Association of Ventilatory Disorders with Respiratory Symptoms, Physical Activity, and Quality of Life in Subjects with Prior Tuberculosis: A National Database Study in Korea
Abstract
:1. Introduction
2. Material and Methods
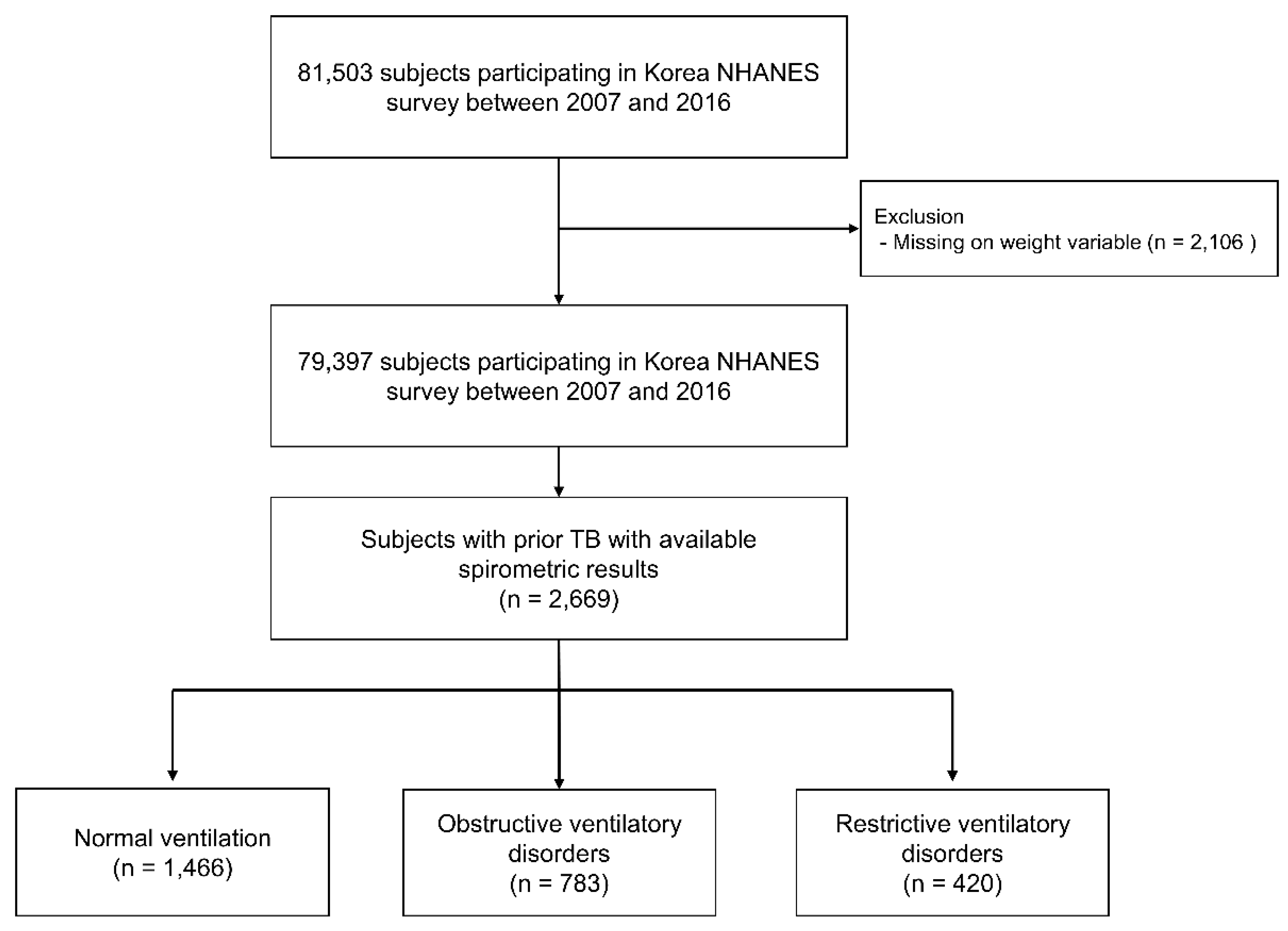
2.1. Study Population
2.2. Measurements
2.3. Definitions of Ventilatory Disorder
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Comparison of Symptoms, Physical Activity, and Quality of Life
3.3. The Impact of Obstructive Ventilatory Disorder and Its Severity on Respiratory Symptoms, Physical Activity Limitations, and EQ-5D Index in Subjects with Prior TB
3.4. The Impact of Restrictive Ventilatory Disorder and Its Severity on Respiratory Symptoms, Physical Activity Limitations, and EQ-5D Index Value in Subjects with Prior TB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2020: Executive Summary; WHO: Geneva, Switzerland, 2020.
- Meghji, J.; Lesosky, M.; Joekes, E.; Banda, P.; Rylance, J.; Gordon, S.; Jacob, J.; Zonderland, H.; MacPherson, P.; Corbett, E.L.; et al. Patient outcomes associated with post-tuberculosis lung damage in Malawi: A prospective cohort study. Thorax 2020, 75, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hnizdo, E.; Singh, T.; Churchyard, G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax 2000, 55, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Plit, M.L.; Anderson, R.; Van Rensburg, C.E.; Page-Shipp, L.; Blott, J.A.; Fresen, J.L.; Feldman, C. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. Eur. Respir. J. 1998, 12, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, J.; Ehrlich, R.I.; Hnizdo, E.; White, N.; Churchyard, G.J. Excess lung function decline in gold miners following pulmonary tuberculosis. Thorax 2010, 65, 1010–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maguire, G.P.; Anstey, N.M.; Ardian, M.; Waramori, G.; Tjitra, E.; Kenangalem, E.; Handojo, T.; Kelly, P.M. Pulmonary tuberculosis, impaired lung function, disability and quality of life in a high-burden setting. Int. J. Tuberc. Lung Dis. 2009, 13, 1500–1506. [Google Scholar] [PubMed]
- Pasipanodya, J.G.; McNabb, S.J.; Hilsenrath, P.; Bae, S.; Lykens, K.; Vecino, E.; Munguia, G.; Miller, T.L.; Drewyer, G.; Weis, S.E. Pulmonary impairment after tuberculosis and its contribution to TB burden. BMC Public Health 2010, 10, 259. [Google Scholar] [CrossRef] [Green Version]
- Ravimohan, S.; Kornfeld, H.; Weissman, D.; Bisson, G.P. Tuberculosis and lung damage: From epidemiology to pathophysiology. Eur. Respir. Rev. 2018, 27. [Google Scholar] [CrossRef] [Green Version]
- Rhee, C.K.; Yoo, K.H.; Lee, J.H.; Park, M.J.; Kim, W.J.; Park, Y.B.; Hwang, Y.I.; Kim, Y.S.; Jung, J.Y.; Moon, J.Y.; et al. Clinical characteristics of patients with tuberculosis-destroyed lung. Int. J. Tuberc. Lung Dis. 2013, 17, 67–75. [Google Scholar] [CrossRef]
- Ralph, A.P.; Kenangalem, E.; Waramori, G.; Pontororing, G.J.; Sandjaja; Tjitra, E.; Maguire, G.P.; Kelly, P.M.; Anstey, N.M. High morbidity during treatment and residual pulmonary disability in pulmonary tuberculosis: Under-recognised phenomena. PLoS ONE 2013, 8, e80302. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, R.I.; Adams, S.; Baatjies, R.; Jeebhay, M.F. Chronic airflow obstruction and respiratory symptoms following tuberculosis: A review of South African studies. Int. J. Tuberc. Lung Dis. 2011, 15, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Manji, M.; Shayo, G.; Mamuya, S.; Mpembeni, R.; Jusabani, A.; Mugusi, F. Lung functions among patients with pulmonary tuberculosis in Dar es Salaam—A cross-sectional study. BMC Pulm. Med. 2016, 16, 58. [Google Scholar] [CrossRef] [Green Version]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.v.; Van der Grinten, C.; Gustafsson, P. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.K.; Paek, D.; Lee, J.O. Normal predictive values of spirometry in Korean population. Tuberc. Respir. Dis. 2005, 58, 230–242. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Shin, S.H.; Gu, S.; Zhao, D.; Kang, D.; Joi, Y.R.; Suh, G.Y.; Pastor-Barriuso, R.; Guallar, E.; Cho, J.; et al. Racial differences in comorbidity profile among patients with chronic obstructive pulmonary disease. BMC Med. 2018, 16, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, P.; Celli, B.; Agustí, A.; Boje Jensen, G.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2015, 373, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabe, K.F.; Hurd, S.; Anzueto, A.; Barnes, P.J.; Buist, S.A.; Calverley, P.; Fukuchi, Y.; Jenkins, C.; Rodriguez-Roisin, R.; van Weel, C.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2007, 176, 532–555. [Google Scholar] [CrossRef]
- Sim, Y.S.; Lee, J.H.; Lee, W.Y.; Suh, D.I.; Oh, Y.M.; Yoon, J.S.; Lee, J.H.; Cho, J.H.; Kwon, C.S.; Chang, J.H. Spirometry and Bronchodilator Test. Tuberc. Respir. Dis. 2017, 80, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Choi, J.C.; Shin, J.W.; Kim, J.Y.; Choi, B.W.; Park, I.W. Pulmonary Impairment in Tuberculosis Survivors: The Korean National Health and Nutrition Examination Survey 2008–2012. PLoS ONE 2015, 10, e0141230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, K.B.; Jiang, C.Q.; Jordan, R.E.; Miller, M.R.; Zhang, W.S.; Cheng, K.K.; Lam, T.H.; Adab, P. Prior TB, smoking, and airflow obstruction: A cross-sectional analysis of the Guangzhou Biobank Cohort Study. Chest 2010, 137, 593–600. [Google Scholar] [CrossRef]
- Snider, G.L.; Doctor, L.; Demas, T.A.; Shaw, A.R. Obstructive airway disease in patients with treated pulmonary tuberculosis. Am. Rev. Respir. Dis. 1971, 103, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Pasipanodya, J.G.; Vecino, E.; Miller, T.L.; Munguia, G.; Drewyer, G.; Fernandez, M.; Slocum, P.; Weis, S.E. Non-hispanic whites have higher risk for pulmonary impairment from pulmonary tuberculosis. BMC Public Health 2012, 12, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Naso, F.C.; Pereira, J.S.; Schuh, S.J.; Unis, G. Functional evaluation in patients with pulmonary tuberculosis sequelae. Rev. Port. Pneumol. 2011, 17, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Miguel-Reyes, J.L.; Gochicoa-Rangel, L.; Pérez-Padilla, R.; Torre-Bouscoulet, L. Functional respiratory assessment in interstitial lung disease. Rev. Investig. Clin. 2015, 67, 5–14. [Google Scholar]
- Pasipanodya, J.G.; Miller, T.L.; Vecino, M.; Munguia, G.; Garmon, R.; Bae, S.; Drewyer, G.; Weis, S.E. Pulmonary impairment after tuberculosis. Chest 2007, 131, 1817–1824. [Google Scholar] [CrossRef]
- De Vallière, S.; Barker, R.D. Residual lung damage after completion of treatment for multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 2004, 8, 767–771. [Google Scholar]
- Gandhi, K.; Gupta, S.; Singla, R. Risk factors associated with development of pulmonary impairment after tuberculosis. Indian J. Tuberc. 2016, 63, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Willcox, P.A.; Ferguson, A.D. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir. Med. 1989, 83, 195–198. [Google Scholar] [CrossRef]
- Kim, C.-J.; Yoon, H.-K.; Park, M.-J.; Yoo, K.-H.; Jung, K.-S.; Park, J.-W.; Lim, S.Y.; Shim, J.J.; Lee, Y.C.; Kim, Y.-S.; et al. Inhaled indacaterol for the treatment of COPD patients with destroyed lung by tuberculosis and moderate-to-severe airflow limitation: Results from the randomized INFINITY study. Int. J. Chronic Obstruct. Pulmon. Dis. 2017, 12, 1589–1596. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.C.; Kim, T.H.; Kim, Y.-J.; Rhee, C.K.; Oh, Y.-M. Effect of tiotropium inhaler use on mortality in patients with tuberculous destroyed lung: Based on linkage between hospital and nationwide health insurance claims data in South Korea. Respir. Res. 2019, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, M.S.; Jankowich, M.D. The Vital Capacity Is Vital: Epidemiology and Clinical Significance of the Restrictive Spirometry Pattern. Chest 2016, 149, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Mannino, D.M.; Doherty, D.E.; Sonia Buist, A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: Findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir. Med. 2006, 100, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.J.; Kim, H.I.; Yang, B.; Kim, T.; Sim, Y.S.; Kang, H.K.; Kim, S.H.; Yoon, H.J.; Choi, H.; Lee, H. Impact of the severity of restrictive spirometric pattern on nutrition, physical activity, and quality of life: Results from a nationally representative database. Sci. Rep. 2020, 10, 19672. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Sherrill, D.L.; Venker, C.; Ceccato, C.M.; Halonen, M.; Martinez, F.D. Morbidity and mortality associated with the restrictive spirometric pattern: A longitudinal study. Thorax 2010, 65, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Nonato, N.L.; Nascimento, O.A.; Padilla, R.P.; de Oca, M.M.; Tálamo, C.; Valdivia, G.; Lisboa, C.; López, M.V.; Celli, B.; Menezes, A.M.; et al. Occurrence of respiratory symptoms in persons with restrictive ventilatory impairment compared with persons with chronic obstructive pulmonary disease: The PLATINO study. Chronic Respir. Dis. 2015, 12, 264–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Total (n = 2669) | Normal Ventilation (n = 1466) | Obstructive Ventilatory Disorder (n = 783) | Restrictive Ventilatory Disorder (n = 420) | p Value | |
|---|---|---|---|---|---|
| Age, years | 57.5 (56.7–58.3) | 53.4 (52.5–65.5) | 64.4 (63.2–65.5) a | 59.6 (57.7–61.4) b,c | <0.001 |
| Male sex | 57.7 (55.2–60.2) | 49.5 (45.9–53.1) | 76.2 (72.1–79.8) a | 52.0 (45.2–58.8) c | <0.001 |
| BMI, kg/m2 | 23.4 (23.3–23.6) | 23.7 (23.5–23.9) | 22.8 (22.5–23.1) a | 23.8 (23.4–24.3) c | <0.001 |
| Smoking history | <0.001 | ||||
| Never-smoker | 48.2 (45.6–50.8) | 55.5 (51.8–59.2) | 28.2 (24.1–32.6) a | 60.6 (53.5–67.2) b,c | |
| Current- or ex-smoker | 51.8 (49.2–54.4) | 44.5 (40.8–59.2) | 71.8 (67.4–75.9) a | 39.4 (32.8–46.5) b,c | |
| Smoking amount, pack-years | 7.3 (6.3–8.2) | 5.5 (4.4–6.6) | 13.3 (10.9–15.5) a | 5.4 (3.4–7.4) b,c | |
| Family income | <0.001 | ||||
| Low | 52.1 (49.2–55.0) | 43.0 (39.2–46.8) | 68.3 (63.4–72.8) a | 55.9 (49.0–62.6) b | |
| High | 47.9 (45.0–50.8) | 57.0 (53.2–60.8) | 31.7 (27.2–36.6) a | 44.1 (37.4–51.0) b | |
| Education | <0.001 | ||||
| High school or less | 79.1 (76.7–81.3) | 74.9 (71.6–78.0) | 86.5 (82.5–89.7) a | 80.4 (73.9–89.7) | |
| More than high school | 20.9 (18.7–23.3) | 25.1 (22.0–28.4) | 13.5 (10.1–17.5) a | 19.6 (14.4–26.1) | |
| Comorbidities | |||||
| Asthma | 4.9 (3.9–6.1) | 2.3 (1.4–3.6) | 10.9 (8.3–14.1) a | 2.8 (1.4–5.3) b,c | <0.001 |
| Diabetes mellitus | 12.4 (10.7–14.3) | 8.3 (6.5–10.5) | 19.1 (15.5–23.4) a | 15.0 (10.9–20.2) b | <0.001 |
| Hypertension | 41.4 (38.7–44.1) | 35.0 (31.7–38.6) | 50.8 (45.7–55.8) a | 47.2 (40.4–54.1) b | <0.001 |
| Dyslipidemia | 44.1 (41.4–47.0) | 42.9 (39.2–46.7) | 44.5 (39.5–49.7) | 48.4 (41.5–55.3) | 0.413 |
| Cardiovascular disease | 5.1 (4.0–6.5) | 4.5 (3.3–6.2) | 5.3 (3.5–7.9) | 7.3 (4.1–12.6) | 0.287 |
| Osteoporosis | 8.2 (6.5–10.3) | 7.4 (5.5–10.3) | 7.7 (4.6–12.5) | 11.9 (7.3–19.0) | 0.297 |
| Arthritis | 15.1 (13.4–16.9) | 15.4 (12.4–18.0) | 12.9 (10.3–16.0) | 18.3 (13.9–23.8) | 0.140 |
| Depression | 4.5 (3.5–5.6) | 4.4 (3.2–6.1) | 4.6 (2.9–7.1) | 4.4 (2.4–7.7) | 0.993 |
| Spirometry | |||||
| FVC, L | 3.5 (3.4–3.5) | 3.7 (3.6–3.8) | 3.4 (3.3–3.5) a | 2.7 (2.6–2.8) b,c | <0.001 |
| FVC, % predicted | 88.7 (88.0–89.4) | 94.4 (93.6–95.1) | 85.5 (84.1–86.9) a | 72.6 (71.5–73.7) b,c | <0.001 |
| FEV1, L | 2.6 (2.5–2.6) | 2.9 (2.8–3.0) | 2.0 (2.0–2.1) a | 2.1 (2.0–2.2) b,c | <0.001 |
| FEV1, % predicted | 84.7 (83.8–85.5) | 94.4 (93.6–95.1) | 70.8 (69.3–72.4) a | 74.3 (73.1–75.6) b,c | <0.001 |
| FEV1/FVC | 73.3 (72.8–74.0) | 78.9 (78.6–79.3) | 60.6 (59.7–61.4) a | 78.0 (77.2–78.7)b,c | <0.001 |
| Total (n = 2669) | Normal Ventilation (n = 1466) | Obstructive Ventilatory Disorder (n = 783) | Restrictive Ventilatory Disorder (n = 420) | p Value | |
|---|---|---|---|---|---|
| Any respiratory symptoms | 16.4 (14.0–19.3) | 12.4 (9.5–16.1) | 22.9 (18.1–28.5) a | 18.6 (12.6–26.6) | 0.001 |
| Cough | 7.3 (5.7–9.2) | 5.4 (3.5–8.3) | 11.6 (8.5–15.8) a | 5.1 (2.8–9.3) | 0.004 |
| Sputum | 13.0 (10.8–15.5) | 9.5 (7.0–12.8) | 18.2 (13.9–23.5) a | 15.1 (9.5–23.2) | 0.004 |
| Dyspnea | 2.0 (1.3–3.1) | 0.9 (0.3–2.5) | 3.8 (2.1–6.7) | 2.4 (0.9–5.9) | 0.020 |
| Physical activity limitations | 15.6 (11.2–21.2) | 5.1 (2.3–10.9) | 27.7 (19.3–38.0) a | 13.0 (6.2–28.4) | <0.001 |
| EQ-5D component | |||||
| Mobility | 18.3 (16.4–20.3) | 14.0 (11.9–16.4) | 24.1 (20.4–28.1) a | 23.5 (18.1–29.8) b | <0.001 |
| Self-care | 5.2 (4.3–6.6) | 3.6 (2.6–5.0) | 7.7 (5.5–10.7) a | 7.3 (4.3–12.4) | 0.005 |
| Usual activity | 12.8 (11.1–14.6) | 9.4 (7.7–11.5) | 16.3 (13.1–20.1) a | 19.1 (14.2–25.2) b | <0.001 |
| Pain/discomfort | 29.0 (26.6–31.5) | 30.7 (26.5–35.2) | 30.1 (26.5–35.2) | 36.3 (29.8–43.4) | 0.015 |
| Anxiety/depression | 13.6 (11.9–15.5) | 13.5 (11.3–16.1) | 13.5 (10.6–17.1) | 13.7 (9.5–17.5) | 0.999 |
| EQ-5D index | 0.93 (0.92–0.93) | 0.94 (0.93–0.94) | 0.91 (0.90–0.93) a | 0.91 (0.89–0.93) b,c | 0.002 |
| Model | Normal (n = 1466) | Obstructive Ventilatory Disorder | |||
|---|---|---|---|---|---|
| Mild (n = 256) | Moderate (n = 432) | Severe (n = 95) | |||
| Respiratory symptoms | Crude model | Reference | 1.52 (0.83, 2.81) | 1.67 (1.03, 2.71) | 8.38 (4.16, 16.88) |
| Adjusted model * | Reference | 1.09 (0.51, 2.33) | 1.05 (0.53, 2.08) | 13.62 (4.64, 39.99) | |
| Cough | Crude model | Reference | 2.45 (1.08, 5.56) | 1.74 (0.87, 3.46) | 4.99 (2.04, 12.28) |
| Adjusted model * | Reference | 1.81 (0.69, 4.76) | 1.13 (0.47, 2.75) | 3.87 (1.14, 13.21) | |
| Sputum | Crude model | Reference | 1.71 (0.91, 3.22) | 1.69 (1.01, 2.84) | 6.84 (3.24, 14.44) |
| Adjusted model * | Reference | 1.34 (0.59, 3.05) | 1.18 (0.56, 2.51) | 11.39 (3.17, 40.94) | |
| Dyspnea | Crude model | Reference | 1.95 (0.43, 8.88) | 2.12 (0.56, 7.98) | 21.58 (5.51, 84.46) |
| Adjusted model * | Reference | 1.41 (0.30, 6.72) | 3.56 (0.68, 18.67) | 21.42 (3.50, 13.13) | |
| Physical activity limitations | Crude model | Reference | 3.55 (0.81, 15.46) | 3.60 (1.25, 10.38) | 38.35 (10.80, 136.15) |
| Adjusted model * | Reference | 7.83 (0.99, 61.27) | 5.59 (0.93, 33.51) | 218.58 (26.82, 1781.12) | |
| EQ-5D index | Crude model | Reference | −0.01 (−0.26, 0.13) | −0.02 (−0.04, −0.001) | −0.07 (−0.11, −0.02) |
| Adjusted model * | Reference | 0.01 (−0.01, 0.04) | −0.003 (−0.03, 0.02) | −0.06 (−0.12, −0.01) | |
| Model | Normal (n = 1466) | Restrictive Ventilatory Disorder | |||
|---|---|---|---|---|---|
| Mild (n = 306) | Moderate (n = 77) | Severe (n = 37) | |||
| Respiratory symptoms | Crude model | Reference | 1.95 (1.09, 3.49) | 0.95 (0.30, 3.06) | 0.59 (0.11, 3.24) |
| Adjusted model * | Reference | 2.10 (1.07, 4.14) | 1.10 (0.28, 4.37) | 0.87 (0.14, 5.55) | |
| Cough | Crude model | Reference | 0.96 (0.39, 2.38) | 0.77 (0.16, 3.80) | 1.11 (0.14, 8.98) |
| Adjusted model * | Reference | 0.57 (0.19, 1.66) | 0.85 (0.14, 4.82) | 1.34 (0.14, 12.55) | |
| Sputum | Crude model | Reference | 2.23 (1.16, 4.26) | 0.42 (0.09, 2.00) | 0.61 (0.08, 4.77) |
| Adjusted model * | Reference | 2.80 (1.34, 5.83) | 0.46 (0.08, 2.72) | 0.84 (0.08, 8.70) | |
| Dyspnea | Crude model | Reference | 1.20 (0.21, 6.82) | 8.56 (1.43, 51.34) | 1.94 (0.20, 18.40) |
| Adjusted model * | Reference | 0.98 (0.12, 7.94) | 18.20 (3.05, 108.58) | 3.04 (0.17, 53.88) | |
| Physical activity limitation | Crude model | Reference | 2.29 (0.46, 11.38) | 3.58 (0.71, 18.02) | 5.34 (0.85, 33.71) |
| Adjusted model * | Reference | 1.92 (0.20, 18.54) | 5.71 (1.14, 28.62) | 9.17 (1.02, 82.22) | |
| EQ-5D index | Crude model | Reference | −0.01 (−0.03, 0.01) | −0.09 (−0.17, −0.01) | −0.05(−0.09, −0.001) |
| Adjusted model * | Reference | −0.01(−0.21, 0.02) | −0.08 (−0.18, 0.02) | −0.021 (−0.08, 0.04) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Choi, H.; Shin, S.H.; Kim, Y.; Moon, J.-Y.; Park, H.Y.; Lee, H. Association of Ventilatory Disorders with Respiratory Symptoms, Physical Activity, and Quality of Life in Subjects with Prior Tuberculosis: A National Database Study in Korea. J. Pers. Med. 2021, 11, 678. https://doi.org/10.3390/jpm11070678
Yang B, Choi H, Shin SH, Kim Y, Moon J-Y, Park HY, Lee H. Association of Ventilatory Disorders with Respiratory Symptoms, Physical Activity, and Quality of Life in Subjects with Prior Tuberculosis: A National Database Study in Korea. Journal of Personalized Medicine. 2021; 11(7):678. https://doi.org/10.3390/jpm11070678
Chicago/Turabian StyleYang, Bumhee, Hayoung Choi, Sun Hye Shin, Youlim Kim, Ji-Yong Moon, Hye Yun Park, and Hyun Lee. 2021. "Association of Ventilatory Disorders with Respiratory Symptoms, Physical Activity, and Quality of Life in Subjects with Prior Tuberculosis: A National Database Study in Korea" Journal of Personalized Medicine 11, no. 7: 678. https://doi.org/10.3390/jpm11070678
APA StyleYang, B., Choi, H., Shin, S. H., Kim, Y., Moon, J.-Y., Park, H. Y., & Lee, H. (2021). Association of Ventilatory Disorders with Respiratory Symptoms, Physical Activity, and Quality of Life in Subjects with Prior Tuberculosis: A National Database Study in Korea. Journal of Personalized Medicine, 11(7), 678. https://doi.org/10.3390/jpm11070678







