefficient and Jaccard similarity coefficient of each method.
| | KCEM | K-Means | Fuzzy C-Means | ICEM |
|---|
| Dice (%) | 86.07% | 83.50% | 83.99% | 83.73% |
| Jaccard (%) | 76.09% | 73.18% | 73.40% | 74.02% |
Table 9.
Non-mass tumor: Confusion matrix.
Table 9.
Non-mass tumor: Confusion matrix.
| | KCEM | K-Means | Fuzzy C-Means | ICEM |
|---|
| Accuracy (%) | 98.41% | 97.57% | 97.66% | 97.74% |
| Precision (%) | 88.42% | 82.38% | 83.26% | 85.16% |
| Recall (%) | 85.42% | 87.48% | 89.31% | 80.58% |
Table 10.
Non-mass tumor: Average execution time of each method for 9 cases.
Table 10.
Non-mass tumor: Average execution time of each method for 9 cases.
| | KCEM | K-Means | Fuzzy C-Means | ICEM |
|---|
| Execution time (s) | 38.385 | 2.075 | 3.7 | 7.594 |
Table 11.
Quantitative results for mass, non-mass, fibroadenoma, and cyst tumors.
Table 11.
Quantitative results for mass, non-mass, fibroadenoma, and cyst tumors.
| Tumor Type | ADC | Signal Decay Slope | D* | D | PF |
|---|
| Mass | 1.21 × 10−3 | −1.89 × 10−4 | 6.10 × 10−3 | 0.84 × 10−3 | 23% |
| Non-mass | 1.49 × 10−3 | −2.14 × 10−4 | 7.53 × 10−3 | 1.03 × 10−3 | 31% |
| Fibroadenoma | 2.08 × 10−3 | −3.14 × 10−4 | 4.09 × 10−3 | 1.26 × 10−3 | 45% |
| Cyst | 2.64 × 10−3 | −3.71 × 10−4 | 4.38 × 10−3 | 1.36 × 10−3 | 52% |