Gut and Endometrial Microbiome Dysbiosis: A New Emergent Risk Factor for Endometrial Cancer
Abstract
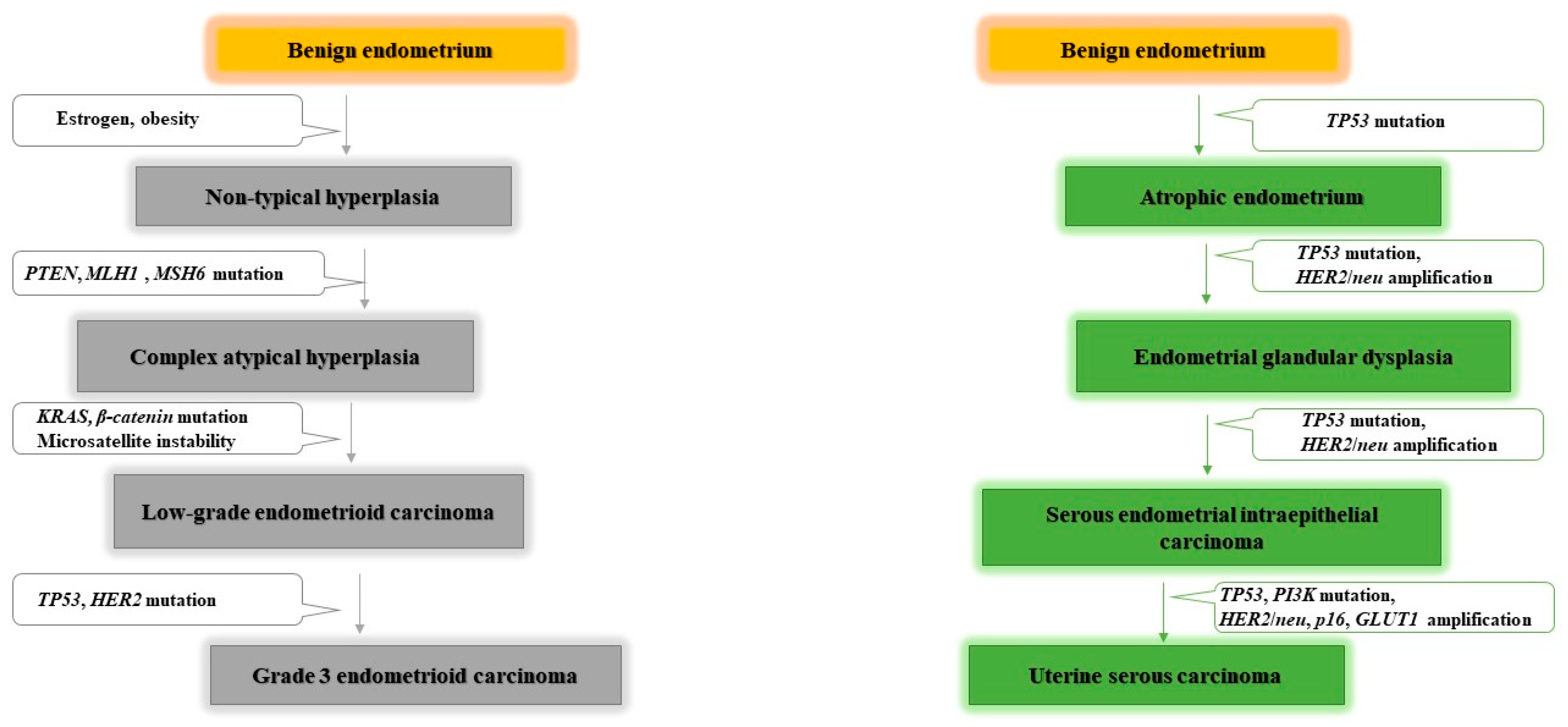
:1. Introduction
2. Endometrial Microbiome
3. Microbiome and Endometrial Cancer
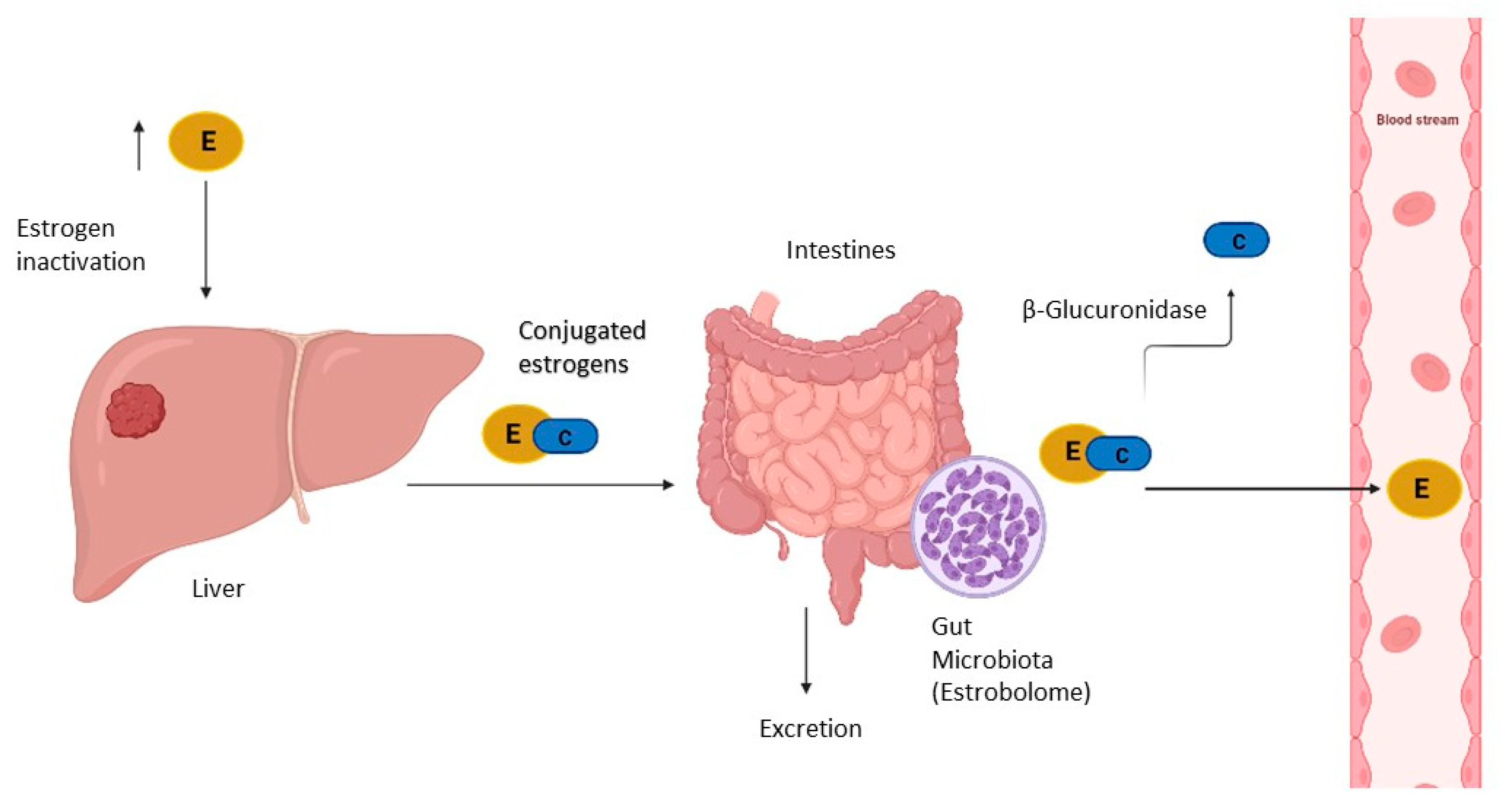
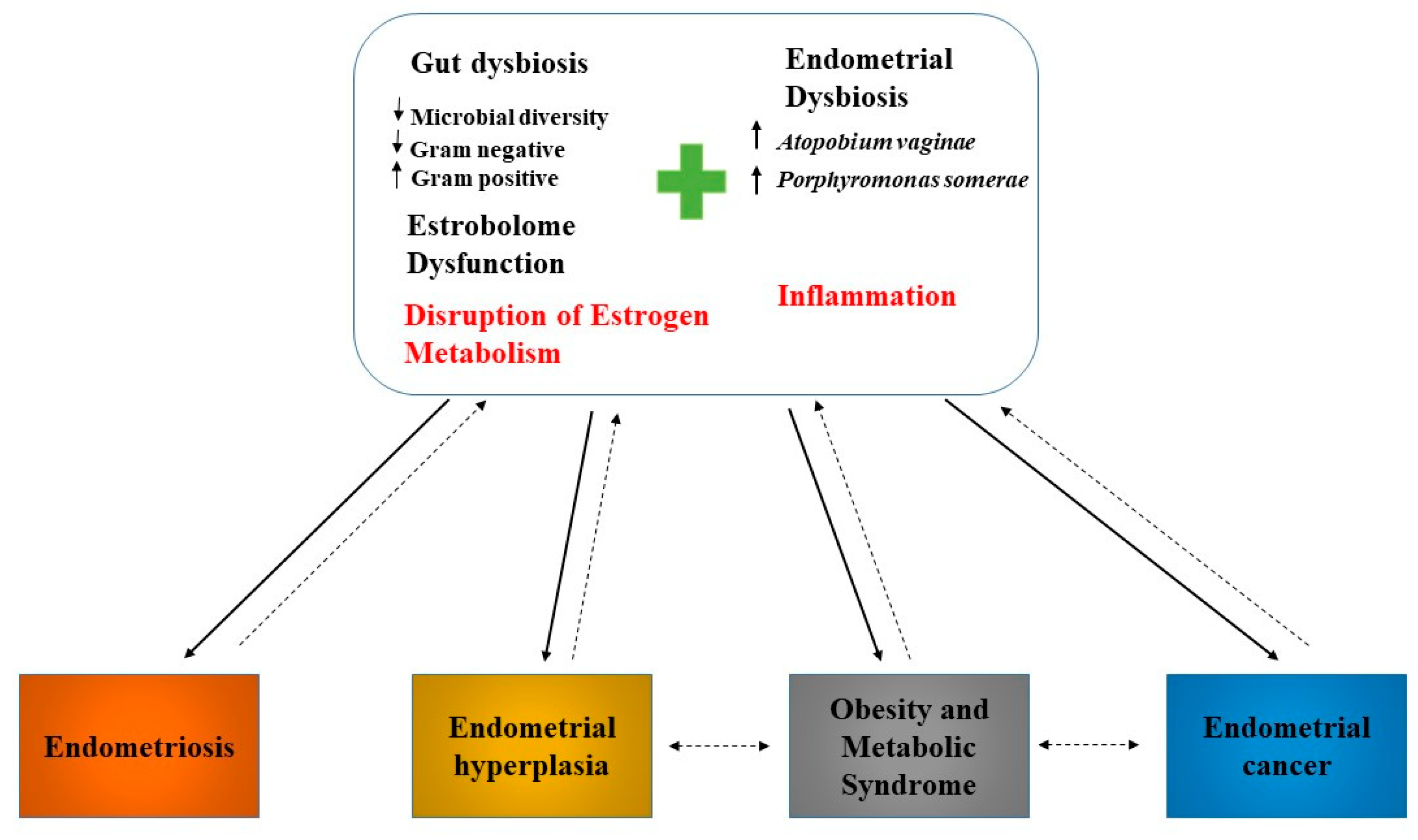
4. Estrogen Metabolism, Gut Microbiota, and Endometrial Cancer
5. Microbiome, Inflammation, and Endometrial Cancer
6. Modulation of Antitumoural Therapies Efficacy and Toxicity by Gut Microbiota
6.1. Immunotherapy
6.2. Chemotherapy
6.3. Radiotherapy
6.4. Targeted Therapy
6.5. Toxicity
7. Modulation of Endometrial Microbiota
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yanaihara, A.; Otsuka, Y.; Iwasaki, S.; Aida, T.; Tachikawa, T.; Irie, T.; Okai, T. Differences in gene expression in the proliferative human endometrium. Fertil. Steril. 2005, 83, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Al Hyassat, S.; Elaiwy, O.; Rashid, S.; Al-Nabet, A.D. Classification of Endometrial Carcinoma: New Perspectives Beyond Morphology. Adv. Anat. Pathol. 2019, 26, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. J. Natl. Cancer Inst. 2017, 110, 354–361. [Google Scholar] [CrossRef]
- Clarke, M.A.; Long, B.J.; Morillo, A.D.M.; Arbyn, M.; Bakkum-Gamez, J.N.; Wentzensen, N. Association of Endometrial Cancer Risk with Postmenopausal Bleeding in Women: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2018, 178, 1210–1222. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Loos, A.H.; Oostindier, M.; Weiderpass, E. Geographic and temporal variations in cancer of the corpus uteri: Incidence and mortality in pre- and postmenopausal women in Europe. Int. J. Cancer 2005, 117, 123–131. [Google Scholar] [CrossRef]
- Bray, F.; Silva, I.D.S.; Moller, H.; Weiderpass, E. Endometrial Cancer Incidence Trends in Europe: Underlying Determinants and Prospects for Prevention. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1132–1142. [Google Scholar] [CrossRef] [Green Version]
- Ketabi, Z.; Mosgaard, B.J.; Gerdes, A.-M.; Ladelund, S.; Bernstein, I. Awareness of Endometrial Cancer Risk and Compliance with Screening in Hereditary Nonpolyposis Colorectal Cancer. Obstet. Gynecol. 2012, 120, 1005–1012. [Google Scholar] [CrossRef]
- Tamura, K.; Kaneda, M.; Futagawa, M.; Takeshita, M.; Kim, S.; Nakama, M.; Kawashita, N.; Tatsumi-Miyajima, J. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int. J. Clin. Oncol. 2019, 24, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Pellat, A.; Netter, J.; Perkins, G.; Cohen, R.; Coulet, F.; Parc, Y.; Svrcek, M.; Duval, A.; André, T. Syndrome de Lynch: Quoi de neuf? Bull. Cancer 2019, 106, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Binder, P.S.; Mutch, D.G. Update on Prognostic Markers for Endometrial Cancer. Women’s Health 2014, 10, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Corrado, G.; Laquintana, V.; Loria, R.; Carosi, M.; De Salvo, L.; Sperduti, I.; Zampa, A.; Cicchillitti, L.; Piaggio, G.; Cutillo, G.; et al. Endometrial cancer prognosis correlates with the expression of L1CAM and miR34a biomarkers. J. Exp. Clin. Cancer Res. 2018, 37, 139. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Levine, R.L.; Cargile, C.B.; Blazes, M.S.; Van Rees, B.; Kurman, R.J.; Ellenson, L.H. PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma. Cancer Res. 1998, 58, 6. [Google Scholar]
- Sherman, M.E. Theories of Endometrial Carcinogenesis: A Multidisciplinary Approach. Mod. Pathol. 2000, 13, 295–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banno, K.; Yanokura, M.; Kisu, I.; Yamagami, W.; Susumu, N.; Aoki, D. MicroRNAs in endometrial cancer. Int. J. Clin. Oncol. 2013, 18, 186–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, N.; Yendluri, V.; Wenham, R.M. The Molecular Biology of Endometrial Cancers and the Implications for Pathogenesis, Classification, and Targeted Therapies. Cancer Control. 2009, 16, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.-S. Molecular Carcinogenesis of Endometrial Cancer. Taiwan J. Obstet. Gynecol. 2007, 46, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Buhtoiarova, T.N.; Brenner, C.A.; Singh, M. Endometrial Carcinoma: Role of Current and Emerging Biomarkers in Resolving Persistent Clinical Dilemmas. Am. J. Clin. Pathol. 2016, 145, 8–21. [Google Scholar] [CrossRef]
- Van Nyen, T.; Moiola, C.P.; Colas, E.; Annibali, D.; Amant, F. Modeling Endometrial Cancer: Past, Present, and Future. Int. J. Mol. Sci. 2018, 19, 2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulsa, M.; Urasińska, E. Triple negative endometrial cancer. Ginekol. Polska 2017, 88, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Walther-António, M.R.S.; Chen, J.; Multinu, F.; Hokenstad, A.; Distad, T.J.; Cheek, E.H.; Keeney, G.L.; Creedon, D.J.; Nelson, H.; Mariani, A.; et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016, 8, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [Green Version]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Fadrosh, D.; Chang, K.; Silver, M.; Viscidi, R.P.; Burke, A.E.; Ravel, J.; Gravitt, P.E. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2014, 21, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, C.M.; Haick, A.; Nkwopara, E.; Garcia, R.; Rendi, M.; Agnew, K.; Fredricks, D.; Eschenbach, D. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am. J. Obstet. Gynecol. 2015, 212, 611.e1–611.e9. [Google Scholar] [CrossRef] [Green Version]
- Fang, R.-L.; Chen, L.-X.; Shu, W.-S.; Yao, S.-Z.; Wang, S.-W.; Chen, Y.-Q. Barcoded sequencing reveals diverse intrauterine microbiomes in patients suffering with endometrial polyps. Am. J. Transl. Res. 2016, 8, 1581–1592. [Google Scholar]
- Verstraelen, H.; Vilchez-Vargas, R.; Desimpel, F.; Jauregui, R.; Vankeirsbilck, N.; Weyers, S.; Verhelst, R.; De Sutter, P.; Pieper, D.H.; Van De Wiele, T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ 2016, 4, e1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Song, X.; Chunwei, Z.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Winters, A.D.; Romero, R.; Gervasi, M.T.; Gomez-Lopez, N.; Tran, M.R.; Garcia-Flores, V.; Pacora, P.; Jung, E.; Hassan, S.S.; Hsu, C.-D.; et al. Does the endometrial cavity have a molecular microbial signature? Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Lu, W.; He, F.; Lin, Z.; Liu, S.; Tang, L.; Huang, Y.; Hu, Z. Dysbiosis of the endometrial microbiota and its association with inflammatory cytokines in endometrial cancer. Int. J. Cancer 2021, 148, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Rajagopala, S.V.; Vashee, S.; Oldfield, L.M.; Suzuki, Y.; Venter, J.C.; Telenti, A.; Nelson, K.E. The Human Microbiome and Cancer. Cancer Prev. Res. 2017, 10, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Meng, W.; Wang, B.; Qiao, L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014, 345, 196–202. [Google Scholar] [CrossRef]
- Mayrand, M.-H.; Duarte-Franco, E.; Rodrigues, I.; Walter, S.D.; Hanley, J.; Ferenczy, A.; Ratnam, S.; Coutlée, F.; Franco, E. Human Papillomavirus DNA versus Papanicolaou Screening Tests for Cervical Cancer. N. Engl. J. Med. 2007, 357, 1579–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myšák, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duškova, J. Porphyromonas gingivalis: Major Periodontopathic Pathogen Overview. J. Immunol. Res. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hokenstad, A.; Mariani, A.; Walther-Antonio, M. Vaginal detection of Porphyromonas somerae is indicative of endometrial cancer diagnosis. Gynecol. Oncol. 2017, 145, 76. [Google Scholar] [CrossRef]
- Gonzalez-Bosquet, J.; Pedra-Nobre, S.; Devor, E.; Thiel, K.; Goodheart, M.; Bender, D.; Leslie, K. Bacterial, Archaea, and Viral Transcripts (BAVT) Expression in Gynecological Cancers and Correlation with Regulatory Regions of the Genome. Cancers 2021, 13, 1109. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Hokenstad, A.N.; Chen, J.; Sung, J.; Jenkins, G.D.; Chia, N.; Nelson, H.; Mariani, A.; Walther-Antonio, M.R.S. Postmenopause as a key factor in the composition of the Endometrial Cancer Microbiome (ECbiome). Sci. Rep. 2019, 9, 19213–19216. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendling, W.; Palmeira-De-Oliveira, A.; Biber, S.; Prasauskas, V. An update on the role of Atopobium vaginae in bacterial vaginosis: What to consider when choosing a treatment? A mini review. Arch. Gynecol. Obstet. 2019, 300, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Groothuis, P.; Dassen, H.; Romano, A.; Punyadeera, C. Estrogen and the endometrium: Lessons learned from gene expression profiling in rodents and human. Hum. Reprod. Updat. 2007, 13, 405–417. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Weelden, W.J.; Massuger, L.F.A.G.; Pijnenborg, J.M.A.; Romano, A. Enitec Anti-estrogen Treatment in Endometrial Cancer: A Systematic Review. Front. Oncol. 2019, 9, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, B.T. Functional role of estrogen metabolism in target cells: Review and perspectives. Carcinogenesis 1998, 19, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plottel, C.S.; Blaser, M.J. Microbiome and Malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaud, D.; Tailliez, P.; Anba-Mondoloni, J. Genetic characterization of the β-glucuronidase enzyme from a human intestinal bacterium, Ruminococcus gnavus. Microbiology 2005, 151, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–Gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollet, R.M.; D’Agostino, E.H.; Walton, W.G.; Xu, Y.; Little, M.S.; Biernat, K.A.; Pellock, S.J.; Patterson, L.M.; Creekmore, B.; Isenberg, H.N.; et al. An Atlas of β-Glucuronidases in the Human Intestinal Microbiome. Structure 2017, 25, 967–977.e5. [Google Scholar] [CrossRef] [Green Version]
- Parida, S.; Sharma, D. The Microbiome–Estrogen Connection and Breast Cancer Risk. Cells 2019, 8, 1642. [Google Scholar] [CrossRef] [Green Version]
- Łaniewski, P.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. The microbiome and gynaecological cancer development, prevention and therapy. Nat. Rev. Urol. 2020, 17, 232–250. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Modugno, F.; Ness, R.B.; Chen, C.; Weiss, N.S. Inflammation and Endometrial Cancer: A Hypothesis. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2840–2847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dossus, L.; Rinaldi, S.; Becker, S.; Lukanova, A.; Tjonneland, A.; Olsen, A.; Stegger, J.; Overvad, K.; Chabbert-Buffet, N.; Jimenez-Corona, A.; et al. Obesity, inflammatory markers, and endometrial cancer risk: A prospective case–control study. Endocr. Relat. Cancer 2010, 17, 1007–1019. [Google Scholar] [CrossRef]
- Zhao, Y.; Nichols, J.E.; Bulun, S.E.; Mendelson, C.; Simpson, E.R. Aromatase P450 Gene Expression in Human Adipose Tissue. Role of a Jak/Stat Pathway in Regulation of the Adipose-Specific Promoter. J. Biol. Chem. 1995, 270, 16449–16457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, Q.; Liu, B.-Y.; Liao, Y.; Zhang, H.-J.; Yang, T.-T.; He, Y.-Y.; Xia, Y.-H.; Lu, W.; He, X.-Y.; Chen, Z.; et al. Activation of a positive feedback loop involving IL-6 and aromatase promotes intratumoral 17β-estradiol biosynthesis in endometrial carcinoma microenvironment. Int. J. Cancer 2014, 135, 282–294. [Google Scholar] [CrossRef]
- St-Germain, M.-E.; Gagnon, V.; Parent, S.; Asselin, E. Regulation of COX-2 protein expression by Akt in endometrial cancer cells is mediated through NF-κB/IκB pathway. Mol. Cancer 2004, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Microbiome, Inflammation, and Cancer. Cancer J. 2014, 20, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Pelzer, E.S.; Willner, D.; Buttini, M.; Huygens, F. A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie Van Leeuwenhoek 2018, 111, 933–943. [Google Scholar] [CrossRef]
- Caselli, E.; Soffritti, I.; D’Accolti, M.; Piva, I.; Greco, P.; Bonaccorsi, G. Atopobium Vaginae And Porphyromonas Somerae Induce Proinflammatory Cytokines Expression In Endometrial Cells: A Possible Implication For Endometrial Cancer? Cancer Manag. Res. 2019, 11, 8571–8575. [Google Scholar] [CrossRef] [Green Version]
- Keita, M.; Bessette, P.; Pelmus, M.; AinMelk, Y.; Aris, A. Expression of interleukin-1 (IL-1) ligands system in the most common endometriosis-associated ovarian cancer subtypes. J. Ovarian Res. 2010, 3, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirata, T.; Osuga, Y.; Hamasaki, K.; Yoshino, O.; Ito, M.; Hasegawa, A.; Takemura, Y.; Hirota, Y.; Nose, E.; Morimoto, C.; et al. Interleukin (IL)-17A Stimulates IL-8 Secretion, Cyclooxygensase-2 Expression, and Cell Proliferation of Endometriotic Stromal Cells. Endocrinology 2008, 149, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.O.; Stephens, N.D.; Qualls, C.R.; Fligelman, T.; Wang, T.; Lin, C.-Y.; Burton, E.H.; Griffith, J.K.; Pollard, J.W. The clinical significance of inflammatory cytokines in primary cell culture in endometrial carcinoma. Mol. Oncol. 2012, 7, 41–54. [Google Scholar] [CrossRef]
- Tong, H.; Feng, H.; Hu, X.; Wang, M.-F.; Song, Y.-F.; Wen, X.-L.; Li, Y.-R.; Wan, X.-P. Identification of Interleukin-9 Producing Immune Cells in Endometrial Carcinoma and Establishment of a Prognostic Nomogram. Front. Immunol. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.A.; Fleming, G.F.; Lastra, R.R.; Lee, N.K.; Moroney, J.W.; Son, C.H.; Tatebe, K.; Veneris, J.L. Current recommendations and recent progress in endometrial cancer. CA Cancer J. Clin. 2019, 69, 258–279. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, J. Gastrointestinal complications of pelvic radiotherapy: Are they of any importance? Gut 2005, 54, 1051–1054. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steven, A.; Fisher, S.A.; Robinson, B.W.; Fong, K.M.; Van Zandwijk, N. Immunotherapy for lung cancer. Respirology 2016, 21, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Ventriglia, J.; Paciolla, I.; Pisano, C.; Cecere, S.C.; Di Napoli, M.; Tambaro, R.; Califano, D.; Losito, N.; Scognamiglio, G.; Setola, S.V.; et al. Immunotherapy in ovarian, endometrial and cervical cancer: State of the art and future perspectives. Cancer Treat. Rev. 2017, 59, 109–116. [Google Scholar] [CrossRef]
- Yang, Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Investig. 2015, 125, 3335–3337. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, L.B.; Salama, A. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-pd-l1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Routy, B.; Le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borella, F.; Carosso, A.R.; Cosma, S.; Preti, M.; Collemi, G.; Cassoni, P.; Bertero, L.; Benedetto, C. Gut Microbiota and Gynecological Cancers: A Summary of Pathogenetic Mechanisms and Future Directions. ACS Infect. Dis. 2021, 7, 987–1009. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Mao, Q.; Xia, W.; Dong, G.; Yu, C.; Jiang, F. Gut Microbiota Shapes the Efficiency of Cancer Therapy. Front. Microbiol. 2019, 10, 1050. [Google Scholar] [CrossRef] [Green Version]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Cramer, P.; Bresalier, R.S. Gastrointestinal and Hepatic Complications of Immune Checkpoint Inhibitors. Curr. Gastroenterol. Rep. 2017, 19, 3. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [Green Version]
- Grisham, R.N.; Adaniel, C.; Hyman, D.M.; Ma, W.; Iasonos, A.; Aghajanian, C.; Konner, J. Gemcitabine for Advanced Endometrial Cancer: A Review of the MSKCC Experience. Int. J. Gynecol. Cancer 2012, 22, 807–811. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-M.; Chieng, W.-W.; Huang, S.-W.; Hsu, L.-J.; Jan, M.-S. The synergistic tumor growth-inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Nishio, S.; Shimokawa, M.; Tasaki, K.; Nasu, H.; Yoshimitsu, T.; Matsukuma, K.; Terada, A.; Tsuda, N.; Kawano, K.; Ushijima, K. A phase II trial of irinotecan in patients with advanced or recurrent endometrial cancer and correlation with biomarker analysis. Gynecol. Oncol. 2018, 150, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.P.; Pellock, S.J.; Biernat, K.A.; Walton, W.G.; Wallace, B.D.; Creekmore, B.C.; Letertre, M.M.; Swann, J.; Wilson, I.D.; Roques, J.R.; et al. Targeted inhibition of gut bacterial β-glucuronidase activity enhances anticancer drug efficacy. Proc. Natl. Acad. Sci. USA 2020, 117, 7374–7381. [Google Scholar] [CrossRef] [Green Version]
- Yan, A.; Culp, E.; Perry, J.; Lau, J.T.; MacNeil, L.T.; Surette, M.G.; Wright, G.D. Transformation of the Anticancer Drug Doxorubicin in the Human Gut Microbiome. ACS Infect. Dis. 2018, 4, 68–76. [Google Scholar] [CrossRef]
- Rigby, R.; Carr, J.; Orgel, K.; King, S.L.; Lund, P.K.; Dekaney, C.M. Intestinal bacteria are necessary for doxorubicin-induced intestinal damage but not for doxorubicin-induced apoptosis. Gut Microbes 2016, 7, 414–423. [Google Scholar] [CrossRef] [Green Version]
- Hong, B.-Y.; Sobue, T.; Choquette, L.; Dupuy, A.K.; Thompson, A.; Burleson, J.A.; Salner, A.L.; Schauer, P.K.; Joshi, P.; Fox, E.; et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 2019, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, C.; Corleto, J.; Ruegger, P.M.; Logan, G.D.; Peacock, B.B.; Mendonca, S.; Yamaki, S.; Adamson, T.; Ermel, R.; McKemy, D.; et al. Dominant Role of the Gut Microbiota in Chemotherapy Induced Neuropathic Pain. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- DeMaria, S.; Formenti, S.C. Radiation as an immunological adjuvant: Current evidence on dose and fractionation. Front. Oncol. 2012, 2, 153. [Google Scholar] [CrossRef] [Green Version]
- Barker, H.E.; Paget, J.T.E.; Khan, A.; Harrington, K. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Cao, H.; Cover, T.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble Proteins Produced by Probiotic Bacteria Regulate Intestinal Epithelial Cell Survival and Growth. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef] [Green Version]
- Ciorba, M.A.; Riehl, T.E.; Rao, M.S.; Moon, C.; Ee, X.; Nava, G.; Walker, M.R.; Marinshaw, J.M.; Stappenbeck, T.S.; Stenson, W.F. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 2011, 61, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Delia, P. Use of probiotics for prevention of radiation-induced diarrhea. World J. Gastroenterol. 2007, 13, 912. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, T.; Dong, P.; Ihira, K.; Kudo, M.; Watari, H. Molecular-targeted therapies and precision medicine for endometrial cancer. Jpn. J. Clin. Oncol. 2019, 49, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Di Modica, M.; Gargari, G.; Regondi, V.; Bonizzi, A.; Arioli, S.; Belmonte, B.; De Cecco, L.; Fasano, E.; Bianchi, F.; Bertolotti, A.; et al. Gut Microbiota Condition the Therapeutic Efficacy of Trastuzumab in HER2-Positive Breast Cancer. Cancer Res. 2021, 81, 2195–2206. [Google Scholar] [CrossRef] [PubMed]
- Flórez, A.B.; Sierra, M.; Ruas-Madiedo, P.; Mayo, B. Susceptibility of lactic acid bacteria, bifidobacteria and other bacteria of intestinal origin to chemotherapeutic agents. Int. J. Antimicrob. Agents 2016, 48, 547–550. [Google Scholar] [CrossRef] [Green Version]
- Berstein, L.; Maximov, S.; Gershfeld, E.; Meshkova, I.; Gamajunova, V.; Tsyrlina, E.; Larionov, A.; Kovalevskij, A.; Vasilyev, D. Neoadjuvant therapy of endometrial cancer with the aromatase inhibitor letrozole: Endocrine and clinical effects. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 105, 161–165. [Google Scholar] [CrossRef]
- Cao, Y.; Jiang, C.; Jia, Y.; Xu, D.; Yu, Y. Letrozole and the Traditional Chinese Medicine, Shaofu Zhuyu Decoction, Reduce Endometriotic Disease Progression in Rats: A Potential Role for Gut Microbiota. Evid. Based Complement. Altern. Med. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Chase, D.; Goulder, A.; Zenhausern, F.; Monk, B.; Herbst-Kralovetz, M. The vaginal and gastrointestinal microbiomes in gynecologic cancers: A review of applications in etiology, symptoms and treatment. Gynecol. Oncol. 2015, 138, 190–200. [Google Scholar] [CrossRef]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.A.; Des Varannes, S.B.; Massart, S.; Moreau, P.; Potel, G.; De La Cochetière, M.F.; Batard, E.; et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, M.J.; Harmsen, H.J.M.; de Bont, E.S.J.M.; Tissing, W.J.E. The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis. PLoS Pathog. 2010, 6, e1000879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benner, M.; Ferwerda, G.; Joosten, I.; Van Der Molen, R.G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Updat. 2018, 24, 393–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, N.M.; Sola-Leyva, A.; Saez-Lara, M.J.; Plaza-Diaz, J.; Tubić-Pavlović, A.; Romero, B.; Clavero, A.; Mozas-Moreno, J.; Fontes, J.; Altmäe, S. New Opportunities for Endometrial Health by Modifying Uterine Microbial Composition: Present or Future? Biomolecules 2020, 10, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenoll, E.; Moreno, I.; Sánchez, M.; Garcia-Grau, I.; Silva, Á.; González-Monfort, M.; Genovés, S.; Vilella, F.; Seco-Durban, C.; Simón, C.; et al. Selection of New Probiotics for Endometrial Health. Front. Cell. Infect. Microbiol. 2019, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Verstraelen, H.; Loening-Baucke, V.; Swidsinski, S.; Mendling, W.; Halwani, Z. Presence of a Polymicrobial Endometrial Biofilm in Patients with Bacterial Vaginosis. PLoS ONE 2013, 8, e53997. [Google Scholar] [CrossRef] [Green Version]
- Dizzell, S.; Nazli, A.; Reid, G.; Kaushic, C. Protective Effect of Probiotic Bacteria and Estrogen in Preventing HIV-1-Mediated Impairment of Epithelial Barrier Integrity in Female Genital Tract. Cells 2019, 8, 1120. [Google Scholar] [CrossRef] [Green Version]
- Kyono, K.; Hashimoto, T.; Kikuchi, S.; Nagai, Y.; Sakuraba, Y. A pilot study and case reports on endometrial microbiota and pregnancy outcome: An analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod. Med. Biol. 2019, 18, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef]
- Romero, R.; Gomez-Lopez, N.; Winters, A.D.; Jung, E.; Shaman, M.; Bieda, J.; Panaitescu, B.; Pacora, P.; Erez, O.; Greenberg, J.M.; et al. Evidence that intra-amniotic infections are often the result of an ascending invasion—A molecular microbiological study. J. Périnat. Med. 2019, 47, 915–931. [Google Scholar] [CrossRef]
- Vornhagen, J.; Armistead, B.; Santana-Ufret, V.; Gendrin, C.; Merillat, S.; Coleman, M.; Quach, P.; Boldenow, E.; Alishetti, V.; Leonhard-Melief, C.; et al. Group B streptococcus exploits vaginal epithelial exfoliation for ascending infection. J. Clin. Investig. 2018, 128, 1985–1999. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Sample Size | Sample Type | Methods | Finding |
|---|---|---|---|---|---|
| Mitchell et al. | 2018 | Women underwent hysteroctomy for benign disease without cancer indications (n = 58). | Vaginal and endometrial swabs. | Bacterial 16S rRNA sequencing. | ↑Lactobacillus iners (45%), Lactobacillus crispatus (33%), Gardnerella vaginalis, Prevotella spp., Atopobium vaginae, and Sneathia. |
| Fang et al. | 2016 | -Patients with only endometrial polyps (n = 10). -Patients with both endometrial polyps and chronic endometritis (n = 10). -Healthy women (n = 10). | Vaginal and endometrial swabs. | Bacterial 16S rRNA genes sequencing. | ↑Lactobacillus, Gardnerella, Bifidobacterium, Streptococcus, and Alteromonas in healthy group compared to endometrial polyps and chronic endometriosis group. |
| Verstraelen et al. | 2016 | Women with various reproductive conditions, without uterine anomalies (n = 90). | Endometrial biopsy: tissue and mucus. | 16S rRNA gene V1–2 region | Uterine microbiome dominated by Bacteroides (B. xylanisolvens, B. thetaiotaomicron, and B. fragilis) and Pelomonas. |
| Chen et al. | 2017 | Reproductive age women operated for conditions not known to involve infection (n = 95). | Endometrial swab and tissue. | 16S rRNA amplicon sequencing | ↑Pseudomonas, Acinetobacter, Vagococcus, and Sphingobium in the endometrium |
| Winters et al. | 2019 | Women (n = 25) underwent a hysterectomy for fibroids (23) and endometrial hyperplasia (2). | Endometrial swab. | Sequencing of the 16S rRNA gene. | ↑Acinetobacter, Pseudomonas, Comamonadaceae, and Cloacibacterium in endometrium. |
| Lu et al. | 2020 | Women undergone a hysterectomy for benign disease and any stage of endometrial cancer (n = 50). | Endometrial tissue. | 16S rRNA gene sequencing for bacterial communities. | ↑Rhodococcus, Phyllobacterium, Sphingomonas, Bacteroides, and Bifidobacterium. |
| Author | Year | Simple Size | Sample Type | Methods | Finding |
|---|---|---|---|---|---|
| Walther-António et al. | 2016 | -Patients with endometrial cancer (n = 17). -Patients with endometrial hyperplasia (n = 4). -Patients with benign uterine conditions (n = 10). | Endometrial swab and scrabe. | 16S rRNA sequencing of V3-V5 region. | Patients with endometrial cancer: ↑Firmicutes (Anaerostipes, ph2, Dialister, Peptoniphilus, 1–68, Ruminococcus, and Anaerotruncus), Spirochaetes (Treponema), Actinobacteria (Atopobium), Bacteroidetes (Bacteroides and Porphyromonas), and Proteobacteria (Arthrospira). Close correlation between Atopobium vaginae and Porphyromonas spp. and endometrial cancer, especially when the vaginal pH is high (˃4.5). |
| Lu et al. | 2020 | Patients undergone a hysterectomy for benign disease and any stage of endometrial cancer (n = 50). | Endometrial cancer tissue. | 16S rRNA gene sequencing for bacterial communities. | ↓Local microbiome diversity in patients with endomatrial cancer. ↑Micrococcus asociated with an inflammatory profile in endometrial cancer patients. |
| Gonzalez-Bosquet et al. | 2021 | -Patients with high grade serous ovarian cancer (HGSC) (n = 112). -Patients with endometrioid endometrial cancer (EEC) (n = 62). -Women with normal fallopian tubes, and no risk factors for cancer (n = 12). | Frozen ovarian and endometrial tumour tissue. | 16S rRNA gene sequencing. | -93 bacterial, archaea, and viral transcripts (BAVTs) were differentially expressed between HGSC and EEC. -The diversity of BAVT species decreased in EEC, and even more in HGSC compared to normal samples. |
| Walsh et al. | 2019 | Patients with a variety of uterine conditions (n = 148): -Patients without endometrial cancer (n = 75). -Patients with Type I endometrial cancer (n = 56). -Patients with Type II endometrial cancer (n = 10). -Patients with complex atypical hyperplasia (n = 7). | Uterine, fallopian and ovarian samples (swabs and scrapes) | -Amplification and sequencing of V3-V5 region of the 16S rRNA gene. | In endometrial cancer patients: ↑Porphyromonas somerae. ↑Anaerococcus tetradius, Anaerococcus lactolyticus, Peptoniphilus coxii, and Campylobacter ureolyticus related to postmenopause status, facilitating the subsequent colonization by Porphyromonas somerae. |
| Antitumoural Therapy | Author | Year | Sample Type Analized | Methods | Finding | |
|---|---|---|---|---|---|---|
| Immunotherapy | anti-PD-L1 | Sivan et al. | 2015 | Two groups of mice with subcutaneous melanomas and different intestinal microbiota, from different laboratories. | Transfer of faecal material within both group of mice before tumour implantation. 16S rRNA sequencing analysis | Gut microbiome of responder group: ↑Bifidobacterium |
| anti-PD-1 | Routy et al. | 2018 | Mice with non-small-cell lung cancer and renal carcinoma (responders to blocking immune checkpoints, and non-responders). | Faecal microbiota transplantation and 16S ribosomal rRNA of faecal samples | Gut microbiome of responder group: ↑Firmicutes (Clostridiales), ↑Alistipes, ↑Ruminococcus, ↑Eubacterium spp. ↑Akkermansia muciniphila ↑Enterococcus hirae, ↓Bifidobacterium adolescentis, ↓Bifidobacterium longum ↓Parabacteroides distasonis Gut microbiome of non-responder group: ↑Corynebacterium aurimucosum, ↑Staphylococcus haemolyticus | |
| Gopalakrishnan et al. | 2018 | Patients with metastatic melanoma (n = 112) | 16S rRNA gene sequencing of oral, buccal and faecal samples, and tumour biopsies at treatment initiation and 6 months after treatment initiation. | Gut microbiome of responders group: ↑Diversity, ↑Ruminococcaceae ↑Faecalibacterium Non-responders group gut microbiome: ↓Diversity, ↑Bacteroidales | ||
| anti-CTLA-4 | Chaput et al. | 2017 | Patients with metastatic melanoma (n = 26) | 16S rRNA gene sequencing at baseline and before each ipilimumab infusion in faecal samples. | Gut microbiome of responders group: ↑Faecalibacterium, ↑other Firmicutes Non-responders gut microbiome: ↑Bacteroidetes | |
| Cramer et al. | 2017 | Patients with metastatic melanoma and received ipilimumab (n = 34) | 16S rRNA gene amplification and multiparallel sequencing of faecal samples. | Bacteroides fragilis enhances the effect of immunological treatment with anti-CTLA-4 | ||
| Vétizou et al. | 2015 | Mice with sarcomas | High-throughput pyrosequencing of 16S rRNA gene amplicons of faeces. | Gut microbiome of responders group: ↑Bacteroides fragilis ↑Bacteroides thetaiotaomicron ↑Burkholderiales ↑Burkholderia cepacia | ||
| Chemotherapy | cyclophosphamide | Ma et al. | 2019 | Mice treated with cyclophosphamide | High-throughput 454 pyrosequencing in faecal samples. Quantitative PCR targeting the domain bacteria and specific bacterial groups. | Gut microbiome involved in enhacing treatment response: Enterococcus hirae Lactobacillus johnsonii Lactobacillus murinus Segmented filamentous bacteria Barnesiella intestinihominis |
| Gemcitabine | Chen et al. | 2020 | Transgenic mice with pancreatic cancer | Probiotic oral gavage of Lactobacillus paracasei and Lactobacillus reuteri. 16S rRNA Amplicon Sequencing. | ↑Lactobacillus paracasei, Lactobacillus reuteri enhacing treatment response in transgenic mice. | |
| Irinotecan | Bhatt et al. | 2020 | Tumour xenograft model | 16S rRNA Amplicon Sequencing | Changes in the composition of the intestinal microbiota induced by irinotecan: ↑Proteobacteria (Enterobacteriaceae), ↑Verrucomicrobia ↑Akkermansia muciniphila | |
| Doxorubicin | Hong et al. | 2019 | Patients with chemotherapeutic treatment for a solid tumour (n = 30) and non-cancer controls (n = 30) | Amplification and sequencing of 16S rRNA gene and ITS 1 DNA | Gut dysbiosis induced by doxorubicin: ↓Streptococcus, ↓Actinomyces ↓Gemella, ↓Granulicatella ↓Veillonella, ↑Fusobacterium nucleatum ↑Prevotella oris | |
| Yan et al. | 2018 | Healthy donors | 16S rRNA Amplicon Sequencing of faecal samples | ↑Raoultella planticola, Klebsiella pneumoniae and Escherichia coli involved in doxorubicin inactivation and degradation. | ||
| Paclitaxel | Ramakrishna et al. | 2019 | Two groups of mice: sensitive and resistant to Paclitaxel-induced pain. | 16S rRNA gene sequencing. | ↓Akkermansia muciniphila | |
| Toxicity induced by chemotherapy | Van Vliet et al. | 2010 | Mice colonic tissues | Elisa for measurement of intestinal permeability. PCR Mucosal cytokine measurements | Gut dysbosis associated to toxicity induced by chemotherapy: ↓Bifidobacteria ↓Lactobacillus, ↓Faecalibacterium ↓Clostridium | |
| Montassier et al. | 2015 | Patients with non-Hodgkin’s lymphoma who received the same myeloablative conditioning regimen and no other concomitant therapy such as antibiotic (n = 28) | Amplification and sequencing of 16S rRNA genes in faecal samples | Gut dysbosis associated to toxicity induced by chemotherapy: ↑Bacteroides, ↑Enterococcus ↑Enterobacteriaceae ↓Firmicutes (Ruminococcaceae, Lachnospiraceae), ↓Actinobacteria (Bifidobacterium), ↑Citrobacter, ↓Ruminococcus, ↓Coprococcus, ↓Dorea, ↓Lachnospira, ↓Roseburia | ||
| Radiotherapy | Yan et al. | 2007 | Colon organ culture | Purification and analizing of proteins from Lactobacillus | Lactobacillus rhamnosus, Lactobacillus casei and Lactobacillus acidophilus have protective roles in minimizing the damage caused by radiation therapy | |
| Ciorba et al. | 2012 | Mice small intestine | Protein and nucleic acid analysis | Administration of Lactobacillus rhamnosus before radiotherapy decreases epithelial apoptosis and stimulates crypt survival in mice guts | ||
| Delia et al. | 2007 | Patients who underwent surgery for sigmoid, rectal, or cervical cancer (n = 429) | Probiotic oral gavage of Lactobacillus spp. and Bifidobacterium spp. | Microbiome that can reduce gut toxicity induced by radiotherapy: Lactobacillus (L. casei, L. plantarum, L. acidophilus, and L. delbrueckii subsp. bulgaricus) Bifidobacteria (B. longum, B. breve, and B. infantis) | ||
| Targeted therapy | Trastuzumab | Di Modica et al. | 2021 | Female mice with breast cancer Patients with breast cancer (n = 24) | Faecal microbial transplantation in mice and faecal sample analysis of variable region V3 and V4 of the 16S rRNA gene for mice and human. | Gut microbiome of responders: ↑Clostridiales (Lachnospiraceae), ↑Bifidobacteriaceae, ↑Turicibacteracea, ↑Bacteroidales (Prevotellaceae) Gut microbiome of non-responders: ↑Bacteroidetes (Bacteroidia) |
| Erlotinib and gefitinib | Flórez et al. | 2016 | Bacterial strains | Determination of minimum inhibitory concentrations | 34 species of lactic acid bacteria, Bifidobacteria, and other intestinal bacteria are resistant to treatment with erlotinib and gefitinib | |
| Letrozole | Cao et al. | 2020 | Rats (8 normal controls and 30 with endometriosis) | Amplification and sequencing of 16S rRNA genes in faecal samples | ↓Firmicutes/Bacteroidetes ratio, ↓inflammation, ↓Ruminococcaceae | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutriq, S.; González-González, A.; Plaza-Andrades, I.; Laborda-Illanes, A.; Sánchez-Alcoholado, L.; Peralta-Linero, J.; Domínguez-Recio, M.E.; Bermejo-Pérez, M.J.; Lavado-Valenzuela, R.; Alba, E.; et al. Gut and Endometrial Microbiome Dysbiosis: A New Emergent Risk Factor for Endometrial Cancer. J. Pers. Med. 2021, 11, 659. https://doi.org/10.3390/jpm11070659
Boutriq S, González-González A, Plaza-Andrades I, Laborda-Illanes A, Sánchez-Alcoholado L, Peralta-Linero J, Domínguez-Recio ME, Bermejo-Pérez MJ, Lavado-Valenzuela R, Alba E, et al. Gut and Endometrial Microbiome Dysbiosis: A New Emergent Risk Factor for Endometrial Cancer. Journal of Personalized Medicine. 2021; 11(7):659. https://doi.org/10.3390/jpm11070659
Chicago/Turabian StyleBoutriq, Soukaina, Alicia González-González, Isaac Plaza-Andrades, Aurora Laborda-Illanes, Lidia Sánchez-Alcoholado, Jesús Peralta-Linero, María Emilia Domínguez-Recio, María José Bermejo-Pérez, Rocío Lavado-Valenzuela, Emilio Alba, and et al. 2021. "Gut and Endometrial Microbiome Dysbiosis: A New Emergent Risk Factor for Endometrial Cancer" Journal of Personalized Medicine 11, no. 7: 659. https://doi.org/10.3390/jpm11070659
APA StyleBoutriq, S., González-González, A., Plaza-Andrades, I., Laborda-Illanes, A., Sánchez-Alcoholado, L., Peralta-Linero, J., Domínguez-Recio, M. E., Bermejo-Pérez, M. J., Lavado-Valenzuela, R., Alba, E., & Queipo-Ortuño, M. I. (2021). Gut and Endometrial Microbiome Dysbiosis: A New Emergent Risk Factor for Endometrial Cancer. Journal of Personalized Medicine, 11(7), 659. https://doi.org/10.3390/jpm11070659






