The Safety and Effect of Local Botulinumtoxin A Injections for Long-Term Management of Chronic Pain in Post-Herpetic Neuralgia: Literature Review and Cases Report Treated with Incobotulinumtoxin A
Abstract
:1. Introduction
2. Mechanisms and Sites of Action
3. Results
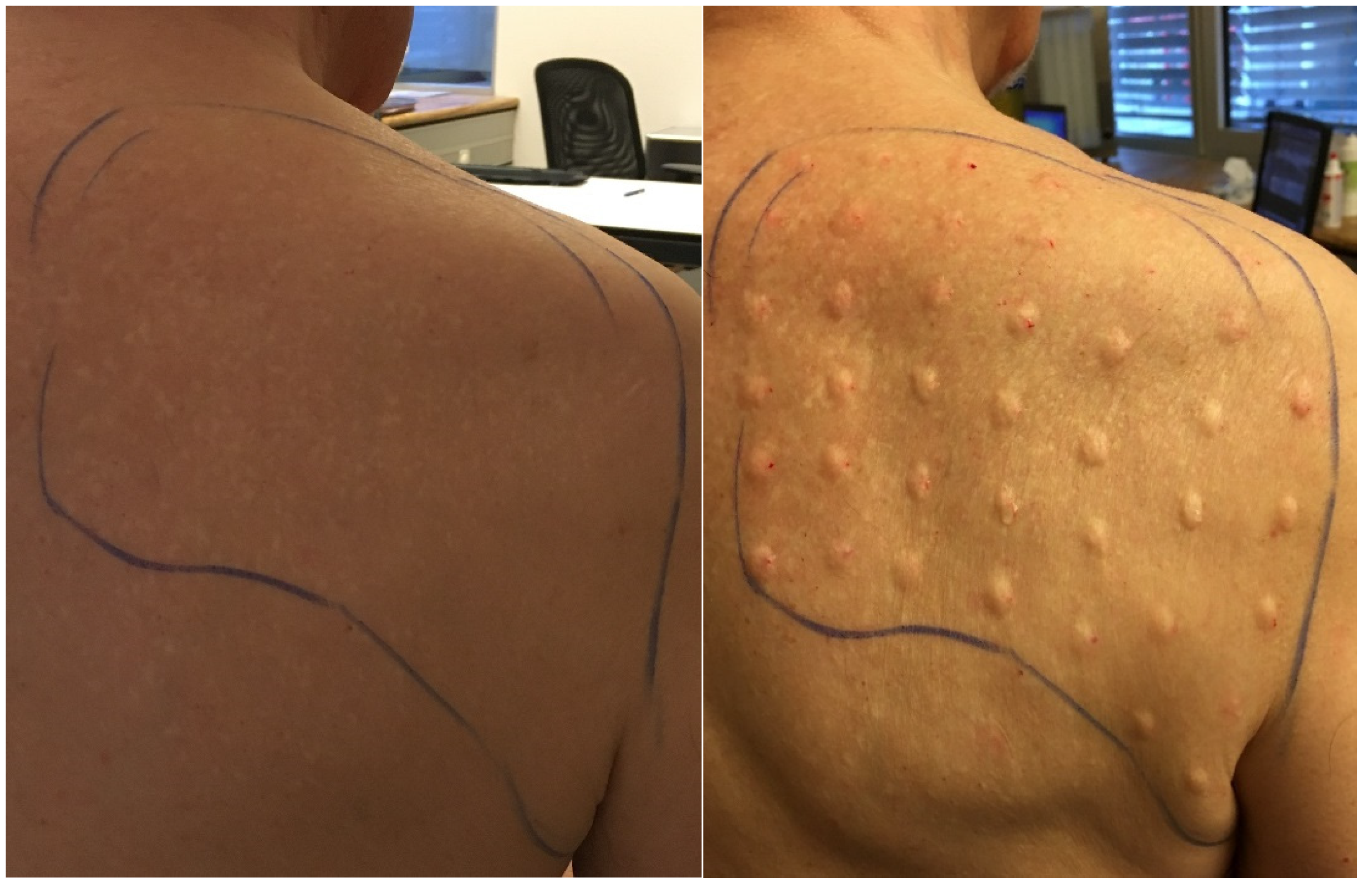
3.1. Case 1
3.2. Case 2
3.3. Case 3
4. Discussion
4.1. Applied Botulinumtoxin A
4.2. Doses and Injection Technique
4.3. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wasner, G. Diagnostik Neuropathischer Schmerzen [Diagnosis of Neuropathic Pain]. Leitlinien für Diagnostik und Therapie in der Neurologie. 2012. Available online: https://dgn.org/wp-content/uploads/2013/01/030-132l_S1_Neuropathische_Schmerzen_Diagnostik_2012_verlaengert.pdf (accessed on 8 May 2018).
- Oxman, M.N.; Levin, M.J.; Johnson, G.R.; Schmader, K.E.; Straus, S.E.; Gelb, L.D.; Arbeit, R.D.; Simberkoff, M.S.; Gershon, A.A.; Davis, L.E.; et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 2005, 352, 2271–2284. [Google Scholar] [CrossRef] [Green Version]
- Goßrau, G. Postherpetic neuralgia. Nervenarzt 2015, 86, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, E.; Piotrowska, A.; Popiolek-Barczyk, K.; Mika, J. Botulinum Toxin Type A—A Modulator of Spinal Neuron—Glia Interactions under Neuropathic Pain Conditions. Toxins 2018, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Hatch, M.N.; Cushing, T.R.; Carlson, G.D.; Chang, E.Y. Neuropathic pain and SCI: Identification and treatment strategies in the 21st century. J. Neurol. Sci. 2017, 384, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Baron, R. Pharmakologisch Nicht Interventionelle Therapie Chronisch Neuropathischer Schmerzen [Pharmacological, Non-Interventional Therapies of Chronic Neuropathic Pain]. Kapitel Kopfschmerzen und Andere Schmerzen. Leitlinien für Diagnostik und Therapie in der Neurologie 2012. Available online: https://dgn.org/wp-content/uploads/2013/01/030-114l_S1_Neuropathischer_Schmerzen_Therapie_2014-verlaengert.pdf (accessed on 8 May 2018).
- Cohen, J.I. Clinical practice: Herpes zoster. N. Engl. J. Med. 2013, 369, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, G.M. Unmet need in the treatment of postherpetic neuralgia. Am. J. Manag. Care 2013, 19, S207–S213. [Google Scholar] [PubMed]
- Brin, M.F.; Fahn, S.; Moskowitz, C.; Friedman, A.; Shale, H.M.; Greene, P.E.; Blitzer, A.; List, T.; Lange, D.; Lovelace, R.E.; et al. Localized injections of botulinum toxin for the treatment of focal dystonia and hemifacial spasm. Mov. Disord. 1987, 2, 237–254. [Google Scholar] [CrossRef]
- Durham, P.L.; Cady, R.; Cady, R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: Implications for migraine therapy. Headache 2004, 44, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lucioni, A.; Bales, G.T.; Lotan, T.L.; McGehee, D.S.; Cook, S.P.; Rapp, D.E. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int. 2008, 101, 366–370. [Google Scholar] [CrossRef] [PubMed]
- McMahon, H.T.; Foran, P.; Dolly, J.O.; Verhage, M.; Wiegant, V.M.; Nicholls, D.G. Tetanus toxin and botulinum toxins type A and B inhibit glutamate, gamma-aminobutyric acid, aspartate, and met-enkephalin release from synaptosomes clues to the locus of action. J. Biol. Chem. 1992, 267, 21338–21343. [Google Scholar] [CrossRef]
- Wissel, J.; Ganapathy, V.; Ward, A.B.; Borg, J.; Ertzgaard, P.; Herrmann, C.; Haggstrom, A.; Sakel, M.; Ma, J.; Dimitrova, R.; et al. Onabotulinumtoxin A Improves Pain in Patients with Post-Stroke Spasticity: Findings From a Randomized, Double-Blind, Placebo-Controlled Trial. J. Pain Symptom Manag. 2016, 52, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, A.W. The therapeutic potential of botulinum toxin. Derm. Surg. 2004, 30, 452–455. [Google Scholar]
- Burstein, R.; Zhang, X.C.; Levy, D.; Aoki, K.R.; Brin, M.F. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: Therapeutic implications for migraine and other pains. Cephalalgia 2014, 34, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.C.; Wakita, M.; Xie, D.J.; Yamaga, T.; Iwata, S.; Torii, Y.; Harakawa, T.; Ginnaga, A.; Kozaki, S.; Akaike, N. Inhibition of membrane Na+ channels by A type botulinum toxin at femtomolar concentrations in central and peripheral neurons. J. Pharmacol. Sci. 2012, 118, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Drinovac, V.; Bach-Rojecky, L.; Matak, I.; Lacković, Z. Involvement of mu-opioid receptors in antinociceptive action of botulinum toxin type A. Neuropharmacology 2013, 70, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Filippi, G.M.; Errico, P.; Santarelli, R.; Bagolini, B.; Manni, E. Botulinum A toxin effects on rat jaw muscle spindles. Acta Otolaryngol. 1993, 113, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Rand, M.J.; Whaler, B.C. Impairment of sympathetic transmission by botulinum toxin. Nature 1965, 206, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, F.; Rossi, C.; Gianfranceschi, L.; Rossetto, O.; Caleo, M. Long-Distance Retrograde Effects of Botulinum Neurotoxin A. J. Neurosci. 2008, 28, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Zychowska, M.; Rojewska, E.; Makuch, W.; Luvisetto, S.; Pavone, F.; Marinelli, S.; Przewlocka, B.; Mika, J. Participation of pro- and anti-nociceptive interleukins in botulinum toxin A-induced analgesia in a rat model of neuropathic pain. Eur. J. Pharmacol. 2016, 791, 377–388. [Google Scholar] [CrossRef]
- Mika, J.; Rojewska, E.; Makuch, W.; Korostynski, M.; Luvisetto, S.; Marinelli, S.; Pavone, F.; Przewlocka, B. The effect of botulinum neurotoxin A on sciatic nerve injury-induced neuroimmunological changes in rat dorsal root ganglia and spinal cord. Neuroscience 2011, 175, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Popiolek-Barczyk, K.; Pavone, F.; Mika, J. Comparison of the Expression Changes after Botulinum Toxin Type A and Minocycline Administration in Lipopolysaccharide-Stimulated Rat Microglial and Astroglial Cultures. Front. Cell. Infect. Microbiol. 2017, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Namazi, H. Intravesical botulinum toxin A injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis: A molecular mechanism. Urology 2008, 72, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Yoshimura, N.; Huang, C.C.; Wu, M.; Chiang, P.H.; Chancellor, M.B. Intraprostatic botulinum toxin a injection inhibits cyclooxygenase-2 expression and suppresses prostatic pain on capsaicin induced prostatitis model in rat. J. Urol. 2008, 180, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Jain, M.; Jain, S. Subcutaneous Injection of Botulinum Toxin-A in Postherpetic Neuralgia During Pregnancy. Ann. Indian Acad. Neurol. 2017, 20, 430. [Google Scholar] [CrossRef]
- Emad, M.R.; Emad, M.; Taheri, P. The Efficacy of Intradermal Injection of Botulinum Toxin in Patients with Post-Herpetic Neuralgia. Iran Red Crescent Med. J. 2011, 13, 323–327. [Google Scholar] [PubMed]
- Jain, P.; Jain, M.; Jain, S. Subcutaneous Injection of Botulinum Toxin in Patients with Post Herpetic Neuralgia. A Preliminary Study. J. Assoc. Physicians India 2018, 66, 48–49. [Google Scholar] [PubMed]
- Liu, H.T.; Tsai, S.K.; Kao, M.C.; Hu, J.S. Botulinum toxin A relieved neuropathic pain in a case of post-herpetic neuralgia. Pain Med. 2006, 7, 89–91. [Google Scholar] [CrossRef] [Green Version]
- Ruiz Huete, C.; Bermejo, P. Botulinum toxin type A in the treatment of neuropathic pain in a case of postherpetic neuralgia. Neurologia 2008, 23, 259–262. [Google Scholar]
- Ranoux, D.; Attal, N.; Morain, F.; Bouhassira, D. Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Ann. Neurol. 2008, 64, 274–283. [Google Scholar] [CrossRef]
- Sotiriou, E.; Apalla, Z.; Panagiotidou, D.; Ioannidis, D. Severe post-herpetic neuralgia successfully treated with botulinum toxin A: Three case reports. Acta Derm Venereol. 2009, 89, 214–215. [Google Scholar]
- Xiao, L.; Mackey, S.; Hui, H.; Xong, D.; Zhang, Q.; Zhang, D. Subcutaneous injection of botulinum toxin a is beneficial in postherpetic neuralgia. Pain Med. 2010, 11, 1827–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apalla, Z.; Sotiriou, E.; Lallas, A.; Lazaridou, E.; Ioannides, D. Botulinum Toxin A in Postherpetic Neuralgia, A Parallel, Randomized, Double-Blind, Single-Dose. Placebocontrolled Trial. Clin. J. Pain 2013, 29, 857–864. [Google Scholar] [CrossRef]
- Ponce, O.R.M.; Guerrero, S.E.L.; Tirado, S.A. Botulinumtoxintype A (onabotulinum toxin A) in the management of post herpetic neuralgia. Dermatol. Rev. Mex. 2013, 57, 18–21. [Google Scholar]
- Li, D.; Xiao, L. Combining Botulinum Toxin (A) Injection With Peripheral Nerve Stimulation in a Patient for Intractable Ophthalmic Postherpetic Neuralgia. Neuromodulation 2015, 18, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; de Andrade, D.C.; Adam, F. Safety and efficacy of repeated injections of botulinum toxin A in peripheral neuropathic pain (BOTNEP): A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2016, 15, 555–565. [Google Scholar] [CrossRef]
- Eitner, L.; Vollert, J.; Maier, C.; Attal, N. Botulinumtoxin-A-Injektionen bei neuropathischem Schmerz: Eine Post-hoc-Subgruppenanalyse bei Patienten mit peripherer Nervenverletzung [Botulinum toxin A injections in neuropathic pain: A post-hoc subgroup analysis of patients with peripheral nerve injury]. Schmerz 2017, 31, 524–526. [Google Scholar]
- Ding, X.D.; Zhong, J.; Liu, Y.P.; Chen, H.X. Botulinum as a Toxin for Treating Post-herpetic Neuralgia. Iran. J. Public Health 2017, 46, 608–611. [Google Scholar]
- Hu, Y.; Zou, L.; Qi, X.; Lu, Y.; Zhou, X.; Mao, Z.; Chen, X.; Liu, K.; Yang, Y.; Wu, Z.; et al. Subcutaneous botulinum toxin-A injection for treating postherpetic neuralgia. Derm. Ther. 2020, 33, e13181. [Google Scholar] [CrossRef]
- Attal, N. Pharmacological treatments of neuropathic pain: The latest recommendations. Rev. Neurol. 2019, 175, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Safarpour, Y.; Jabbari, B. Botulinum toxin treatment of pain syndromes—An evidence based review. Toxicon 2018, 147, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Frevert, J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mårtensson, L.; Nyberg, K.; Wallin, G. Subcutaneous versus intracutaneous injections of sterile water for labour analgesia: A comparison of perceived pain during administration. BJOG 2000, 107, 1248–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Publications | BoNT A | Study Type | N | I. T. | Doses | Follow-Up | Results |
|---|---|---|---|---|---|---|---|
| Hu 2020 [29] | Ona A (Chi-Botox) | control Study | 33 | s.c. | 10–20 × 5 IU | 16 w | Significant pain reduction (VAS) |
| Ding 2017 [30] | Ona A (Chi-Botox) | Cohort study | 58 | s.c. | 10–20 × 5 IU | 6 m | Significant pain reduction (VAS) |
| Attal 2016 [31] | Ona A | RCT (total n = 66) | 5 | s.c. | Up to 60 × 5 IU, every 12 w | 24 w | Significant pain reduction |
| Ponce 2013 [32] | Ona A | Cohort Study | 12 | i.c./s.c. | 8–10 × 2.5 IU | 3 m | Significant pain reduction |
| Apalla 2013 [33] | Ona A | RCT | 30 | s.c. | 40 × 5 IU | 20 w | greater than 50% pain reduction in VAS, improvement of sleep disorder |
| Xiao 2010 [34] | Ona A | RCT | 60 | s.c. | Up to 40 × 5 IU | 3 m | Significant pain reduction (mean decreased 4.5 in VAS), improvement of sleep disorder, decreased opioid use |
| Sotiriou 2009 [35] | Ona A | Case series | 3 | s.c. | 20 × 5 IU | 12 w | VAS reduction from 8.3 to 2 |
| Ranoux 2008 [36] | Ona A | RCT (total n = 29) | 4 | i.c. | Up to 40 × 5 IU | 14 w | NNT, 50% pain reduction at 12 weeks |
| Liu 2006 [37] | Ona A | Case report | 1 | s.c. | 20 × 5 IU | 52 d | VAS reduction from 10 to 1 |
| Klein 2004 [14] | Ona A | Case report | 1 | i.c. | 20 IU | 4 m | Completely pain reduction |
| Jain 2018 [28] | Abo A | Cohort Study | 19 | s.c. | 25 × 20 IU | 16 w | Significant pain reduction (VAS) |
| Emad 2011 [27] | Abo A | Cohort Study | 15 | i.c. | 15 U/10 cm2 (4 mL 2% lidocain) | 30 d | pain reduction, but not significant |
| Jain 2017 [26] | Abo A | Case series (in pregnancy) | 2 | s.c. | 500 IU (5 mL 0.9% NaCl) | 16 w | VAS from 9 and 10 to 1 |
| Eitner 2017 [38] | Unknown | RCT, placebo (total n = 66) | 6 | s.c. | Every 1.5–2 cm, 100–300 IU (4 mL 0.9% Nacl) | 24 w | Significant pain reduction (VAS) |
| Ruiz H. 2008 [39] | Unknown | Case report | 1 | i.c. | unknown | 2 m | Dramatically pain reduction |
| Li 2015 [40] | Unknown | Case report | 1 | s.c. | 100 IU | 6 m | Significant improvement in pain relief |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ri, S.; Kivi, A.; Wissel, J. The Safety and Effect of Local Botulinumtoxin A Injections for Long-Term Management of Chronic Pain in Post-Herpetic Neuralgia: Literature Review and Cases Report Treated with Incobotulinumtoxin A. J. Pers. Med. 2021, 11, 758. https://doi.org/10.3390/jpm11080758
Ri S, Kivi A, Wissel J. The Safety and Effect of Local Botulinumtoxin A Injections for Long-Term Management of Chronic Pain in Post-Herpetic Neuralgia: Literature Review and Cases Report Treated with Incobotulinumtoxin A. Journal of Personalized Medicine. 2021; 11(8):758. https://doi.org/10.3390/jpm11080758
Chicago/Turabian StyleRi, Songjin, Anatol Kivi, and Jörg Wissel. 2021. "The Safety and Effect of Local Botulinumtoxin A Injections for Long-Term Management of Chronic Pain in Post-Herpetic Neuralgia: Literature Review and Cases Report Treated with Incobotulinumtoxin A" Journal of Personalized Medicine 11, no. 8: 758. https://doi.org/10.3390/jpm11080758
APA StyleRi, S., Kivi, A., & Wissel, J. (2021). The Safety and Effect of Local Botulinumtoxin A Injections for Long-Term Management of Chronic Pain in Post-Herpetic Neuralgia: Literature Review and Cases Report Treated with Incobotulinumtoxin A. Journal of Personalized Medicine, 11(8), 758. https://doi.org/10.3390/jpm11080758






