Personalized Cancer Medicine in the Media: Sensationalism or Realistic Reporting?
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Coding Instrument
3. Results
3.1. Defining Personalized Medicine
3.2. Benefits and Challenges of Personalized Medicine
3.3. Genetic Testing
3.4. Targeted Therapies Reported
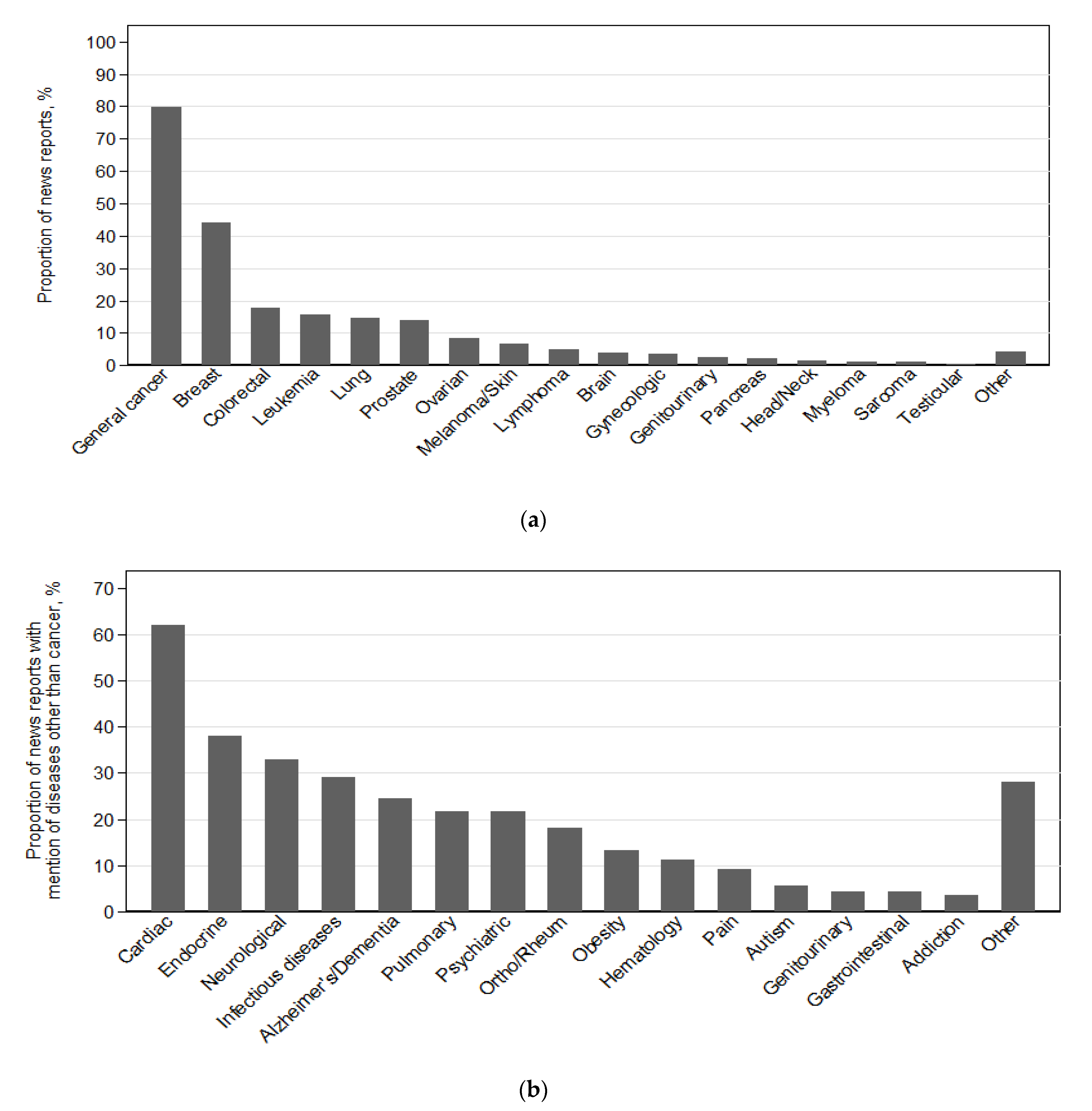
3.5. Types of Cancer and Other Diseases Reported
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Singer, D.S.; Jacks, T.; Jaffee, E. A US “Cancer Moonshot” to accelerate cancer research. Science 2016, 353, 1105–1106. [Google Scholar] [CrossRef]
- National Cancer Institute. Cancer MoonshotSM—Funding Opportunities. Available online: https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/funding (accessed on 5 May 2015).
- Cherny, N.I.; de Vries, E.G.; Emanuel, L.; Fallowfield, L.; Francis, P.A.; Gabizon, A.; Piccart, M.J.; Sidransky, D.; Soussan-Gutman, L.; Tziraki, C. Words matter: Distinguishing “personalized medicine” and “biologically personalized therapeutics”. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 5.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 4 July 2019).
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast and Ovarian (Version 3.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf (accessed on 4 July 2019).
- National Comprehensive Cancer Network. Chronic Myeloid Leukemia (Version 1.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf (accessed on 4 July 2019).
- National Comprehensive Cancer Network. Cutaneous Melanoma (Version 2.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf (accessed on 4 July 2019).
- National Comprehensive Cancer Network. Uveal Melanoma (Version 1.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/uveal.pdf (accessed on 4 July 2019).
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Colorectal (Version 1.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf (accessed on 4 July 2019).
- Rutten, L.J.F.; Arora, N.K.; Bakos, A.D.; Aziz, N.; Rowland, J. Information needs and sources of information among cancer patients: A systematic review of research (1980–2003). Patient Educ. Couns. 2005, 57, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.P.; Kanter, E.J.; Bednarczyk, B.; Tastad, P.L. Importance of the lay press in the transmission of medical knowledge to the scientific community. N. Engl. J. Med. 1991, 325, 1180–1183. [Google Scholar] [CrossRef]
- Rogers, E.M. Diffusion of Innovations; Simon and Schuster: New York, NY, USA, 2010. [Google Scholar]
- Briggs, C.L.; Hallin, D.C. Making Health Public: How News Coverage is Remaking Media, Medicine, and Contemporary Life; Routledge: London, UK, 2016. [Google Scholar]
- Passalacqua, R.; Caminiti, C.; Salvagni, S.; Barni, S.; Beretta, G.D.; Carlini, P.; Contu, A.; Di Costanzo, F.; Toscano, L.; Campione, F. Effects of media information on cancer patients’ opinions, feelings, decision-making process and physician-patient communication. Cancer 2004, 100, 1077–1084. [Google Scholar] [CrossRef]
- Hay, J.; Coups, E.J.; Ford, J.; DiBonaventura, M. Exposure to mass media health information, skin cancer beliefs, and sun protection behaviors in a United States probability sample. J. Am. Acad. Dermatol. 2009, 61, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Grilli, R.; Ramsay, C.; Minozzi, S. Mass media interventions: Effects on health services utilisation. Cochrane Database Syst. Rev. 2002, CD000389. [Google Scholar] [CrossRef]
- Booth, C.M.; Dranitsaris, G.; Gainford, M.C.; Berry, S.; Fralick, M.; Fralick, J.; Sue, J.; Clemons, M. External influences and priority-setting for anti-cancer agents: A case study of media coverage in adjuvant trastuzumab for breast cancer. BMC Cancer 2007, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, R.; Chapman, S.; Salkeld, G.; Holding, S. Media influence on Herceptin subsidization in Australia: Application of the rule of rescue? J. R. Soc. Med. 2008, 101, 305–312. [Google Scholar] [CrossRef]
- Moynihan, R.; Bero, L.; Ross-Degnan, D.; Henry, D.; Lee, K.; Watkins, J.; Mah, C.; Soumerai, S.B. Coverage by the news media of the benefits and risks of medications. N. Engl. J. Med. 2000, 342, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Canales, M.K.; Breslau, E.S.; Nelson, D.E.; Ballard-Barbash, R.R. Did news reporters get it right? Translation of the 2002 hormone study findings. Am. J. Prev. Med. 2008, 34, 61–68. [Google Scholar] [CrossRef]
- Fishman, J.; Ten Have, T.; Casarett, D. Cancer and the media: How does the news report on treatment and outcomes? Arch. Intern. Med. 2010, 170, 515–518. [Google Scholar] [CrossRef]
- Schwartz, L.M.; Woloshin, S. On the prevention and treatment of exaggeration. J. Gen. Intern. Med. 2003, 18, 153–154. [Google Scholar] [CrossRef]
- Nagler, R.H.; Fowler, E.F.; Marino, N.M.; Mentzer, K.M.; Gollust, S.E. The Evolution of Mammography Controversy in the News Media: A Content Analysis of Four Publicized Screening Recommendations, 2009 to 2016. Women’s Health Issues Off. Publ. Jacobs Inst. Women’s Health 2019, 29, 87–95. [Google Scholar] [CrossRef]
- Bubela, T.M.; Caulfield, T.A. Do the print media “hype“ genetic research? A comparison of newspaper stories and peer-reviewed research papers. CMAJ 2004, 170, 1399–1407. [Google Scholar] [CrossRef]
- Holtzman, N.A.; Bernhardt, B.A.; Mountcastle-Shah, E.; Rodgers, J.E.; Tambor, E.; Geller, G. The quality of media reports on discoveries related to human genetic diseases. Community Genet. 2005, 8, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Rogith, D.; Yusuf, R.A.; Hovick, S.R.; Fellman, B.M.; Peterson, S.K.; Burton-Chase, A.M.; Li, Y.; Bernstam, E.V.; Meric-Bernstam, F. Patient knowledge and information-seeking about personalized cancer therapy. Int. J. Med. Inform. 2016, 88, 52–57. [Google Scholar] [CrossRef]
- Finnegan, J.R., Jr.; Viswanath, K.; Hertog, J. Mass media, secular trends, and the future of cardiovascular disease health promotion: An interpretive analysis. Prev. Med. 1999, 29, S50–S58. [Google Scholar] [CrossRef]
- Stamm, K.; Williams, J.W.; Noël, P.H.; Rubin, R. Helping journalists get it right. J. Gen. Intern. Med. 2003, 18, 138–145. [Google Scholar] [CrossRef]
- Martinson, B.E.; Hindman, D.B. Building a health promotion agenda in local newspapers. Health Educ. Res. 2004, 20, 51–60. [Google Scholar] [CrossRef]
- Valenti, J.M.; Tavana, G. Report: Continuing science education for environmental journalists and science writers: In situ with the experts. Sci. Commun. 2005, 27, 300–310. [Google Scholar] [CrossRef]
- National Human Genome Research Institute. Genetic Information Nondiscrimination Act (GINA) of 2008. Available online: https://www.genome.gov/24519851/genetic-information-nondiscrimination-act-of-2008 (accessed on 2 July 2019).
- Gray, S.W.; Cronin, A.; Bair, E.; Lindeman, N.; Viswanath, V.; Janeway, K.A. Marketing of personalized cancer care on the web: An analysis of Internet websites. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Gray, S.W.; Hicks-Courant, K.; Lathan, C.S.; Garraway, L.; Park, E.R.; Weeks, J.C. Attitudes of Patients With Cancer About Personalized Medicine and Somatic Genetic Testing. J. Oncol. Pract. 2012, 8, 329–335. [Google Scholar] [CrossRef]
- Personalized Medicine Coalition. National Survey Finds Americans See Benefit and Value in Personalized Care. Majority of Americans Want Coverage for Personalized Prevention and Treatment Plans. 2014. Available online: https://www.businesswire.com/news/home/20140722005513/en/National-Survey-Finds-Americans-See-Benefit-and-Value-in-Personalized-Care (accessed on 31 May 2015).
- Schleidgen, S.; Klingler, C.; Bertram, T.; Rogowski, W.H.; Marckmann, G. What is personalized medicine: Sharpening a vague term based on a systematic literature review. BMC Med. Ethics 2013, 14, 55. [Google Scholar] [CrossRef]
- Redekop, W.K.; Mladsi, D. The faces of personalized medicine: A framework for understanding its meaning and scope. Value Health J. Int. Soc. Pharm. Outcomes Res. 2013, 16, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Aronson, N. Making personalized medicine more affordable. Ann. N. Y. Acad. Sci. 2015, 1346, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Frueh, F.W. Regulation, reimbursement, and the long road of implementation of personalized medicine—A perspective from the United States. Value Health 2013, 16, S27–S31. [Google Scholar] [CrossRef][Green Version]
- Meckley, L.M.; Neumann, P.J. Personalized medicine: Factors influencing reimbursement. Health Policy 2010, 94, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.; Annemans, L. Reflections on market access for personalized medicine: Recommendations for Europe. Value Health 2013, 16, S32–S38. [Google Scholar] [CrossRef][Green Version]
- Van Allen, E.M.; Wagle, N.; Levy, M.A. Clinical analysis and interpretation of cancer genome data. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 1825–1833. [Google Scholar] [CrossRef]
- Harvey, E.K.; Fogel, C.E.; Peyrot, M.; Christensen, K.D.; Terry, S.F.; McInerney, J.D. Providers’ knowledge of genetics: A survey of 5915 individuals and families with genetic conditions. Genet. Med. 2007, 9, 259. [Google Scholar] [CrossRef]
- Nisbet, M.C.; Lewenstein, B.V. Biotechnology and the American media: The policy process and the elite press, 1970 to 1999. Sci. Commun. 2002, 23, 359–391. [Google Scholar] [CrossRef]
- Horst, M. Public expectations of gene therapy: Scientific futures and their performative effects on scientific citizenship. Sci. Technol. Hum. Values 2007, 32, 150–171. [Google Scholar] [CrossRef]
- Kohn, D.B.; Candotti, F. Gene therapy fulfilling its promise. N. Engl. J. Med. 2009, 360, 518. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, Z.Y.; Tumblin, A.R.; Kato, G.J. Current therapy of sickle cell disease. Haematologica 2006, 91, 7. [Google Scholar]
- Nathwani, A.C.; Tuddenham, E.G.; Rangarajan, S.; Rosales, C.; McIntosh, J.; Linch, D.C.; Chowdary, P.; Riddell, A.; Pie, A.J.; Harrington, C. Adenovirus-associated virus vector–mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011, 365, 2357–2365. [Google Scholar] [CrossRef]
- McGuire, A.L.; Evans, B.J.; Caulfield, T.; Burke, W. Regulating direct-to-consumer personal genome testing. Science 2010, 330, 181–182. [Google Scholar] [CrossRef]
- Hudson, K.L. Genetic testing oversight. Science 2006, 313, 1853. [Google Scholar] [CrossRef][Green Version]
- Hudson, K.; Javitt, G.; Burke, W.; Byers, P. ASHG Statement* on direct-to-consumer genetic testing in the United States. Am. J. Hum. Genet. 2007, 81, 635–637. [Google Scholar] [CrossRef]
- Evans, J.P.; Watson, M.S. Genetic testing and FDA regulation: Overregulation threatens the emergence of genomic medicine. JAMA 2015, 313, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratories. FDA Notification and Medical Device Reporting for Laboratory Developed Tests (LDTs). Available online: https://www.fda.gov/media/89837/download (accessed on 17 August 2015).
- Schwartz, L.M.; Woloshin, S. The media matter: A call for straightforward medical reporting. Ann. Intern. Med. 2004, 140, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Marcon, A.R.; Bieber, M.; Caulfield, T. Representing a “revolution”: How the popular press has portrayed personalized medicine. Genet. Med. 2018, 20, 950. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.J. Race, risk, and recreation in personal genomics: The limits of play. Med. Anthropol. Q. 2013, 27, 550–569. [Google Scholar] [CrossRef]
- Kaye, J. The regulation of direct-to-consumer genetic tests. Hum. Mol. Genet. 2008, 17, R180–R183. [Google Scholar] [CrossRef]

| Personalized Medicine | N (%) |
|---|---|
| Definition of personalized medicine | |
| No (not defined at all) | 110 (28) |
| Yes (clearly defined) | 107 (27) |
| Ambiguous | 179 (45) |
| Benefits of personalized medicine reported * (n = 286) | |
| No | 11 (4) |
| Yes | 275 (96) |
| Challenges of personalized medicine reported * (n = 286) | |
| No | 149 (52) |
| Yes | 137 (48) |
| Genetics | |
| Genetics mentioned | |
| No | 47 (12) |
| Yes | 349 (88) |
| Genomic testing mentioned (n = 349) | |
| No | 62 (18) |
| Yes | 287 (82) |
| Specific genetic tests mentioned (n = 287) | |
| No | 217 (76) |
| Yes | 70 (24) |
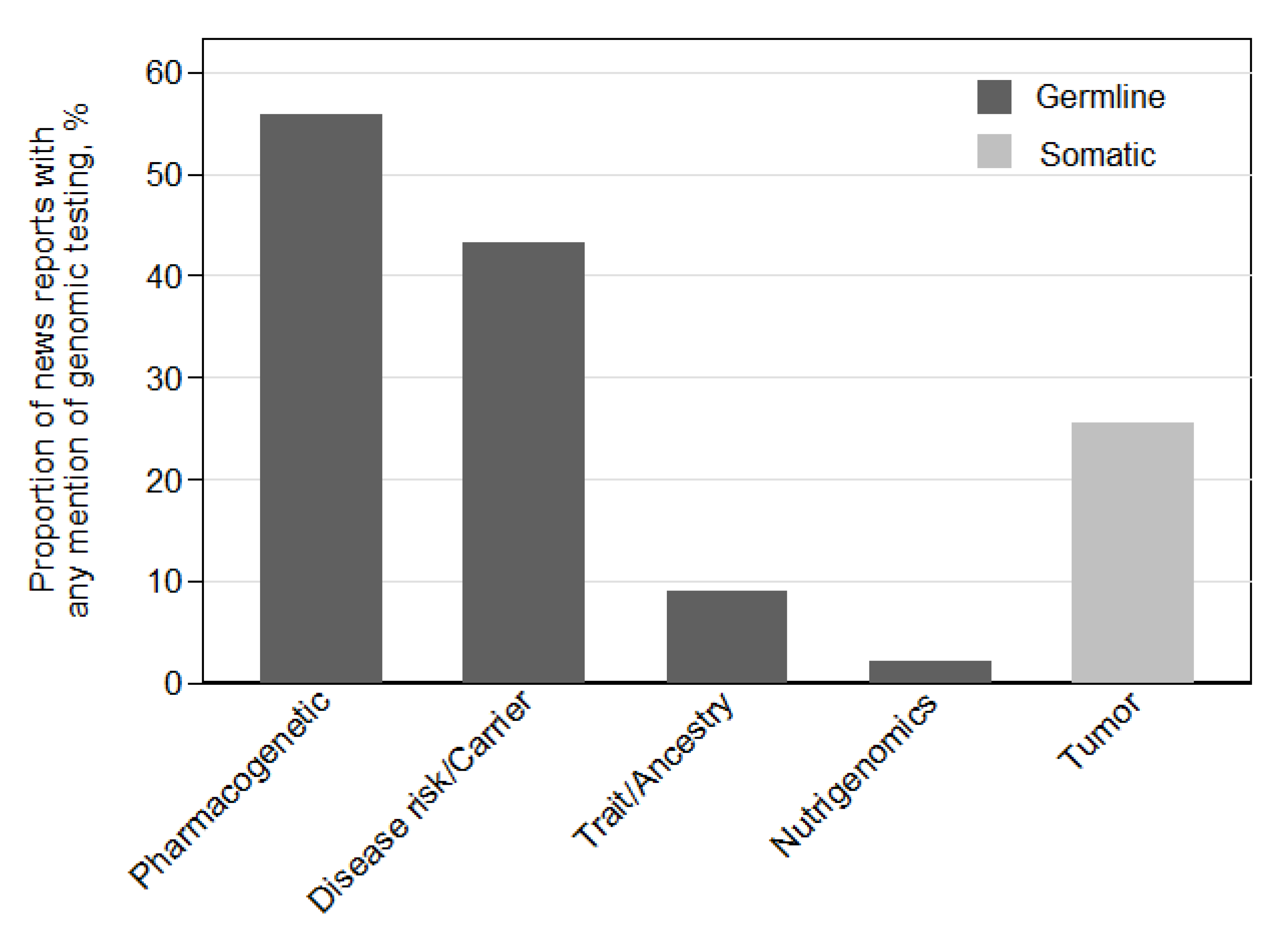
| Germline testing mentioned * (n = 287) | |
| No | 59 (21) |
| Yes | 228 (79) |
| Somatic/tumor testing mentioned * (n = 287) | |
| No | 214 (75) |
| Yes | 73 (25) |
| Targeted therapies mentioned | |
| No | 237 (60) |
| Yes | 159 (40) |
| GINA mentioned | |
| No | 384 (97) |
| Yes | 12 (3) |
| Care Delivery Method | |
| Direct to consumer * (n = 287) | |
| No | 257 (90) |
| Yes | 30 (10) |
| Clinic * (n = 287) | |
| No | 212 (74) |
| Yes | 75 (26) |
| Research * (n = 287) | |
| No | 97 (34) |
| Yes | 190 (66) |
| Exemplars | |
| None | 303 (77) |
| Genetic testing or treatment exemplars | 93 (23) |
| N (%) | |
|---|---|
| Benefits, subtypes (n = 275) | |
| Improve treatments | 244 (89) |
| Improve outcomes (any) | 43 (16) |
| Improve chance of cure | 1 (0.4) |
| Increase survival | 22 (8) |
| Decrease disease recurrence | 6 (2.2) |
| Improve response rate | 4 (1.5) |
| Predict risk of developing disease | 91 (33) |
| Decrease side effects | 83 (30) |
| Decrease cost | 52 (19) |
| Improve prevention | 49 (18) |
| Beneficial to drug developers | 47 (17) |
| Improve diagnostic capabilities | 36 (13) |
| Improve prognostication | 1 (0.4) |
| Other | 41 (15) |
| Challenges, subtypes (n = 137) | |
| Risk of discrimination (any) | 40 (29) |
| Employment | 30 (22) |
| Insurance | 32 (23) |
| Racial | 4 (3) |
| Increase costs | 39 (28) |
| Concerns over privacy | 29 (21) |
| Regulation | 25 (18) |
| Insurance reimbursement/coverage | 22 (16) |
| Detrimental to drug developers | 20 (15) |
| Ethical | 17 (12) |
| Contribution of genetic vs. environmental factors | 11 (8) |
| Need for education (any) | 10 (7) |
| Providers | 7 (5) |
| Patients | 5 (4) |
| Uncertainty regarding application of data | 8 (6) |
| Exacerbation of disparities | 7 (5) |
| Inadequate cancer care delivery systems | 6 (4) |
| Patent law | 6 (4) |
| Other | 67 (49) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hicks-Courant, K.; Shen, J.; Stroupe, A.; Cronin, A.; Bair, E.F.; Wing, S.E.; Sosa, E.; Nagler, R.H.; Gray, S.W. Personalized Cancer Medicine in the Media: Sensationalism or Realistic Reporting? J. Pers. Med. 2021, 11, 741. https://doi.org/10.3390/jpm11080741
Hicks-Courant K, Shen J, Stroupe A, Cronin A, Bair EF, Wing SE, Sosa E, Nagler RH, Gray SW. Personalized Cancer Medicine in the Media: Sensationalism or Realistic Reporting? Journal of Personalized Medicine. 2021; 11(8):741. https://doi.org/10.3390/jpm11080741
Chicago/Turabian StyleHicks-Courant, Katherine, Jenny Shen, Angela Stroupe, Angel Cronin, Elizabeth F. Bair, Sam E. Wing, Ernesto Sosa, Rebekah H. Nagler, and Stacy W. Gray. 2021. "Personalized Cancer Medicine in the Media: Sensationalism or Realistic Reporting?" Journal of Personalized Medicine 11, no. 8: 741. https://doi.org/10.3390/jpm11080741
APA StyleHicks-Courant, K., Shen, J., Stroupe, A., Cronin, A., Bair, E. F., Wing, S. E., Sosa, E., Nagler, R. H., & Gray, S. W. (2021). Personalized Cancer Medicine in the Media: Sensationalism or Realistic Reporting? Journal of Personalized Medicine, 11(8), 741. https://doi.org/10.3390/jpm11080741





