The Impact of the Synergistic Effect of Temperature and Air Pollutants on Chronic Lung Diseases in Subtropical Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Collection of Demographic, Laboratory and Meteorological Factors
2.3. Assessment of Lung Function Status
2.4. Ethics Statement
2.5. Statistical Analysis
3. Results
3.1. Descriptive Statistics of the Demographic, Laboratory, Meteorological Factors and Air Pollutants
3.2. Correlation between Meteorological Factors and Outdoor Air Pollutants
3.3. Associations between Chronic Lung Disease and All Factors
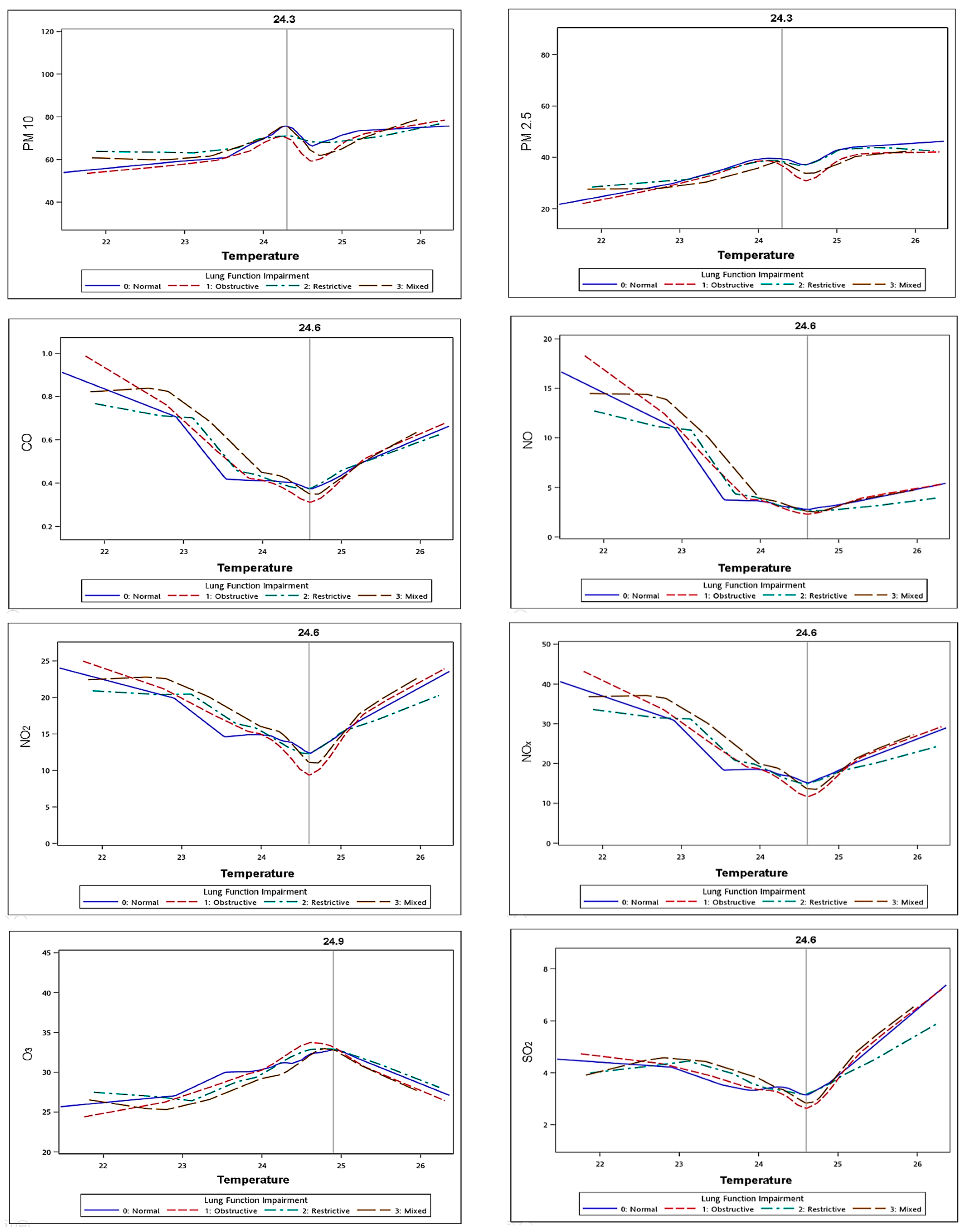
3.4. Interactions between Temperature and Air Pollutants
3.5. Interactions between Temperature and Monitoring Regions
3.6. Correlations between Temperature and Air Pollutants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardization of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doiron, D.; Hoogh, K.; Probst-Hensch, N.; Fortier, I.; Cai, Y.; Matteis, S.D.; Hansell, A.L. Air pollution, lung function and COPD: Results from the population-based UK Biobank study. Eur. Respir. J. 2019, 54, 1802140. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Schikowski, T.; Carsin, A.E.; Cai, Y.; Jacquemin, B.; Sanchez, M.; Vierkötter, A.; Marcon, A.; Keidel, D.; Sugiri, D.; et al. Adult lung function and long-term air pollution exposure. ESCAPE: A multicenter cohort study and meta-analysis. Eur. Respir. J. 2005, 45, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Jong, K.; Vonk, J.; Zijlema, W. Air pollution exposure is associated with restrictive ventilatory patterns. Eur. Respir. J. 2016, 48, 1221–1224. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Carey, I.M.; Kent, A.J.; Staa, T.P.; van Anderson, H.R.; Cook, D.G. Long-term exposure to outdoor air pollution and the incidence of chronic obstructive pulmonary disease in a national English cohort. Occup. Environ. Med. 2015, 72, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.-Q.; Me, X.-D.; Feng, D. Air pollution and chronic airway diseases: What should people know and do? J. Thorac. Dis. 2016, 8, E31. [Google Scholar]
- Sangkharat, K.; Fisher, P.; Thomas, G.N.; Thornes, J.; Pope, F.D. The impact of air pollutants on ambulance dispatches: A systematic review and meta-analysis of acute effects. Environ. Pollut. 2019, 254, 112769. [Google Scholar] [CrossRef]
- Sanyal, S.; Rochereau, T.; Maesano, C.N.; Com-Ruelle, L.; Annesi-Maesano, I. Long-Term Effect of Outdoor Air Pollution on Mortality and Morbidity: A 12-Year Follow-Up Study for Metropolitan France. Int. J. Environ. Res. Public Health 2018, 15, 2487. [Google Scholar] [CrossRef] [Green Version]
- Dong, G.-H.; Zhang, P.; Sun, B.; Zhang, L.; Chen, X.; Ma, N.; Yu, F.; Guo, H.; Huang, H.; Lee, Y.-L.; et al. Long-Term Exposure to Ambient Air Pollution and Respiratory Disease Mortality in Shenyang, China: A 12-Year Population-Based Retrospective Cohort Study. Respiration 2012, 84, 360–368. [Google Scholar] [CrossRef]
- To, T.; Feldman, L.; Simatovic, J.; Gershon, A.S.; Dell, S.; Su, J.; Foty, R.; Licskai, C. Health risk of air pollution on people living with major chronic diseases: A Canadian population-based study. BMJ Open 2015, 5, e009075. [Google Scholar] [CrossRef] [Green Version]
- Valenti, C.; Pozzi, P.; Busia, A.; Mazza, R.; Bossi, P.; Marco, C.D.; Ruprecht, A.A.; Borgini, A.; Boffi, R. Respiratory illness and air pollution from the steel industry: The case of Piquiá de Baixo, Brazil (Preliminary report). Multidiscip. Respir. Med. 2016, 11, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, Composition, and Lung Diseases. Front. Immunol. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Loft, S. Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ. Health Perspect. 2010, 118, 1126–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bind, M.A.; Baccarelli, A.; Zanobetti, A.; Tarantini, L.; Suh, H.; Vokonas, P.; Schwartz, J. Air pollution and markers of coagulation, inflammation and endothelial function: Associations and epigene-environment interactions in an elderly cohort. Epidemiology 2012, 23, 332. [Google Scholar] [CrossRef] [Green Version]
- Paulin, L.; Hansel, N. Particulate air pollution and impaired lung function. F1000Research 2016, 5, F1000. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-W.; Chen, P.-L.; Ren, L.; Lin, Y.-N.; Zhou, J.-P.; Ni, L.; Li, Q.-Y. The cumulative effect of air pollutants on the acute exacerbation of COPD in Shanghai, China. Sci. Total Environ. 2018, 622, 875–881. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, C.; Chen, R.; Wang, C.; Li, J.; Sun, J.; Kan, H.; Cao, J.; Bai, H. Association of fine particulate matter on acute exacerbation of chronic obstructive pulmonary disease in Yancheng, China. Sci. Total Environ. 2019, 650, 1665–1670. [Google Scholar] [CrossRef]
- Abramson, M.J.; Wigmann, C.; Altug, H.; Schikowski, T. Ambient air pollution is associated with airway inflammation in older women: A nested cross-sectional analysis. BMJ Open Respir. Res. 2020, 7, e000549. [Google Scholar] [CrossRef]
- Yu, H.-R.; Lin, C.-H.; Tsai, J.-H.; Hsieh, Y.-T.; Tsai, T.-A.; Tsai, C.-K.; Lee, Y.-C.; Liu, T.-Y.; Tsai, C.-M.; Chen, C.-C.; et al. A multifactorial evaluation of the effects of air pollution and meteorological factors on asthma exacerbation. Int. J. Environ. Res. Public Health 2020, 17, 4010. [Google Scholar] [CrossRef]
- Lee, W.; Choi, H.M.; Kim, D.; Honda, Y.; Guo, Y.-L.L.; Kim, H. Synergic effect between high temperature and air pollution on mortality in Northeast Asia. Environ. Res. 2019, 178, 108735. [Google Scholar] [CrossRef] [PubMed]
- Analitis, A.; Donato, F.D.; Scortichini, M.; Lanki, T.; Basagana, X.; Ballester, F.; Astrom, C.; Paldy, A.; Pascal, M.; Gasparrini, A.; et al. Synergistic Effects of Ambient Temperature and Air Pollution on Health in Europe: Results from the PHASE Project. Int. J. Environ. Res. Public Health 2018, 15, 1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansel, N.N.; McCormack, M.C.; Kim, V. The Effects of Air Pollution and Temperature on COPD. COPD: J. Chronic Obstr. Pulm. Dis. 2016, 13, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.-J.; Lee, W.-S.; Jo, H.-Y.; Kim, C.-H.; Eom, J.-S.; Mok, J.-H.; Kim, M.-H.; Lee, K.; Kim, K.-U.; Lee, M.-K.; et al. Effects of particulate matter on respiratory disease and the impact of meteorological factors in Busan, Korea. Respir. Med. 2017, 124, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Yang, J.-H.; Chiang, C.W.K.; Hsiung, C.-N.; Wu, P.-E.; Chang, L.-C.; Chu, H.-W.; Chang, J.; Song, I.W.; Yang, S.-L.; et al. Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan Biobank project. Hum. Mol. Genet. 2016, 25, 5321–5331. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.-C.; Hsiao, W.W.-W.; Fan, C.-T. Managing “incidental findings” in biobank research: Recommendations of the Taiwan biobank. Comput. Struct. Biotechnol. J. 2019, 17, 1135–1142. [Google Scholar] [CrossRef]
- Hsieh, S.W.; Wu, D.W.; Wang, C.W.; Chen, S.C.; Hung, C.H.; Kuo, C.H. Poor cognitive function is associated with obstructive lung diseases in Taiwanese adults. Int. J. Environ. Res. Public Health 2021, 18, 2344. [Google Scholar] [CrossRef]
- Oliveros, E.; Somers, V.; Sochor, O.; Goel, K.; Lopez-Jimenez, F. The concept of normal weight obesity. Prog. Cardiovasc. Dis. 2014, 56, 426–433. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Ziegler, T.R.; Millson, E.C.; Stecenko, A.A. Body composition and lung function in cystic fibrosis and their association with adiposity and normal-weight obesity. Nutrition 2016, 32, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, C.R.; Celli, B.; Anderson, J.A.; Ferguson, G.T.; Jones, P.W.; Vestbo, J.; Yates, Y.C.; Calverley, P.M.A. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur. Respir. J. 2012, 39, 38–45. [Google Scholar] [CrossRef]
- Huang, K.; Rabold, R.; Schofield, B.; Mitzner, W.; Tankersley, C.G. Age dependent changes of airway and lung parenchyma in C57BL/6J mice. J. Appl. Physiol. 2007, 102, 200–206. [Google Scholar] [CrossRef]
- Glassberg, M.K.; Choi, R.; Manzoli, V.; Shahzeidi, S.; Rauschkolb, P.; Voswinckel, R.; Aliniazee, M.; Xia, X.; Elliot, S.J. 17β-Estradiol replacement reverses age-related lung disease in estrogen-deficient C57BL/6J mice. Endocrinology 2014, 155, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Glassberg, M.K.; Catanuto, P.; Shahzeidi, S.; Aliniazee, M.; Lilo, S.; Rubio, G.A.; Elliot, S.J. Estrogen deficiency promotes cigarette smoke–induced changes in the extracellular matrix in the lungs of aging female mice. Transl. Res. 2016, 178, 107–117. [Google Scholar] [CrossRef]
- Tian, L.; Liang, F.; Guo, Q.; Shi, C.; Xiao, S.; Wu, Z.; Jin, X.; Pan, X. The effects of interaction between particulate matter and temperature on mortality in Beijing, China. Environ. Sci. Process. Impacts 2018, 20, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.; Davis, S.R.; Abramson, M.J.; Mishra, G.; Handelsman, D.J.; Perret, J.L.; Dharmage, S.C. Menopause, lung function and obstructive lungdisease outcomes: A systematic review. Climacteric 2018, 21, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Woodward, A.; Hou, X.-Y.; Zhu, T.; Zhang, J.; Brown, H.; Yang, J.; Qin, R.; Gao, J.; Gu, S.; et al. Modification of the effects of air pollutants on mortality by temperature: A systematic review and meta-analysis. Sci. Total Environ. 2017, 575, 1556–1570. [Google Scholar] [CrossRef]
- Lin, S.; Luo, M.; Walker, R.J.; Liu, X.; Hwang, S.-A.; Chinery, R. Extreme high temperatures and hospital admissions for respiratory and cardiovascular diseases. Epidemiology 2009, 20, 738–746. [Google Scholar] [CrossRef]
- Zanobetti, A.; O’Neill, M.S.; Gronlund, C.J.; Schwartz, J.D. Summer temperature variability and long-term survival among elderly people with chronic disease. Proc. Natl. Acad. Sci. USA 2012, 109, 6608–6613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.-M.; Chen, Y.-T.; Ou, S.-M.; Hsiao, Y.-H.; Li, S.-Y.; Wang, S.-J.; Yang, A.C.; Chen, T.-J.; Perng, D.-W. The effect of cold temperature on increased exacerbation of chronic obstructive pulmonary disease: A nationwide study. PLoS ONE 2013, 8, e57066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.-T.; Kor, C.-T.; Chang, C.-C.; Chai, W.-H.; Soon, M.-S.; Ciou, Y.-S.; Lian, I.B.; Chang, C.C. Association of meteorological factors and air NO2 and O3 concentrations with acute exacerbation of elderly chronic obstructive pulmonary disease. Sci. Rep. 2018, 8, 10192. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.; He, H.; Su, Y.; Sha, Y.; Zong, S. Lag effect of air temperature on the incidence of respiratory diseases in Lanzhou, China. Int. J. Biometeorol. 2020, 64, 83–93. [Google Scholar] [CrossRef]
- Breitner, S.; Wolf, K.; Devlin, R.B.; Diaz-Sanchez, D.; Peters, A.; Schneider, A. Short-term effects of air temperature on mortality and effect modification by air pollution in three cities of Bavaria, Germany: A time-series analysis. Sci. Total Environ. 2014, 485, 49–61. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Chiu, H.-F.; Yang, C.-Y. Coarse particulate air pollution associated with increased risk of hospital admissions for respiratory diseases in a tropical city, Kaohsiung, Taiwan. Int. J. Environ. Res. Public Health 2015, 12, 13053–13068. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, A.; Pecoraro, V.; Zauli, S.; Ottone, M.; Leonardi, G.; Lauriola, P.; Trenti, T. Use of carboxyhemoglobin as a biomarker of environmental CO exposure: Critical evaluation of the literature. Environ. Sci. Pollut. Res. Int. 2017, 24, 25798–25809. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.R. Inhaled Carbon Monoxide: From Toxin to Therapy. Respir. Care. 2017, 62, 1333–1342. [Google Scholar] [CrossRef]
- Nightingale, R.; Lesosky, M.; Flitz, G.; Rylance, S.J.; Meghji, J.; Burney, P.; Balmes, J.; Mortimer, K. Noncommunicable respiratory disease and air pollution exposure in Malawi. Am. J. Respir. Crit. Care Med. 2019, 199, 613–621. [Google Scholar] [CrossRef]
- Marriott, H.; Higenbottam, T. The role of nitric oxide in respiratory disease. Schweiz. Med. Wochenschr. 1997, 127, 709–714. [Google Scholar]
- Gebistorf, F.; Karam, O.; Wetterslev, J.; Afshari, A. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst. Rev. 2016, 6, CD002787. [Google Scholar] [CrossRef]
- Dadvand, P.; Nieuwenhuijsen, M.J.; Agust, À.; Batlle, J.; Benet, M.; Beelen, R.; Cirach, M.; Martinez, D.; Hoek, F.; Basagaña, X.; et al. Air pollution and biomarkers of systemic inflammation and tissue repair in COPD patients. Eur. Respir. J. 2014, 44, 603–613. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-C.; Lu, M.-C.; Lin, Y.-C.; Wu, T.-C.; Hsu, J.-Y.; Jan, M.-S.; Chen, C.-M. Differences in IL-8 in serum and exhaled breath condensate from patients with exacerbated COPD or asthma attacks. J. Formos. Med. Assoc. 2014, 113, 908–914. [Google Scholar] [CrossRef] [Green Version]
- Barck, C.; Sandström, T.; Lundahl, J.; Halldén, G.; Svartengren, M.; Strand, V.; Rak, S.; Bylin, G. Ambient level of NO2 augments the inflammatory response to inhaled allergen in asthmatics. Respir. Med. 2002, 96, 907–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Niu, Y.; Xia, Y.; Liu, C.; Lin, Z.; Wang, W.; Ge, Y.; Lei, X.; Wang, C.; Cai, J.; et al. Effects of personal nitrogen dioxide exposure on airway inflammation and lung function. Environ. Res. 2019, 177, 108620. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Liao, Y.; Li, H.; Yan, S.; Zhao, Z.; Yu, S.; Zhao, Z.; Yu, S.; Fu, Y.; Wang, Z.; et al. Effect of changes in season and temperature on cardiovascular mortality associated with nitrogen dioxide air pollution in Shenzhen, China. Sci. Total Environ. 2019, 697, 134051. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, G.; Geravandi, S.; Idani, E.; Hosseini, S.A.; Baneshi, M.M.; Yari, A.R.; Dobaradaran, S.; Shirali, S.; Marzooni, B.B.; Ghomeishi, A.; et al. An evaluation of hospital admission respiratory disease attributed to sulfur dioxide ambient concentration in Ahvaz from 2011 through 2013. Environ. Sci. Pollut. Res. Int. 2016, 23, 22001–22007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Zhao, L.; Tong, J.; Yan, Y.; Xu, C. Fine particulate matter and sulfur dioxide coexposures Induce Rat Lung Pathological Injury and Inflammatory Responses Via TLR4/p38/NF-κB Pathway. Int. J. Toxicol. 2017, 36, 165–173. [Google Scholar] [CrossRef]
- Malley, C.S.; Henze, D.K.; Kuylenstierna, J.C.I.; Vallack, H.W.; Davila, Y.; Anenberg, S.C.; Turner, M.C.; Ashmore, M.R. Updated Global Estimates of Respiratory Mortality in Adults ≥30 Years of Age Attributable to Long-Term Ozone Exposure. Environ. Health Perspect. 2017, 125, 087021. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.; Zheng, J.; Li, R.; Huang, X.; Zhong, Z.; Zhong, L.; Lin, H. Speciated OVOC and VOC emission inventories and their implications for reactivity-based ozone control strategy in the Pearl River Delta region, China. Sci. Total Environ. 2015, 530, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Wei, Y.; Fang, Z. Ozone Pollution: A Major Health Hazard Worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ma, Z.; Zheng, C.; Shang, Y. Ambient temperature enhanced acute cardiovascular-respiratory mortality effects of PM2.5 in Beijing, China. Int. J. Biometeorol. 2015, 59, 1761–1770. [Google Scholar] [CrossRef]
- Zhang, X.; Diamond, M.L.; Robson, M.; Harrad, S. Sources, emissions, and fate of polybrominated diphenyl ethers and poly chlorinated biphenyls indoors in Toronto, Canada. Environ. Sci. Technol. 2011, 45, 3268–3274. [Google Scholar] [CrossRef] [PubMed]
- Vardoulakis, S.; Giagloglou, E.; Steinle, S.; Davis, A.; Sleeuwenhoek, A.; Galea, K.S.; Dixon, K.; Crawford, J.O. Indoor Exposure to Selected Air Pollutants in the Home Environment: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 8972. [Google Scholar] [CrossRef] [PubMed]
| Total | Normal Spirometry(1) | Obstructive Impairment(2) | Restrictive Impairment(3) | Mixed Impairment(4) | p-Value | |
|---|---|---|---|---|---|---|
| n | 2889 | 1902 | 733 | 154 | 100 | |
| Age (years), mean (SD) | 50.14 (10.64) | 49.51 (10.46) | 50.56(10.68) | 52.61 (11.02) | 55.31 (11.25) | <0.0001 |
| 30–39 | 628 (21.7) | 438 (23.0) | 149 (20.3) | 26 (16.9) | 15 (15.0) | |
| 40–49 | 760 (26.3) | 524 (27.5) | 186 (25.4) | 37 (24.0) | 13 (13.0) | |
| 40–59 | 891 (30.8) | 585 (30.8) | 231 (31.5) | 49 (31.8) | 26 (26.0) | |
| ≥60 | 610 (21.1) | 355 (18.7) | 167 (22.8) | 42 (27.3) | 46 (46.0) | <0.0001 |
| Sex, n (%) | ||||||
| Male | 1323 (45.8) | 903 (47.5) | 322 (43.9) | 66 (42.9) | 32 (32.0) | |
| Female | 1566 (54.2) | 999 (52.5) | 411 (56.1) | 88 (57.1) | 68 (68.0) | 0.0098 |
| Smoking, n (%) | ||||||
| None | 2112 (73.1) | 1382 (72.7) | 535 (73) | 116 (75.3) | 79 (79.0) | |
| Current & Former | 777 (26.9) | 520 (27.3) | 198 (27) | 38 (24.7) | 21 (21.0) | 0.5031 |
| Alcohol consumption, n (%) | ||||||
| None & sometimes | 2605 (90.2) | 1711 (90.0) | 660 (90.0) | 143 (92.9) | 91 (91.0) | |
| Quit | 67 (2.3) | 47 (2.5) | 16 (2.2) | 4 (2.6) | 0 (0.0) | |
| Current | 217 (7.5) | 144 (7.6) | 57 (7.8) | 7 (4.5) | 9 (9.0) | 0.5520 |
| Anthropometric parameter, mean (SD) | ||||||
| Height (cm) | 162.63 (8.26) | 163.09 (8.32) | 162.52 (8.05) | 160.26 (7.57) | 158.28 (7.80) | <0.0001 |
| Weight (kg) | 64.10 (12.10) | 64.49 (12.22) | 64.08 (11.6) | 62.74 (12.94) | 58.82 (10.67) | <0.0001 |
| Body Mass Index mean (kg/m2) | 24.12 (3.45) | 24.13 (3.44) | 24.16 (3.32) | 24.34 (4.22) | 23.38 (3.27) | 0.1457 |
| Body Adiposity Index | 28.60 (3.92) | 28.40 (3.92) | 28.75 (3.79) | 29.62 (4.38) | 29.72 (3.79) | <0.0001 |
| Body Roundness Index | 3.72 (1.13) | 3.70 (1.12) | 3.74 (1.08) | 3.96 (1.38) | 3.70 (1.20) | 0.0475 |
| Biochemical data, mean (SD) | ||||||
| Hematocrit (%) | 44.15 (4.61) | 44.32 (4.64) | 43.86 (4.61) | 44.13 (4.52) | 43.10 (3.86) | 0.0139 |
| Glycohemoglobin (%) | 5.78 (0.79) | 5.73 (0.71) | 5.84 (0.90) | 6.00 (1.13) | 5.82 (0.73) | <0.0001 |
| Albumin (g/dL) | 4.60 (0.24) | 4.60 (0.24) | 4.6 (0.24) | 4.56 (0.25) | 4.54 (0.24) | 0.0241 |
| Serum glutamic pyruvic Transaminase (IU/L) | 24.91 (22.64) | 24.84 (22.51) | 24.93 (24.01) | 27.45 (22.79) | 22.25 (11.92) | 0.3416 |
| Creatinine (mg/dL) | 0.76 (0.44) | 0.77 (0.50) | 0.75 (0.32) | 0.72 (0.22) | 0.68 (0.16) | 0.0534 |
| Comorbidities, n (%) | ||||||
| Hypertension | 309 (10.7) | 197 (10.4) | 78 (10.6) | 23 (14.9) | 11 (11.0) | 0.3662 |
| Diabetes mellitus type 2 | 138 (4.8) | 77 (4.0) | 43 (5.9) | 12 (7.8) | 6 (6.0) | 0.0474 |
| Renal failure | 4 (0.1) | 3 (0.2) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1.0000 |
| Metabolic syndrome | 525 (18.2) | 339 (17.8) | 136 (18.6) | 36 (23.4) | 14 (14.0) | 0.2503 |
| Coronary artery disease | 29 (1.0) | 21 (1.1) | 6 (0.8) | 2 (1.3) | 0 (0.0) | 0.7713 |
| Monitoring region, n (%) | ||||||
| Northern region | 571 (19.8) | 312 (16.4) | 182 (24.8) | 36 (23.4) | 41 (41.0) | |
| Central region | 558 (19.3) | 390 (20.5) | 139 (19.0) | 21 (13.6) | 8 (8.0) | |
| Southern region | 1760 (60.9) | 1200 (63.1) | 412 (56.2) | 97 (63.0) | 51 (51.0) | <0.0001 |
| Environmental factors, mean (SD) | ||||||
| Temperature (°C) | 24.33 (0.77) | 24.31 (0.72) | 24.41 (0.84) | 24.36 (0.93) | 24.18 (1.04) | 0.0040 |
| Relative humidity (%) | 74.29 (2.45) | 74.2 (2.47) | 74.51 (2.37) | 74.22 (2.49) | 74.57 (2.47) | 0.0157 |
| Rainfall (mm/day) | 0.22 (0.05) | 0.22 (0.05) | 0.21 (0.05) | 0.23 (0.05) | 0.23 (0.05) | <0.0001 |
| Air pollution factors, mean (SD) | ||||||
| PM10 (μg/m3) | 68.07 (17.06) | 69.05 (16.99) | 65.72 (17.51) | 68.5 (14.8) | 65.97 (16.56) | <0.0001 |
| PM2.5 (μg/m3) | 37.65 (10.74) | 38.44 (10.74) | 35.88 (10.74) | 38.36 (10.15) | 34.45 (9.56) | <0.0001 |
| CO (ppm) | 0.45 (0.18) | 0.44 (0.17) | 0.45 (0.20) | 0.48 (0.19) | 0.54 (0.26) | <0.0001 |
| NO (ppb) | 4.18 (4.01) | 4.00 (3.64) | 4.31 (4.29) | 4.46 (4.57) | 6.21 (6.34) | <0.0001 |
| NO2 (ppb) | 14.98 (5.65) | 14.9 (5.23) | 14.76 (6.45) | 15.77 (5.33) | 16.9 (6.89) | 0.0011 |
| NOX (ppb) | 19.14 (8.93) | 18.88 (8.19) | 19.06 (9.94) | 20.21 (9.23) | 23.09 (12.45) | <0.0001 |
| O3 (ppb) | 30.91 (3.86) | 30.94 (3.78) | 31.04 (4.04) | 30.71 (3.63) | 29.54 (4.20) | 0.0028 |
| SO2 (ppb) | 3.66 (1.20) | 3.61 (1.09) | 3.70 (1.39) | 3.82 (1.20) | 4.00 (1.42) | 0.0017 |
| Obstructive vs. Normal | Restrictive vs. Normal | Mixed vs. Normal | ||||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | |
| Age group (years) | ||||||
| 30–39 | 1.00 | 1.00 | 1.00 | |||
| 40–49 | 1.00 (0.77–1.3) | 0.9876 | 1.11 (0.65–1.89) | 0.6944 | 0.65 (0.3–1.42) | 0.2809 |
| 40–59 | 0.98 (0.76–1.27) | 0.8847 | 1.08 (0.64–1.81) | 0.7836 | 0.89 (0.44–1.8) | 0.7546 |
| ≥60 | 1.15 (0.86–1.54) | 0.3385 | 1.38 (0.80–2.39) | 0.2532 | 2.64 (1.35–5.13) | 0.0043 |
| Gender | ||||||
| Male | 1.00 | 1.00 | 1.00 | |||
| Female | - | - | - | - | ||
| Anthropometric parameters | ||||||
| Height | 0.99 (0.98–1.01) | 0.2861 | 0.96 (0.94–0.98) | <0.0001 | 0.93 (0.91–0.96) | <0.0001 |
| Weight | - | - | - | - | ||
| Body Adiposity Index | - | - | - | - | ||
| Body Roundness Index | - | - | - | - | ||
| Biochemical data | ||||||
| Hematocrit | - | - | - | - | ||
| Glycohemoglobin | 1.13 (1.01–1.27) | 0.0312 | 1.28 (1.09–1.51) | 0.0028 | 0.94 (0.69–1.28) | 0.7093 |
| Albumin | - | - | - | - | ||
| Creatinine | - | - | - | - | ||
| Comorbidities | ||||||
| Diabetes mellitus type 2 | - | - | - | - | ||
| Monitoring region | ||||||
| Northern region | 1.00 | 1.00 | 1.00 | |||
| Central region | 0.94 (0.59–1.50) | 0.7913 | 0.32 (0.13–0.78) | 0.0124 | 0.07 (0.02–0.24) | <0.0001 |
| Southern region | 0.61 (0.39–0.97) | 0.0351 | 0.31 (0.13–0.74) | 0.0083 | 0.14 (0.05–0.44) | 0.0007 |
| Meteorological factors | ||||||
| Temperature | 1.58 (1.35–1.86) | <0.0001 | 1.30 (0.96–1.77) | 0.0906 | 1.87 (1.26–2.77) | 0.0020 |
| Relative humidity | 1.06 (1.01–1.11) | 0.0104 | 1.07 (0.99–1.16) | 0.0870 | 1.15 (1.03–1.28) | 0.0108 |
| Rainfall | - | - | - | - | ||
| Air pollution factors | ||||||
| PM10 | - | - | - | - | ||
| PM2.5 | 0.97 (0.96–0.99) | 0.0002 | 1.00 (0.97–1.03) | 0.9868 | 0.97 (0.93–1.01) | 0.0930 |
| CO | 1.19 (0.45–3.14) | 0.7219 | 20.3 (3.14–131.12) | 0.0016 | 0.87(0.08–9.77) | 0.9122 |
| NO | - | - | - | - | ||
| NO2 | 1.01 (0.91–1.13) | 0.8136 | 1.39 (1.14–1.7) | 0.0012 | 1.58 (1.25–1.99) | 0.0001 |
| NOX | 0.98 (0.91–1.06) | 0.6126 | 0.76 (0.66–0.87) | 0.0001 | 0.86 (0.73–1.02) | 0.0809 |
| O3 | - | - | - | - | ||
| SO2 | 1.26 (1.09–1.45) | 0.0015 | 0.91 (0.68–1.22) | 0.5256 | 0.80 (0.56–1.13) | 0.2058 |
| Low Temperature | High Temperature | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obstructive vs. Normal | Restrictive vs. Normal | Mixed vs. Normal | Obstructive vs. Normal | Restrictive vs. Normal | Mixed vs. Normal | |||||||
| Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | |
| PM10 | 0.99 (0.98–0.99) | 0.0084 | 0.99 (0.97–1.01) | 0.3815 | 0.98 (0.96–1.01) | 0.1297 | 0.99 (0.99–1.00) | 0.1169 | 1.01 (0.99–1.02) | 0.1942 | 1.01 (0.99–1.02) | 0.5185 |
| PM2.5 | 0.98 (0.96–0.99) | 0.0133 | 0.97 (0.93–1.00) | 0.0845 | 0.87 (0.82–0.93) | <0.0001 | 0.98 (0.97–0.99) | 0.0015 | 1.02 (1.00–1.04) | 0.0458 | 1.00 (0.98–1.02) | 0.9675 |
| CO | 1.21 (0.88–1.66) | 0.2421 | 1.78 (0.98–3.25) | 0.0596 | 5.35 (2.88–9.94) | <0.0001 | 0.93 (0.63–1.39) | 0.7307 | 4.21 (1.77–9.99) | 0.0011 | 2.07 (0.84–5.11) | 0.1163 |
| NO | 1.03 (1.01–1.06) | 0.0041 | 1.05 (1.01–1.09) | 0.0137 | 1.11 (1.07–1.15) | <0.0001 | 1.06 (0.94–1.18) | 0.3491 | 0.86 (0.69–1.06) | 0.1604 | 1.14 (0.87–1.49) | 0.3313 |
| NO2 | 1.02 (0.99–1.04) | 0.2000 | 1.04 (0.99–1.09) | 0.0659 | 1.13 (1.08–1.19) | <0.0001 | 1.00 (0.98–1.03) | 0.7627 | 1.06 (1.01–1.12) | 0.0251 | 1.07 (1.00–1.14) | 0.0406 |
| NOX | 1.01 (1.00–1.03) | 0.0312 | 1.02 (1.00–1.05) | 0.0252 | 1.06 (1.04–1.08) | <0.0001 | 1.01 (0.98–1.03) | 0.6624 | 1.04 (0.99–1.08) | 0.1144 | 1.05 (0.99–1.11) | 0.0582 |
| O3 | 0.88 (0.83–0.93) | 0.8177 | 0.97 (0.92–1.02) | 0.2219 | 0.88 (0.83–0.93) | <0.0001 | 0.88 (0.75–1.03) | 0.0008 | 0.98 (0.87–1.11) | 0.7681 | 0.88 (0.75–1.03) | 0.1116 |
| SO2 | 1.04 (0.92–1.19) | 0.5211 | 1.29 (1.01–1.65) | 0.0402 | 1.77 (1.36–2.30) | <0.0001 | 1.12 (1.00–1.24) | 0.0426 | 1.17 (0.96–1.41) | 0.1189 | 1.38 (1.08–1.78) | 0.0104 |
| Northern and Central Region | Southern Region | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obstructive vs. Normal | Restrictive vs. Normal | Mixed vs. Normal | Obstructive vs. Normal | Restrictive vs. Normal | Mixed vs. Normal | |||||||
| Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | |
| PM10 | 0.99 (0.98–0.00) | 0.0769 | 1.02 (0.99–0.04) | 0.0626 | 1.01 (0.99–1.03) | 0.1390 | 0.99 (0.98–1.00) | 0.1266 | 0.96 (0.94–0.99) | 0.0029 | 0.98 (0.95–1.02) | 0.3101 |
| PM2.5 | 0.98(0.96–0.99) | 0.0048 | 1.00 (0.97–0.03) | 0.8962 | 0.98 (0.95–1.01) | 0.2379 | 0.97 (0.96–0.99) | 0.0030 | 1.00 (0.97–1.03) | 0.8837 | 0.97 (0.93–1.02) | 0.2641 |
| CO | 0.85 (0.66–0.10) | 0.2096 | 1.48 (0.89–0.46) | 0.1276 | 2.42 (1.44–4.06) | 0.0008 | 4.17 (2.08–8.37) | <0.0001 | 11.58 (3.29–40.81) | 0.0001 | 18.85 (3.53–100.59) | 0.0006 |
| NO | 1.00 (0.98–0.03) | 0.7094 | 1.03 (0.99–0.08) | 0.1062 | 1.08 (1.04–1.12) | 0.0002 | 1.28 (1.15–1.44) | <0.0001 | 0.89 (0.70–1.14) | 0.3524 | 1.33 (1.02–1.73) | 0.0349 |
| NO2 | 0.99 (0.97–0.00) | 0.1122 | 1.03 (0.99–0.06) | 0.1903 | 1.06 (1.02–1.10) | 0.0033 | 1.13 (1.08–1.18) | <0.0001 | 1.09 (1.01–1.18) | 0.0263 | 1.25 (1.13–1.37) | <0.0001 |
| NOX | 1.00 (0.98–0.01) | 0.4423 | 1.02 (0.99–0.04) | 0.1333 | 1.04 (1.02–1.06) | 0.0006 | 1.09 (1.06–1.13) | <0.0001 | 1.05 (0.99–1.11) | 0.1367 | 1.16 (1.08–1.25) | <0.0001 |
| O3 | 1.01 (0.98–0.04) | 0.4920 | 0.95 (0.90–0.02) | 0.1542 | 0.89 (0.83–0.95) | 0.0004 | 0.99 (0.95–1.03) | 0.5165 | 1.01 (0.94–1.09) | 0.7119 | 0.91 (0.82–1.01) | 0.0838 |
| SO2 | 0.95 (0.86–0.05) | 0.3577 | 1.19 (0.99–0.44) | 0.0658 | 1.34 (1.10–1.63) | 0.0031 | 1.65 (1.45–1.89) | <0.0001 | 1.29 (1.00–1.65) | 0.0491 | 1.83 (1.4–2.38) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.-W.; Chen, S.-C.; Tu, H.-P.; Wang, C.-W.; Hung, C.-H.; Chen, H.-C.; Kuo, T.-Y.; Wang, C.-F.; Lai, B.-C.; Chen, P.-S.; et al. The Impact of the Synergistic Effect of Temperature and Air Pollutants on Chronic Lung Diseases in Subtropical Taiwan. J. Pers. Med. 2021, 11, 819. https://doi.org/10.3390/jpm11080819
Wu D-W, Chen S-C, Tu H-P, Wang C-W, Hung C-H, Chen H-C, Kuo T-Y, Wang C-F, Lai B-C, Chen P-S, et al. The Impact of the Synergistic Effect of Temperature and Air Pollutants on Chronic Lung Diseases in Subtropical Taiwan. Journal of Personalized Medicine. 2021; 11(8):819. https://doi.org/10.3390/jpm11080819
Chicago/Turabian StyleWu, Da-Wei, Szu-Chia Chen, Hung-Pin Tu, Chih-Wen Wang, Chih-Hsing Hung, Huang-Chi Chen, Tzu-Yu Kuo, Chen-Feng Wang, Bo-Cheng Lai, Pei-Shih Chen, and et al. 2021. "The Impact of the Synergistic Effect of Temperature and Air Pollutants on Chronic Lung Diseases in Subtropical Taiwan" Journal of Personalized Medicine 11, no. 8: 819. https://doi.org/10.3390/jpm11080819






