Abstract
Cellular therapy is an emerging field in clinical and personalised medicine. Many adult mesenchymal stem/progenitor cells (MSC) or pluripotent derivatives are being assessed simultaneously in preclinical trials for their potential treatment applications in chronic and degenerative human diseases. Endometrial mesenchymal stem/progenitor cells (eMSC) have been identified as clonogenic cells that exist in unique perivascular niches within the uterine endometrium. Compared with MSC isolated from other tissue sources, such as bone marrow and adipose tissue, eMSC can be extracted through less invasive methods of tissue sampling, and they exhibit improvements in potency, proliferative capacity, and control of culture-induced differentiation. In this review, we summarize the potential cell therapy and tissue engineering applications of eMSC in pelvic organ prolapse (POP), emphasising their ability to exert angiogenic and strong immunomodulatory responses that improve tissue integration of novel surgical constructs for POP and promote vaginal tissue healing.
1. Introduction
Pelvic organ prolapse (POP) is a common urogynaecological disorder that affects one in four women across all age groups, or over 50% of postmenopausal parous women with a history of vaginal birth [1,2]. POP is defined as the descent of the anterior and/or posterior vaginal wall, the apex of the vagina, or the vault, into or past the vaginal introitus, and presents significant yet hidden clinical burdens of disease with bowel, bladder, or sexual dysfunction that profoundly impact the quality of life of sufferers [3]. Up to 19% of women have a lifetime risk of undergoing reconstructive surgery for POP [4,5], with a 30–35% risk of reoperation due to recurrent anatomical failure [6], or adverse events associated with primary surgery [7].
In the recent past, urogynaecologists have implemented polypropylene (PP) mesh to augment POP surgery in an attempt to reduce the risk of anatomical failure. This mesh comprised synthetic, non-degradable macroporous monofilament fibres that caused serious adverse events in some women when implanted vaginally; including infection, retraction, exposure, and erosion [8]. The frequency and severity of these post-operative complications culminated in the Food and Drug Administration (FDA) issuing warnings against their use, and the subsequent prohibition of their use in transvaginal POP surgery in Australia, New Zealand, USA, and UK [9]. The adverse events associated with use of PP mesh in pelvic reconstructive surgery has been attributed to a lack of biomechanical compatibility of synthetic mesh with the unique dynamics of vaginal tissue, which in turn is associated with an exaggerated foreign body response that results in chronic fibrosis [10].
Thus, there is a critical need to provide novel surgical constructs that are not only safe and surgically efficacious, but also congruent with the native tissue, to maximise post-operative tissue healing and mesh integration. Tissue engineered surgical constructs consisting of endometrial mesenchymal stem cells (eMSC), combined with mesh, have demonstrated improved surgical outcomes in pre-clinical models of POP surgery, with a more favourable immune response and improved biomechanical properties of vaginal tissue [11]. This review will highlight key points in the trajectory of eMSC discovery, emphasising their potential benefit when combined with novel meshes in enhancing tissue integration and modulating inflammatory responses after mesh augmented pelvic reconstructive surgeries.
2. Pelvic Organ Prolapse
2.1. Aetiology
POP is a significant urogynaecological disorder that profoundly impacts the lives of millions of women worldwide due to consequent bladder, bowel, and sexual dysfunction [12]. Risk factors include multiple vaginal births (>3), difficult obstetric history, macrosomia, pregnancy, ageing, obesity, diabetes, hypertension, chronic straining, and coughing [13]. Other significant risk factors include the use of forceps, and other gynaecological procedures such as hysterectomy for other clinical indications [5,14,15].
2.2. Anatomy, Pathophysiology, and Biomechanics
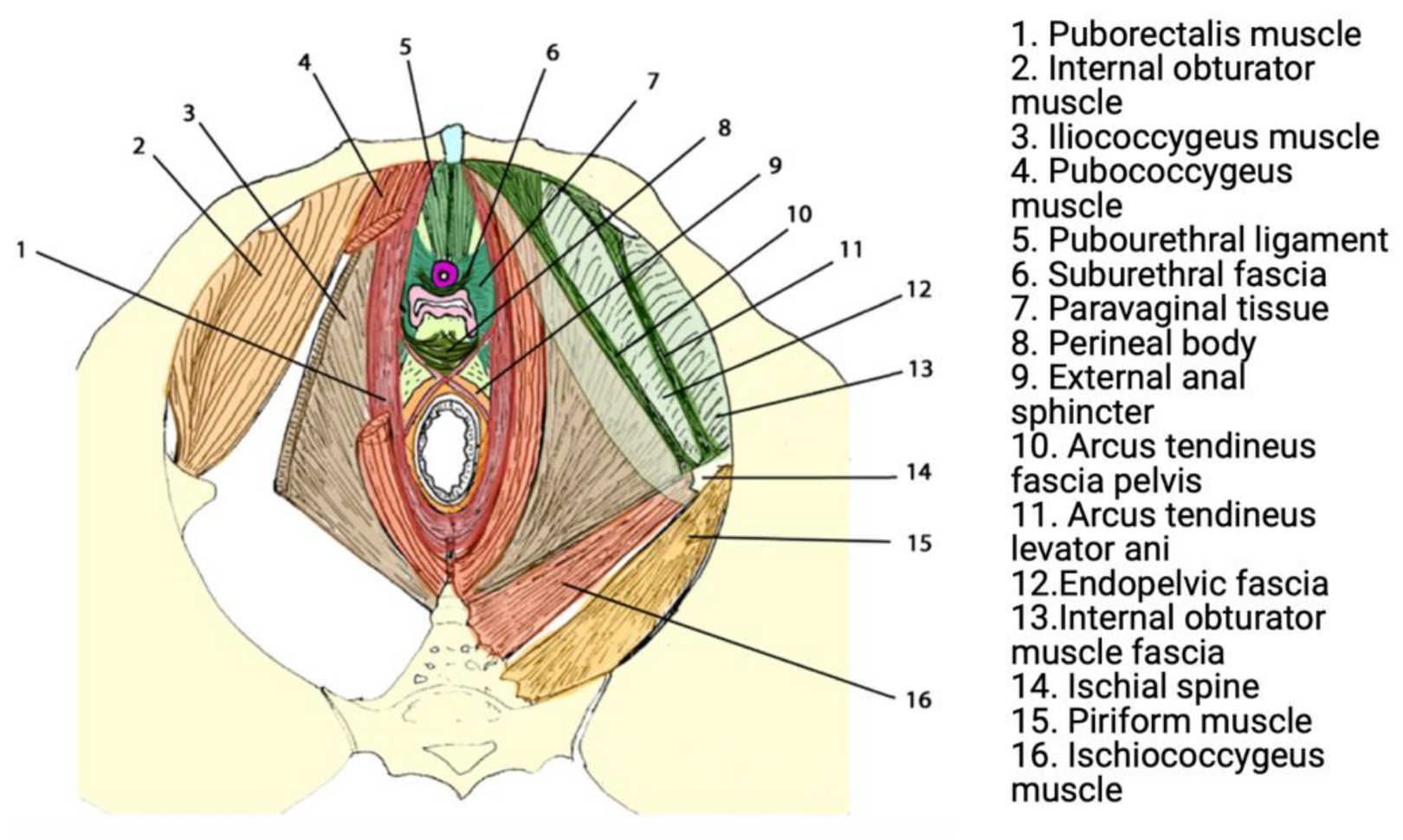
Urogenital prolapse results from anatomic deficits in the ligamentous, fascial, and muscular structures that support the pelvic organs in sequence [16]. These structures have the crucial role of supporting abdominopelvic viscera through tonic contraction, passive resistance to increases in intra-pelvic/abdominal pressure (e.g., coughing), and crucially contribute to urinary and faecal continence. The most significant structure contributing to the pelvic floor is the levator ani muscle (LAM), which comprises three sling-shaped muscles known as the puborectalis, pubococcygeus, and iliococcygeus [17,18] (Figure 1).
Vaginal birth results in stress to the vaginal wall and surrounding ligamentous, fascial, and muscular structures, beyond their critical stretch limit, resulting in tissue damage through non-elastic deformation [19]. During labour, pressure from strong uterine contractions and the transiting foetal head are directed to the LAM muscle, particularly the medial portion of the pubococcygeus. This results in an increased stretch ratio of up to 3.2, in contrast to a previous non-introitus stretch ratio of 2.17, which damages muscle fibres through non-elastic deformation [20,21,22].
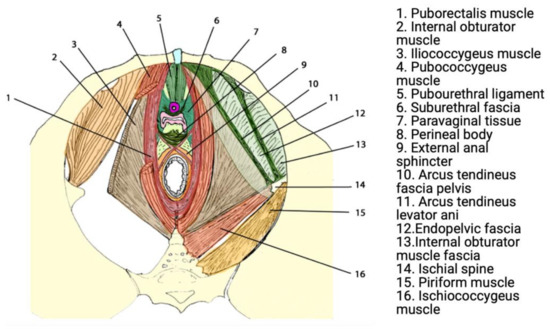
Figure 1.
Functional anatomy of the pelvic floor. Reprinted with permission from Lamblin et al. (2016) [23]. Copyright Springer Nature, 2015.
Figure 1.
Functional anatomy of the pelvic floor. Reprinted with permission from Lamblin et al. (2016) [23]. Copyright Springer Nature, 2015.
2.3. Treatment of POP
Conservative treatment of POP incorporates non-operative approaches such as weight loss, avoiding exacerbating activities (lifting, constipation, and coughing), pelvic floor muscle training (PFMT), and various vaginal pessaries [24,25]. Failure of these treatments necessitates surgery in an estimated 20% of women, which includes native tissue repairs and/or mesh augmentation [4]. The operative approach is dependent on unique patient and pathological factors such as age, weight, physical activity level, severity of prolapse and symptoms, and expected durability of repair [25].
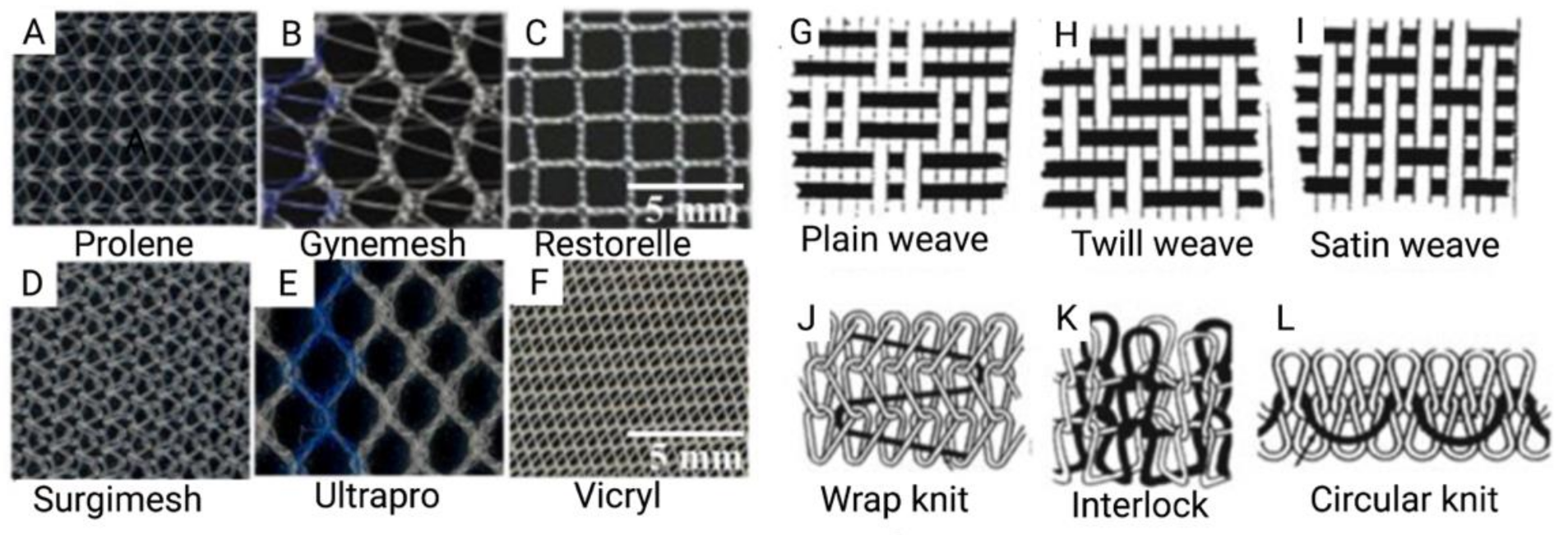
In the past, pelvic reconstructive surgeons had to balance the higher risk of anatomical failure with native tissue repair with the risk of post-operative complications from mesh augmented repair [26]. Many surgeons chose to optimize the durability of the repair, and thus the increased use of synthetic non-degradable PP meshes for POP surgery followed [27,28,29,30] (Figure 2).
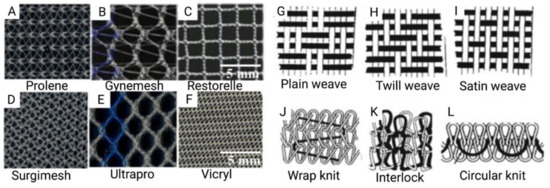
Figure 2.
(A–F) Commercially available prosthetic meshes for POP; (G–L) Illustrations of mesh topography demonstrating different weave and knit types. Reprinted from Pott et al. (2012) [31]. Copyright: © 2021 by the authors.
2.4. Clinical Adversities
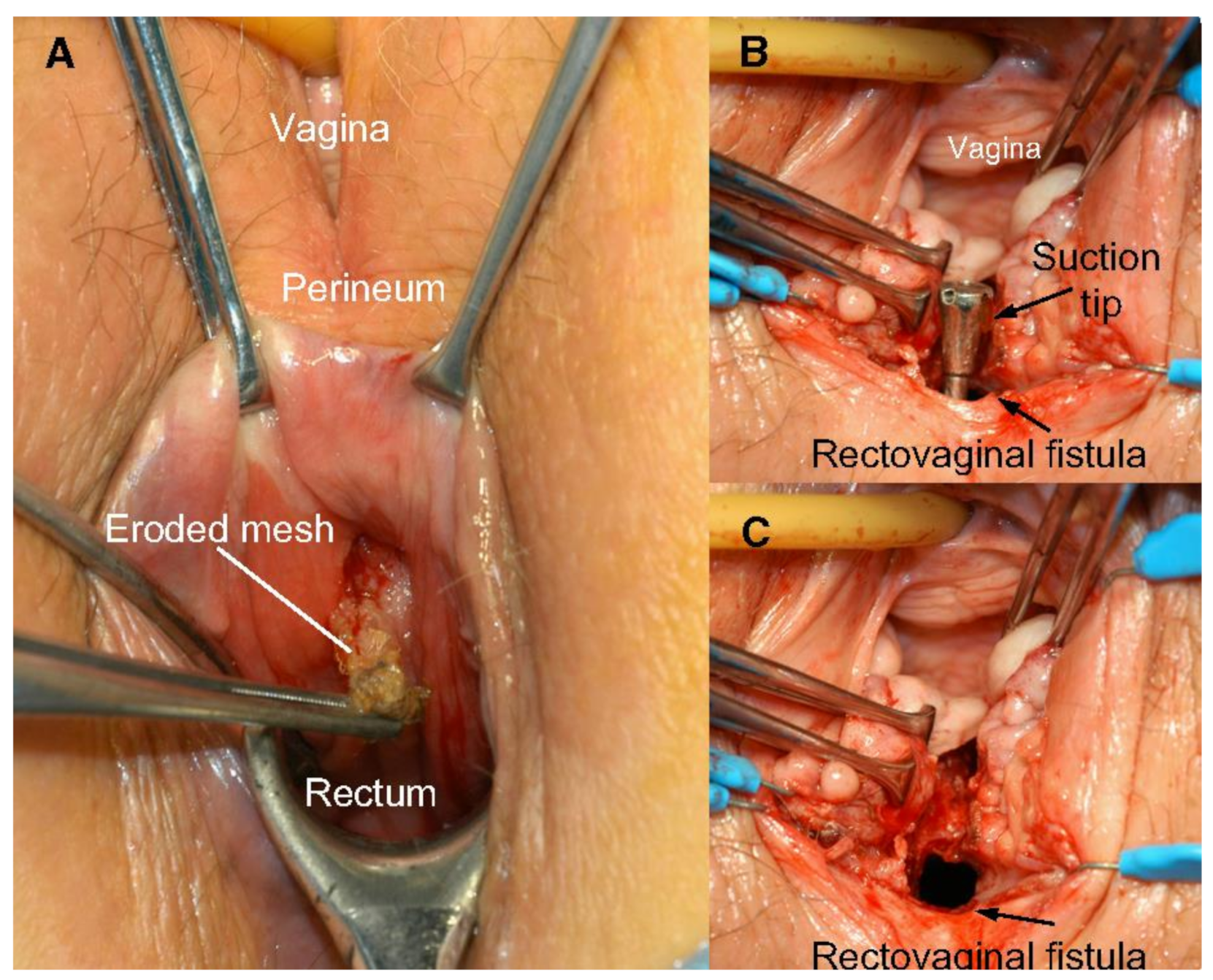
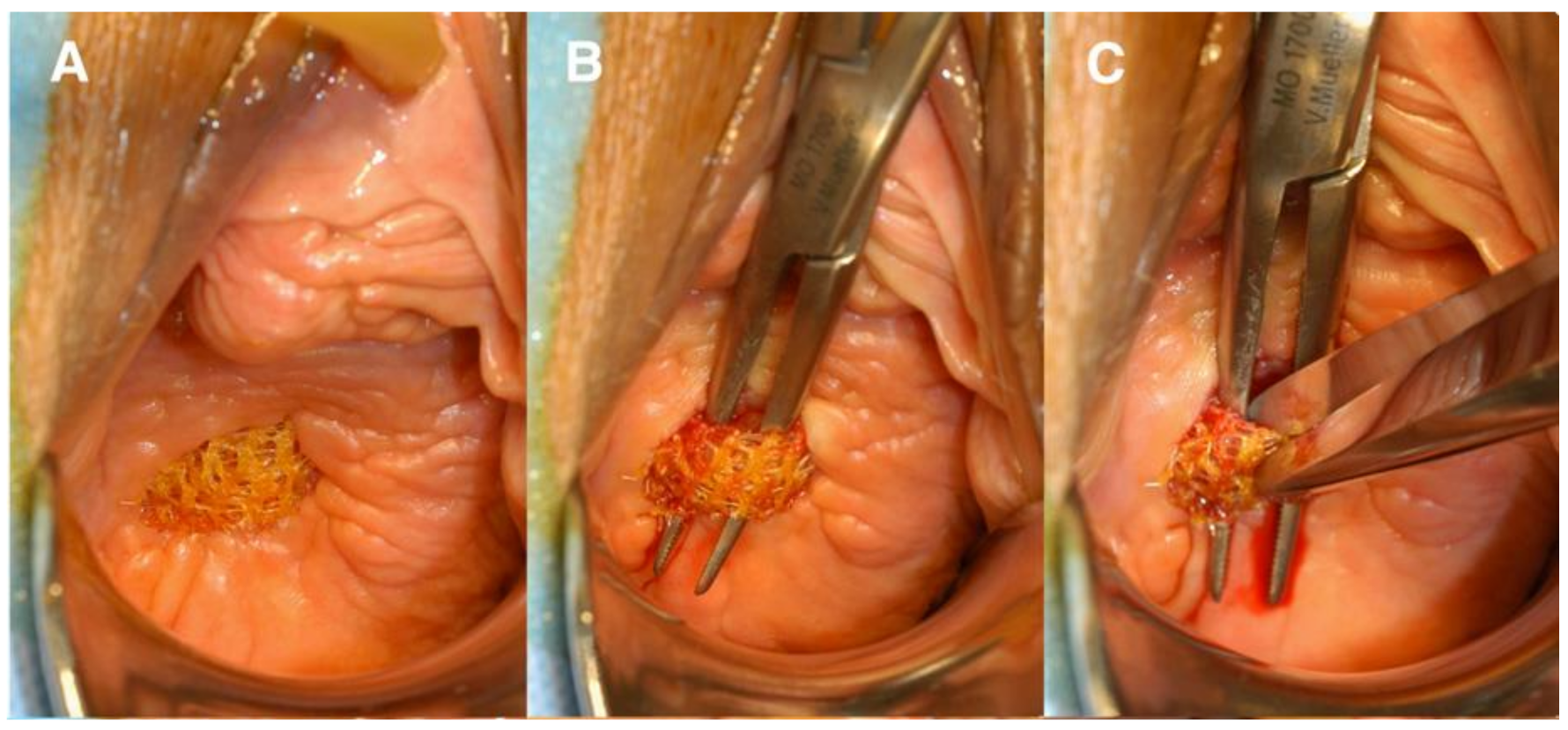
The use of transvaginal PP mesh resulted in post-operative complications including chronic pelvic and sexual pain, infection, extrusion, and erosion of mesh [32]. Mesh exposure occurs when the mesh extrudes from the vaginal mucosa, while mesh erosion occurs when mesh invades into other surrounding structures such as the bladder, rectum, or urethra [9] (Figure 3 and Figure 4). Even after optimized tertiary management of mesh complications, the impact of these adverse effects cause physical and emotional pain, in addition to the discomfort caused by original pelvic floor dysfunction. Feelings of anxiety, desperation, hopelessness, and abandonment are some detrimental emotional corollaries of adverse events that can impact a woman’s quality of life in the long-term [33].
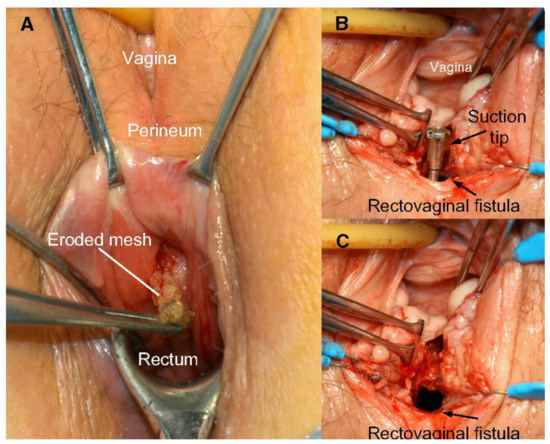
Figure 3.
(A) Rectovaginal fistula formation secondary to mesh erosion through posterior vaginal wall; (B) rectal side of rectovaginal fistula after mesh removal; and (C) vaginal side of rectovaginal fistula after mesh removal. Reprinted with permission from Margulies et al. (2012) [34]. Copyright Elsevier, 2008.
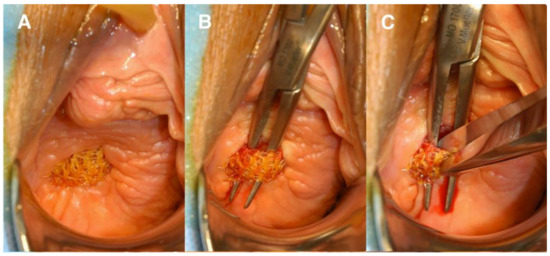
Figure 4.
(A) Anterior vaginal wall with mesh exposure; (B) undermining the mesh; (C) transecting the mesh. Reprinted with permission from Margulies et al. (2012) [34]. Copyright Elsevier, 2008.
The adverse events associated with synthetic mesh has been attributed to a number of factors. Firstly, an incompatibility of various mesh characteristics such as size, weight, stiffness, thickness, and porosity to comply with the unique dynamics of vaginal tissue [35]. Secondly, a suboptimal foreign body response resulting in increased matrix metalloproteinase (MMP) activity, which disrupts the integrity of the histological layers of the vagina through tissue degradation, particularly affecting the vaginal adventitia and smooth muscle fibres of the muscularis layer [36,37,38]. Transvaginal meshes used in vaginal repair should promote healthy tissue formation and remodelling while minimizing the host foreign body response.
At present, there is a large void in the surgical management of vaginal prolapse which presents a need for novel bioengineered surgical constructs. New generations of mesh must consider the unique properties of the vaginal tissue they are designed to mimic in their nanostructure topography, porosity, and stiffness. The amalgamation of highly specialised disciplines such as tissue engineering, stem cell therapy and personalised medicine provide important approaches and tools to respond to this challenge.
3. Mesenchymal Stem/Progenitor Cells
The primary focus of cell-based therapies in the past has been adult multipotent mesenchymal stem or stromal cells (MSC). These cells have been pursued for their clonogenicity, proliferative capacity, differentiation to mesodermal lineages, secretion of angiogenic factors, and many other growth-promoting factors [39,40,41]. MSC provide therapeutic potential through their interaction with the both the innate and adaptive immune systems, by direct cell-cell contact and/or MSC secretion of immunosuppressive factors such as Indoleamine 2,3 deoxygenase (IDO), prostaglandin E2 (PGE2), nitric oxide (NO), human leukocyte antigen G5 (HLA-G5), IL-10, IL-6, and TGF-β [40]. This results in the inhibition of T and B cell proliferation, conversion of Th17 cells toward regulatory T cells (Tregs), and macrophage switching from M1 pro-inflammatory phenotype to anti-inflammatory M2 polarisation [42,43,44]. By acting in a paracrine manner, MSC possess the ability to promote endogenous cell proliferation, stimulate angiogenesis, and reduce fibrosis to effect repair of damaged host tissues [45].
MSC have typically been isolated from bone marrow through plastic adherence, and have also been extracted from other tissue including umbilical cord and unfractionated adipose tissue. However, MSC isolated from these sites have had limited clinical efficacy, due to their variable potency, lack of reproducibility in cell culture, and high rates of culture-induced spontaneous differentiation to fibroblasts [43]. For instance, the number of MSC in bone marrow significantly decreases with ageing, with an estimated tenfold loss for each decade of life [46]. Another major hurdle of MSC clinical translation has been the often painful and invasive techniques of tissue sampling involved in sourcing MSC, such as bone marrow aspiration or liposuction [47].
Human endometrial MSC (eMSC) have emerged as a novel source of therapeutic immunomodulatory cells isolated from an endometrial biopsy specimen, collected in an office-based procedure without anaesthesia, or harvested at the time of hysteroscopy and hysterectomy performed for other clinical indications [47]. The ease of sampling eMSC presents the opportunity to overcome one of the hurdles associated with clinical translation of bone marrow and adipose MSC. In their natural environment, eMSC are highly proliferative perivascular cells, functioning in generating vasculature to support rapid endometrial growth each menstrual cycle [39].
During the first 4–10 days of each menstrual cycle, 5–10 mm of new endometrial mucosa known as the functionalis grows from the basalis (0.5–1 mm thick) not shed during menstruation, into which an embryo subsequently implants [48]. If implantation does not occur, the vascularised and secretory endometrium sheds during days 1–4 of the subsequent menstrual cycle. Within 48 h of endometrial shedding, rapid repair/re-epithelialization of the endometrial surface occurs to cover the exposed basalis surface followed by regeneration of the functionalis layer [49]. This process repeats itself around 400 times across a woman’s reproductive life. Gene profiling has demonstrated that the lysed stroma of menstrual endometrium is enriched in genes involved in extracellular matrix (ECM) biosynthesis and degradation [50]. The ability for eMSC to promote the deposition of new ECM makes it an attractive source for technical applications in novel therapeutic POP constructs.
eMSC were first identified in the perivascular stroma of mouse endometrium, as stromal label-retaining cells (LRCs) with about 13% located around blood vessels and 13% at the endometrial–myometrial junction [51]. Studies on human endometrium obtained from hysterectomy tissue demonstrated the existence of small numbers of stromal cells capable of forming colonies initiated by stromal stem/progenitor cells or colony-forming unit fibroblasts (CFU- F) [52], thereby indicating their pluripotent potential. Though a greater percentage of CFU-F was observed in proliferative endometrium under the influence of rising circulating oestrogen levels, they were also detected in the non-cycling atrophic endometrium from postmenopausal women and women on oral contraceptives [53,54].
However, like all MSC, the challenges of eMSC lie in isolating them from the perivascular stroma, and limiting spontaneous differentiation to non-clonogenic fibroblasts during the culture expansion [55,56]. The identity of clonogenic eMSC was revealed by showing their enrichment in endometrial stromal cells, colocalizing two perivascular markers, CD140b (PDGFRb) and CD146 and their perivascular location (Figure 5) [55]. Gene profiling distinguished CD140b+CD146+ MSC within a perivascular niche in human endometrium from CD140b+CD146− endometrial fibroblasts, which differentially expressed 762 other genes (Figure 5) [57]. The use of these markers allows prospective isolation and colocalization of eMSC, and supports the existence of eMSC within subpopulations of pericytes in human endometrium [58]. A single marker, W5C5 or Sushi Domain-containing 2 (SUSD2) is now used to purify and isolate rare perivascular eMSC from surrounding endometrial stromal cells [58]. SUSD2 enables prospective isolation of eMSC from freshly isolated cell suspensions using magnetic bead sorting, providing a more clonogenic population than obtained by flow cytometry sorting, which adversely affects cell viability [59,60].
A small molecule transforming growth factor-beta receptor (TGFRβ) inhibitor, A83-01, aids culture expansion of eMSC and other reproductive MSC, by maintaining them in the undifferentiated state promoting MSC proliferation, and preventing apoptosis and senescence [61,62]. Previous studies using functional assays and whole transcriptome sequencing have demonstrated that A83-01 promoted proliferation and increased cloning efficiency in premenopausal SUSD2 eMSC, providing insight into their biological activity [61,62,63]. The potency of A83-01 treated eMSC was also demonstrated by the increased expression of novel bone marrow MSC (bmMSC) potency genes TWIST1, TWIST2, JAG1, LIFR, and SLIT2 in a novel assay of A83-01 treated MSC [62,64]. A83-01 treated eMSC are posited to improve tissue regeneration through the upregulation of pro-angiogenic factors and inhibition of anti-angiogenic factors, improved cell migratory capacity, promoting angiogenesis demonstrated by increased endothelial cell tube formation, and downregulation of fibrosis-associated gene transcription [62]. Furthermore, RNA sequencing studies have confirmed that eMSC secrete exosomes, potentially explaining their paracrine immunomodulatory effects [62]. A83-01 treated eMSC differentially expressed classic exosome markers, CD63, CD9, CD81, and CD82, confirming their paracrine immunomodulatory potential that could be exploited in regenerative or personalized medicine [62]. We are currently investigating whether or not eMSC produce exosomes distinguished from other types of MSC; however, data is insufficient to comment on this at present.
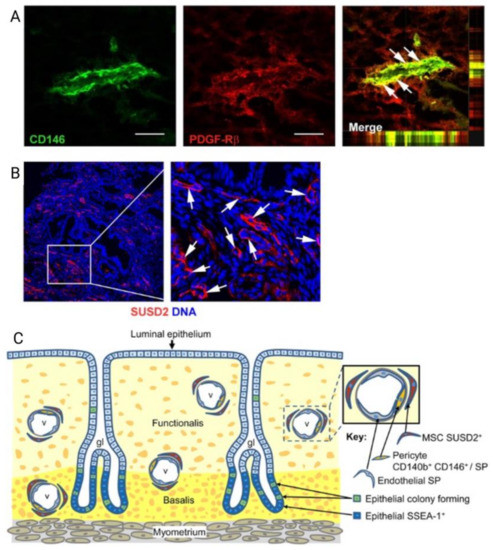
Figure 5.
Localisation of human endometrial mesenchymal stem cells (eMSC). (A) Immunofluorescence images of human endometrium showing colocalization markers CD146 and platelet derived growth factor receptor beta (PDGF-Rβ, CD140b) to reveal the perivascular niche of eMSC (merge in the right of panel A); (B) perivascular Sushi Domain containing-2 (SUSD2) expression showing perivascular location (white arrows); and (C) Schematic showing the perivascular identity of co-expressing CD146 and PDGFRβ/CD140b and SUSD2+ eMSC in the endometrial basalis and functionalis layer, indicating eMSC will be shed into menstrual blood. Scale bar in (A) = 50 µm. Reprinted with permission from Gargett et al. (2010) [65]. Copyright Oxford University Press, 2010.
Figure 5.
Localisation of human endometrial mesenchymal stem cells (eMSC). (A) Immunofluorescence images of human endometrium showing colocalization markers CD146 and platelet derived growth factor receptor beta (PDGF-Rβ, CD140b) to reveal the perivascular niche of eMSC (merge in the right of panel A); (B) perivascular Sushi Domain containing-2 (SUSD2) expression showing perivascular location (white arrows); and (C) Schematic showing the perivascular identity of co-expressing CD146 and PDGFRβ/CD140b and SUSD2+ eMSC in the endometrial basalis and functionalis layer, indicating eMSC will be shed into menstrual blood. Scale bar in (A) = 50 µm. Reprinted with permission from Gargett et al. (2010) [65]. Copyright Oxford University Press, 2010.
Similar to other MSC, eMSC exert strong immunomodulatory and anti-inflammatory effects primarily via paracrine cross-talk with cells of the innate and adaptive immune system, including macrophages, T cells, and NK cells. These effects may be harnessed to enhance the success of transvaginal mesh surgery reducing pathological foreign body responses, thereby promoting tissue integration. In addition, their perivascular identity makes them good candidates for regenerative medicine through the regulation of angiogenesis, inflammation, and fibrosis [11,66] (see Table 1).

Table 1.
Summary of endometrial MSC (eMSC)/stromal cell discoveries combined with novel tissue engineered scaffolds for applications in pelvic organ prolapse (POP).
Given eMSC demonstrate angiogenic activity, concerns have been raised about their potential tumorigenicity and carcinogenicity. In an immunosuppressed xenograft rat model of brain stroke, menstrual blood stromal fibroblasts not only failed to stimulate, but also inhibited tumour growth and showed no evidence of tumour formation [77]. Similar anti-tumourigenic properties have not been demonstrated in eMSC to date, though no cancer due to culture expanded MSC has been diagnosed in pre-clinical or clinical studies [60]. Genomic studies have demonstrated that eMSC cultured in A83-01-containing medium may promote the stability of telomeres, through upregulated genes such as TERC (Telomerase RNA Component), TERF1 and 2 (Telomeric Repeat Binding Factors 1 and 2), TINF2 (TERF1 Interacting Nuclear Factor 2), TERF2IP (TERF2 Interacting Protein), TNKS (Tankyrase) and POT1 (Protection of Telomere 1), which collaborate to regulate telomere length and protect cells from chromosomal damage [62]. Nonetheless, the potential for genomic instability needs to be further assessed through transcriptomics, karyotyping and in animal models.
In addition to their enhanced therapeutic potential, another strength of eMSC is their retrieval through a relatively less invasive and cost-effective method of sampling that is not limited by the patient’s age, menopausal status, or medical comorbidities. Furthermore, by drawing upon the principles of personalised medicine, there is an exciting opportunity to use autologous eMSC derived from each patient to create a patient-specific tissue engineered cellular surgical construct.
4. Engineering Novel Meshes with eMSC
Tissue engineering involves the creation of a tissue implant or substitute comprising a scaffold seeded with stem/progenitor cells, growth factors and/or drugs for restoring normal tissue function [38,39]. The biomaterial scaffold functions as physical support but also provides a three-dimensional nanostructured environment onto which therapeutic mesenchymal stem/progenitor cells may adhere, to accelerate and promote tissue regeneration and repair. Recently, it has been proposed that tissue engineering approaches combining autologous stem/progenitor cells with biomaterials fabrication could drastically improve the outcomes of pelvic reconstructive surgery [39,78].
4.1. eMSC Non-Degradable Tissue Engineered Mesh
Our group has aimed to test the therapeutic potential of tissue engineering constructs in multiple small and large animal pre-clinical models. A rat skin fascial defect model was first implemented to assess an FDA-approved non-degradable polymer, polyamide (PA), which was warp-knitted to generate a mesh subsequently coated in cross-linked gelatin (PAG) for eMSC seeding and delivery [79]. The PAG meshes seeded with eMSC promoted more neovascularization and neo-tissue formation surrounding mesh filaments compared with unseeded PAG mesh. A more favourable response was also observed in the eMSC/PAG implanted tissue, where there was a switch to the M2 wound healing macrophage phenotype after early M1 inflammatory macrophage infiltration. Long-term chronic inflammation in the mesh–tissue complex was reduced in eMSC-seeded PAG mesh at 90 days, compared with PAG mesh alone [11,79]. Collagen fibres deposited around PAG eMSC-seeded mesh were crimped and more organized, improving the stiffness of the mesh/tissue complex and therefore the overall biomechanical properties of the tissue. However, eMSC only persisted for in-vivo for 2 weeks, emphasising that the more favourable tissue response was likely generated from paracrine anti-inflammatory effects that persisted long after the eMSC were reabsorbed [79]. A similar tissue engineered construct has been assessed in a large animal pre-clinical model, where PAG mesh was used either alone or seeded with autologous ovine eMSC. This study demonstrated that a simpler two step procedure of inserting uncoated polyamide (PA) mesh followed by the application of autologous eMSC in a gelatin hydrogel crosslinked in situ with blue light drastically improved tissue–mesh integration and resulted in no cases of mesh erosion [72].
4.2. eMSC Degradable Tissue Engineered Mesh
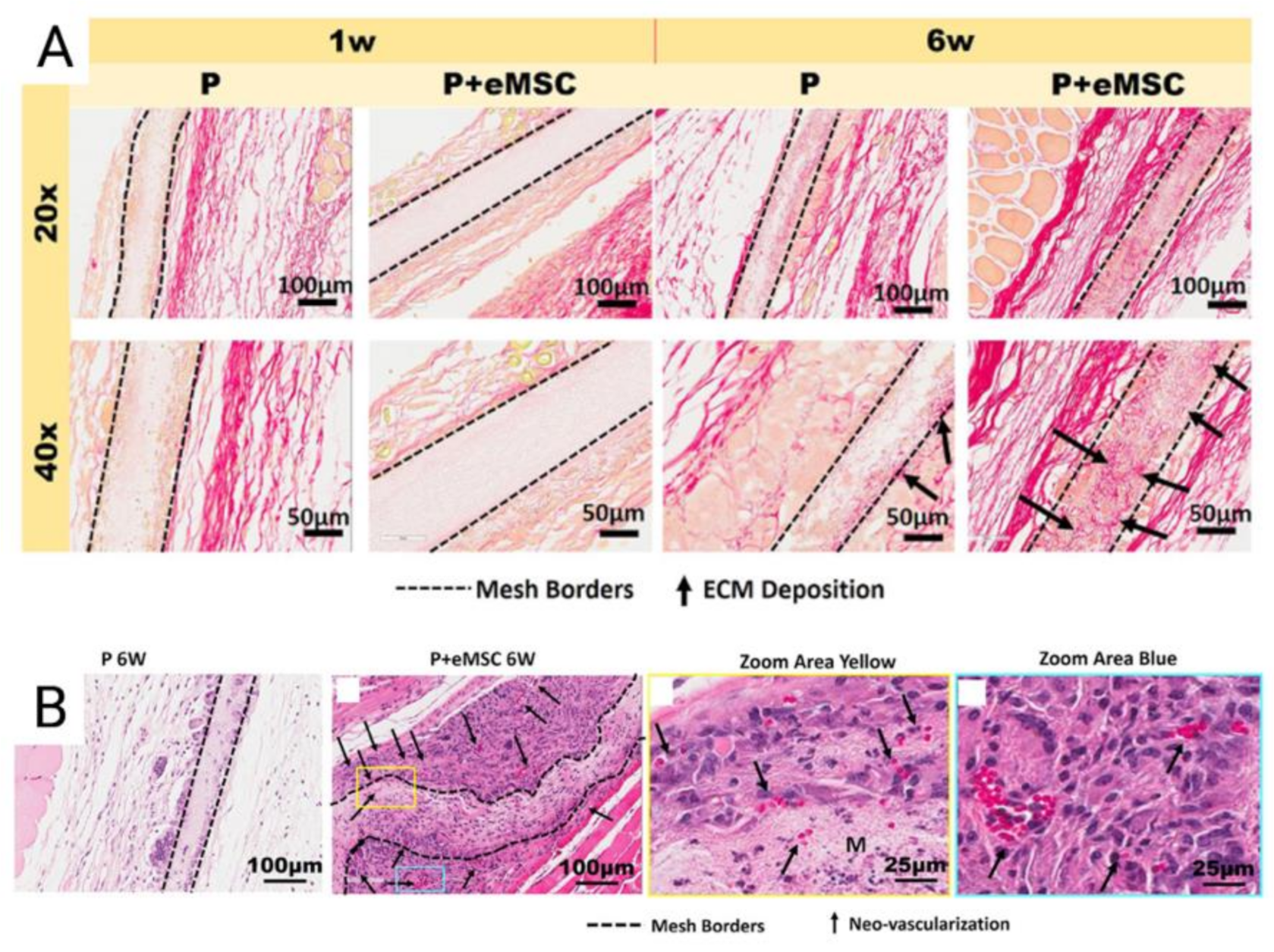
Tissue engineering of degradable electrospun meshes with eMSC have demonstrated improvements in mesh integration. Electrospinning is an emerging versatile method of mesh construction that uses electric forces to draw charged threads of polymer solution together to create ultrafine meshes that allow for host immune cells to adhere in both nanofibrous and microfibrous formats [80,81]. These emerging biomimetic electrospun meshes produce an ECM-like topography that mimic the ECM of vaginal tissue of women with POP at the nanoscale [73]. Nanostructured meshes also provide a larger surface area for the delivery of eMSC and other adsorbing proteins and growth factors [19,73]. Poly-L-lactide-co-ε-caprolactone (PLCL) is a biocompatible, elastic, and flexible polymer investigated by several groups for novel tissue engineering applications, as its elastic modulus is well matched to the nanoarchitecture of vaginal tissue [81]. The properties of biomimetic PLCL were maximized by blending it with gelatin and incorporating eMSC in a mouse skin wound repair model. Blending of PLCL with gelatin forms nanostructured meshes and significantly increased the hydrophilicity and pore size of the meshes [73]. As a result, such blended meshes could be completely infiltrated by therapeutic eMSC despite a pore size of less than 3 μm. The presence of eMSC prevented rapid degradation of PLCL mesh, more effectively recruited host cells into the scaffolds, improved neovascularisation and successfully promoted an M2 macrophage phenotype to sustain a favourable inflammatory response [73]. Furthermore, eMSC seeded mesh resulted in an upregulation of genes controlling ECM deposition, cell adhesion and angiogenesis in tissues (Figure 6) [73].
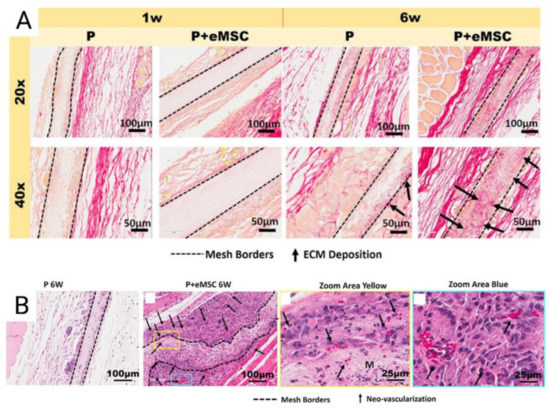
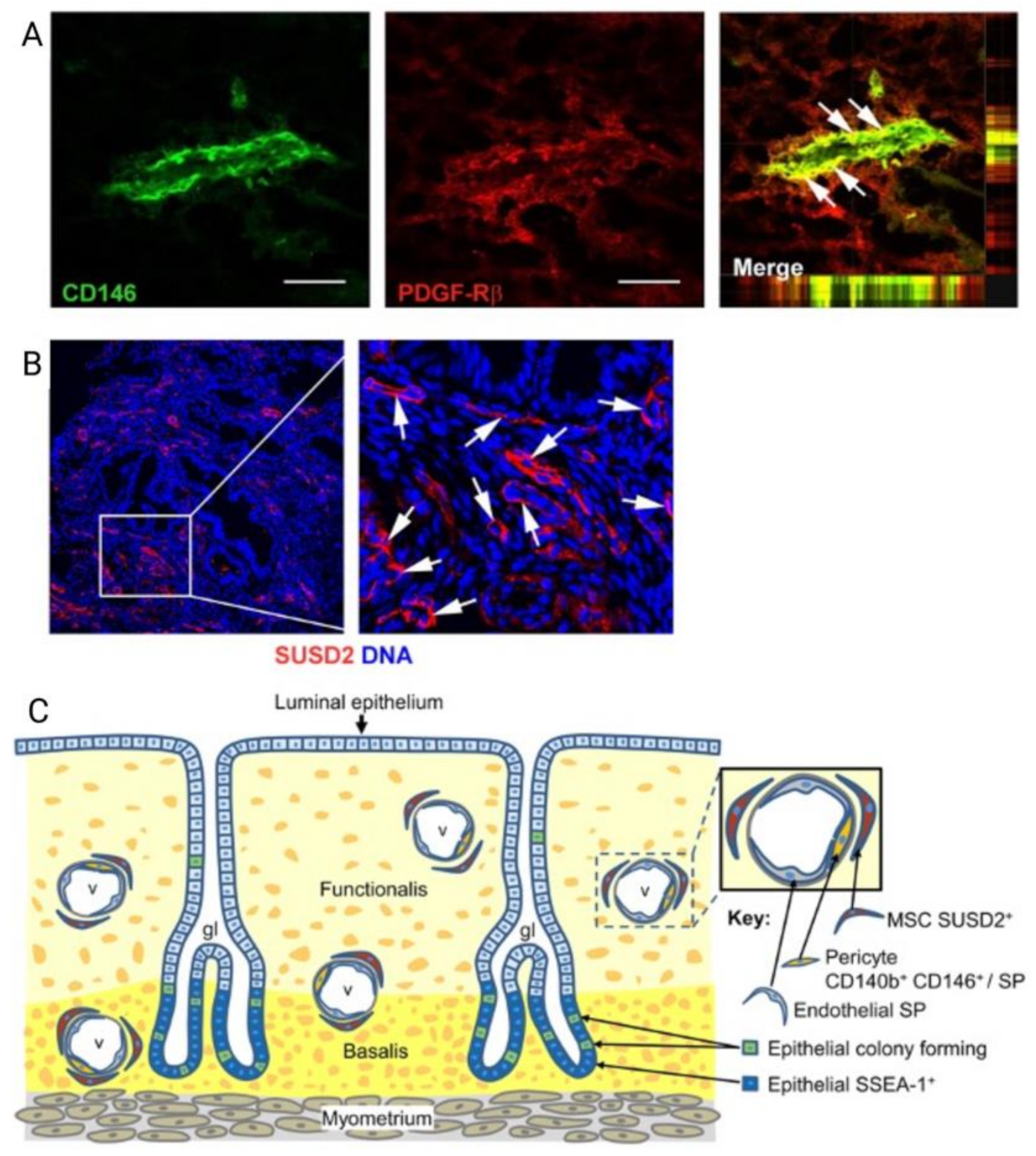
Figure 6.
(A) Explanted Poly-L-lactide-co-ε-caprolactone (PLCL) nanomeshes with and without endometrial mesenchymal stem/stromal cells (eMSC) showing Picro-Sirius red staining of collagen, at 1 and 6 weeks explantation; (B) 6-week nanomesh explants with and without eMSC demonstrating improved neovascularisation (black arrows) within mesh area (yellow) with eMSC compared to without. Reprinted with permission from Mukherjee et al. (2020) [66]. Copyright: © 2021 by the authors.
In another study, PLCL meshes spun with fibrinogen (PLCL/Fg) have been shown to promote faster neo-vascularisation, better collagen fibre organisation, muscle regeneration, and no tissue erosion when compared with other polypropylene meshes, in a canine abdominal defect model [82]. This PLCL/Fg mesh was also trialled in human pelvic floor reconstruction (n = 38), with significant improvements in some aspects of POP-Q measurements when compared with the PP mesh group. Polylactic acid (PLA) meshes seeded with human and rat adipose-derived MSC implanted in a rodent abdominal wall defect model demonstrated improved macrophage infiltration, angiogenesis, ECM formation, and remodelling as indicated by increased deposition of collagen III [83]. PLCL meshes combined with eMSC have also demonstrated significant downregulation of several inflammatory genes in a 6-week mouse model, including Tnf, Il1b, Ccl4, Ccl5, Ccl7, Ccl12, Ccl19, Cxcl1, Cxcll-2, Ccr1, and Ccr7, and upregulation of anti-inflammatory genes involved in the M2 macrophage response like Arg1 and Mrc1 [66]. Thus, nanofiber electrospun mesh combined with eMSC have demonstrated a more favourable biomimetic and immunomodulatory profile that has the potential for applications in POP.
In a recent landmark pre-clinical study, our team developed the first known 3D-bioprinted polycaprolactone (PCL) mesh and evaluated its performance in vitro and in vivo. This mesh was constructed by a two-step process involving melt electrospinning (MES) followed by 3D bioprinting of therapeutic mCherry-labelled SUSD2+ eMSC in an aloe-vera-alginate hydrogel, and subsequently implanted under the skin of NOD scid gamma (NSG) mice. In vivo, acute foreign body response assessment revealed that eMSC bioprinted on electrospun mesh improved host tissue integration, eMSC retention, and promoted an anti-inflammatory M2 macrophage phenotype characterised by F4/80+CD206+ colocalization (Figure 7 and Figure 8) [74].
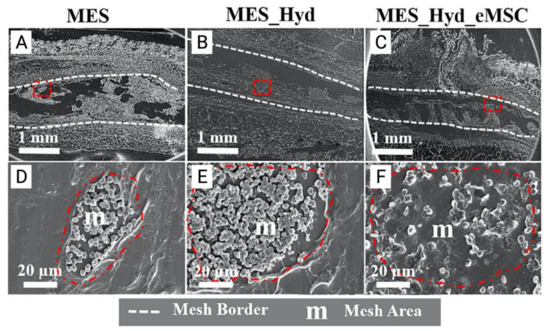
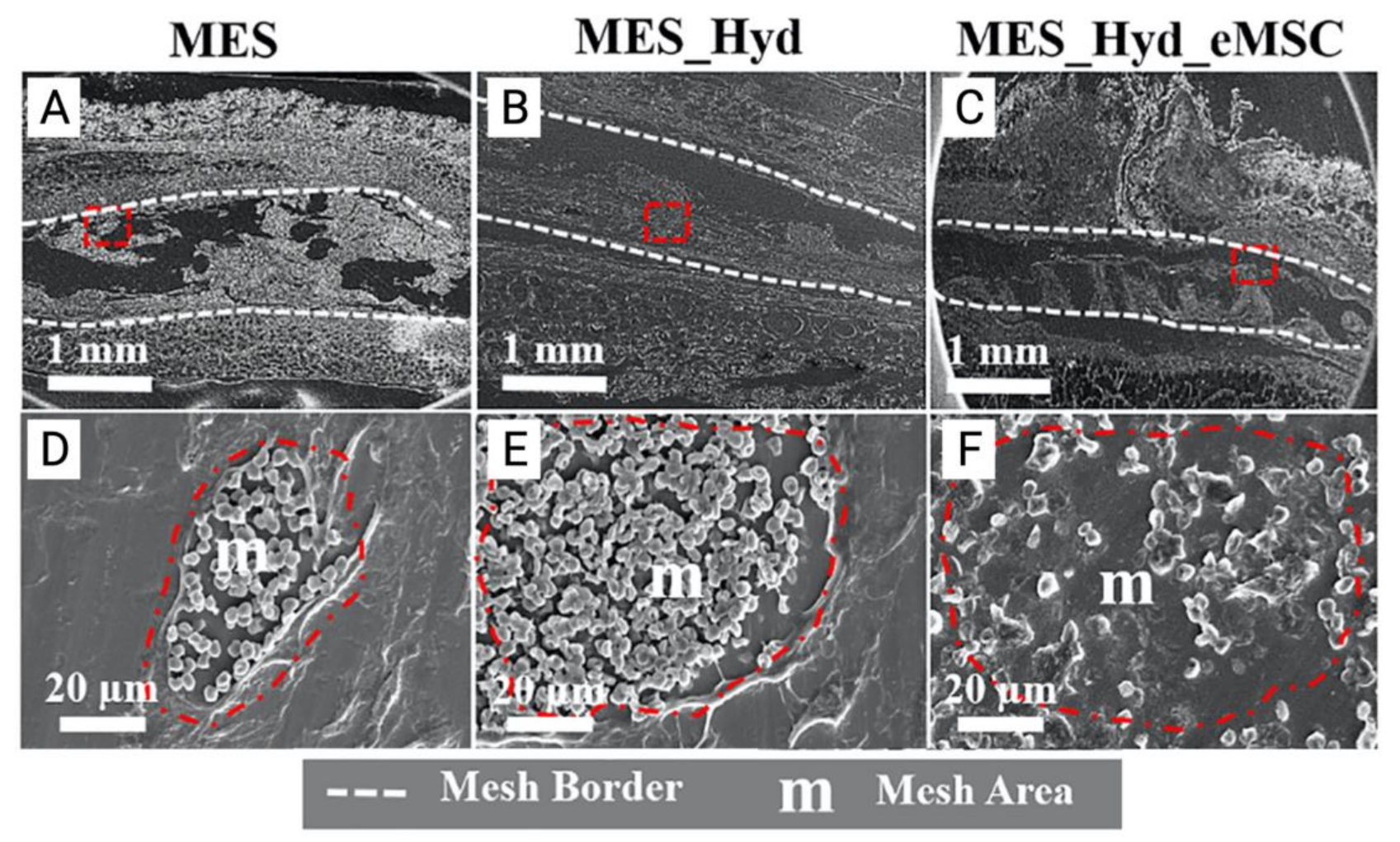
Figure 7.
(A–C) 3D printed polycaprolactone (PCL) mesh explants visualised by electron microscopy one week after implantation. (D–E) Cross section of melt electrospun mesh (MES), MES_ Hydrogel and MES_Hydrogel_eMSC constructs (red dotted area) demonstrating improved integration with host tissue and neo-ECM formation (F). Reprinted with permission from Paul et al. (2019) [74]. Copyright Elsevier, 2019.
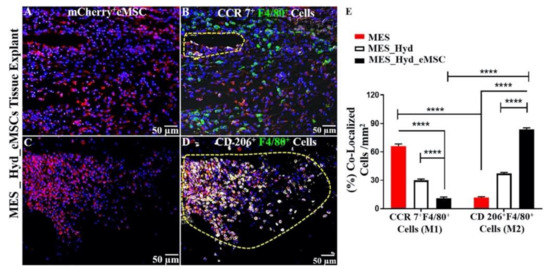
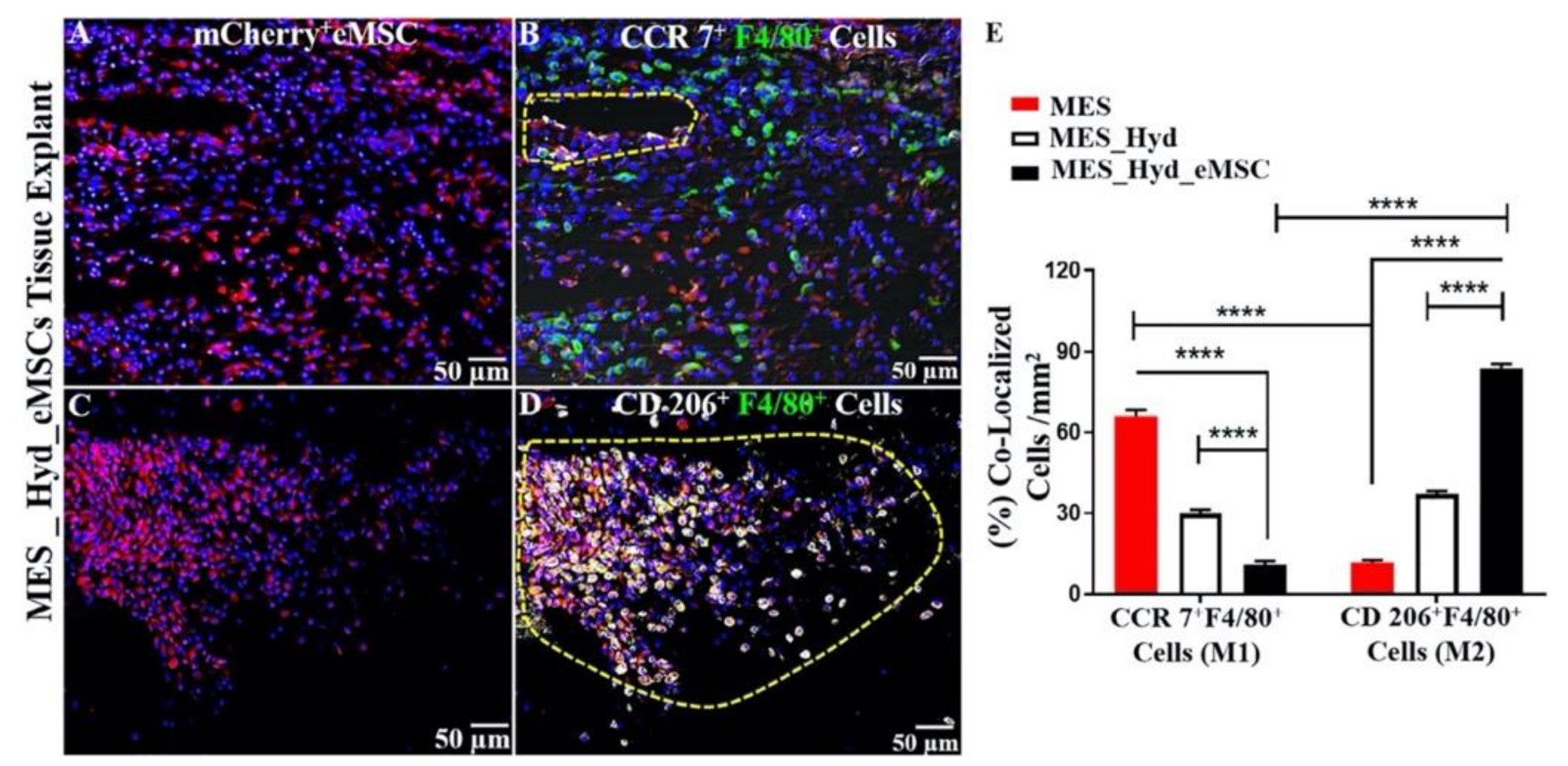
Figure 8.
3D printed polycaprolactone (PCL) explants from NSG mice implanted for 1 week. (A,C) Demonstration of m-cherry+ eMSC retention; (B) recruitment of CCR7+ F4/80+ M1 Macrophages; (D) recruitment of CD 206+F4/80+M2 Macrophages (yellow cells in enclosed area); and (E) quantification of co-localized M2/M1 Macrophages showing relative predominance of CD 206+M2 macrophages (****; p < 0.0001). Reprinted with permission from Paul et al. (2019) [74]. Copyright Elsevier, 2019.
A novel aloe vera (AV)–alginate (ALG) hydrogel engineered to incorporate therapeutic SUSD2+ eMSC was used to effectively deliver cells following a simulated birth injury in a rat model [76]. In untreated subjects, vaginal trauma resulted in fibrotic healing, with a significant reduction in smooth muscle content and increased elastin, which increased tissue stiffness. Mice receiving AV-ALG hydrogel with eMSC, demonstrated a more favourable inflammatory response with a lower M1:M2 ratio. This resulted in reversal of fibrosis and restoration of vaginal connective tissue structure, with increased organised collagen deposition and reduced overall tissue stiffness [74]. Further studies are exploring the use of AV-ALG hydrogel in a large pre-clinical ovine animal model of birth injury, to optimize therapeutic cell delivery to vaginal tissue immediately after the simulated injury. This hydrogel-loaded MSC presents great potential as an immediate treatment for vaginal birth injury representing the first ever secondary preventative therapy for the development of POP.
Irrespective of the method of biomaterial fabrication, it is imperative that new-generation meshes and hydrogels incorporate and deliver therapeutic cells such as eMSC that activate a favourable host immune response, rather than maladaptive chronic immune responses as seen with previous synthetic meshes [84,85]. To avoid mesh erosion, novel surgical constructs must have the desirable fibre alignment, stiffness, porosity, and topography to interact well with host vaginal tissue. Thus, personalised bioengineered constructs that are congruent with the topography of host vaginal tissue, and combined with therapeutic stem/progenitor cells, will promote the integration of mesh with native tissue. At the micro- and nanoscale level, cellular therapy has the potential to aid neovascularisation and deposition of neo-tissue that is more immunologically competent, resulting in a favourable surgical outcome for POP [86,87].
4.3. Large Pre-Clinical Animal Models of POP
Biomaterials combining eMSC with nanofiber meshes may be of great promise for improving POP surgery, though they have mostly been analysed in xenogenic rodent models of skin wound repair or abdominal hernia [39]. To avoid the complications associated with the rapid translation of non-degradable abdominal hernia mesh to transvaginal POP surgery under the 510(k) pathway, preclinical studies using large animal models is an imperative step in developing novel tissue engineered therapies. The domestic ewe has proven a good large animal model to perform pre-clinical studies in pelvic reconstructive surgery [88]. Anatomical, biomechanical, biochemical, and histological studies in nulliparous, primiparous, and multiparous ewes demonstrated similar rates of POP after vaginal delivery to that observed in humans, due to a large foetal head to body ratio and relatively comparable pelvic anatomy [88,89]. The diameter and length of the ovine and human vagina are relatively similar, and the ovine pelvic architecture relies on three levels of support, similar to the DeLancey model described in humans [90,91]. Both ovine and human vaginal wall consist of four histological zones of epithelium, lamina propria, muscularis, and adventitia [92]. Nulliparous, primiparous, and multiparous ewes demonstrated that parity results in greater displacements of the vaginal wall when using a modified POP-quantification (POP-Q) system adapted for sheep [93], which histologically correlated with an increased elastin and lower collagen content of vaginal wall, and thinning of the muscularis layer [89]. The modified POP-Q system has been adapted from the human POP-Q system, to assesses vaginal wall laxity at distinct anatomical landmarks in sheep, thereby providing an objective and reproducible measure of POP in anterior, posterior, and vault compartments. In this method, tissue forceps are used to assess maximum displacement of tissue at a reference point 3 cm proximal to the introitus at three possible points: Aa, 3 cm above the introitus on the anterior vaginal wall (range −3 above to +3 cm); Ap, 3 cm above the introitus (posterior wall); and Ba, above the urethra (anterior wall) (−3 cm to total vaginal length). Additional measurements include the length of the genital hiatus (GH), which is the vaginal orifice anterior to posterior (cm) and perineal body (PB), the distance from the middle of the anus to the edge of vaginal introitus with tail pulled back [93], (see Supplementary Figure S1). Cervical descent is not measured in the modified POP-Q as ewes have very long and relatively narrow vaginas (12–13 cm), with no obvious cervical descent [93].
Tissue engineered mesh inserted transvaginally in large animal models will aid the validation of these constructs prior to clinical translation by assessing their integration with host tissue and foreign body response through histological analysis, immunoassays and gene-profiling. In addition, the surgical and biomechanical efficacy of engineered mesh can be assessed at the nano- and macro-scale by implementing the modified POP-Q system, assessing tissue tensile strength testing, and collagen content analysis [74,76,89,93]. Research has commenced with the completion of multiple xenogenic small and large animal studies assessing eMSC/PAG constructs. Based on these findings, multiple heterologous small and large animal studies are underway to assess the efficacy of other biomimetic degradable materials such as PLCL and 3D PCL meshes seeded with eMSC (Figure 9). These animal models will be crucial in further assessing the efficacy of locally delivered eMSC and further determine their mechanism of action.
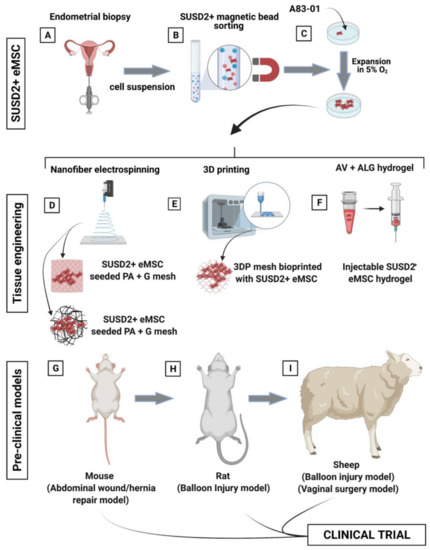
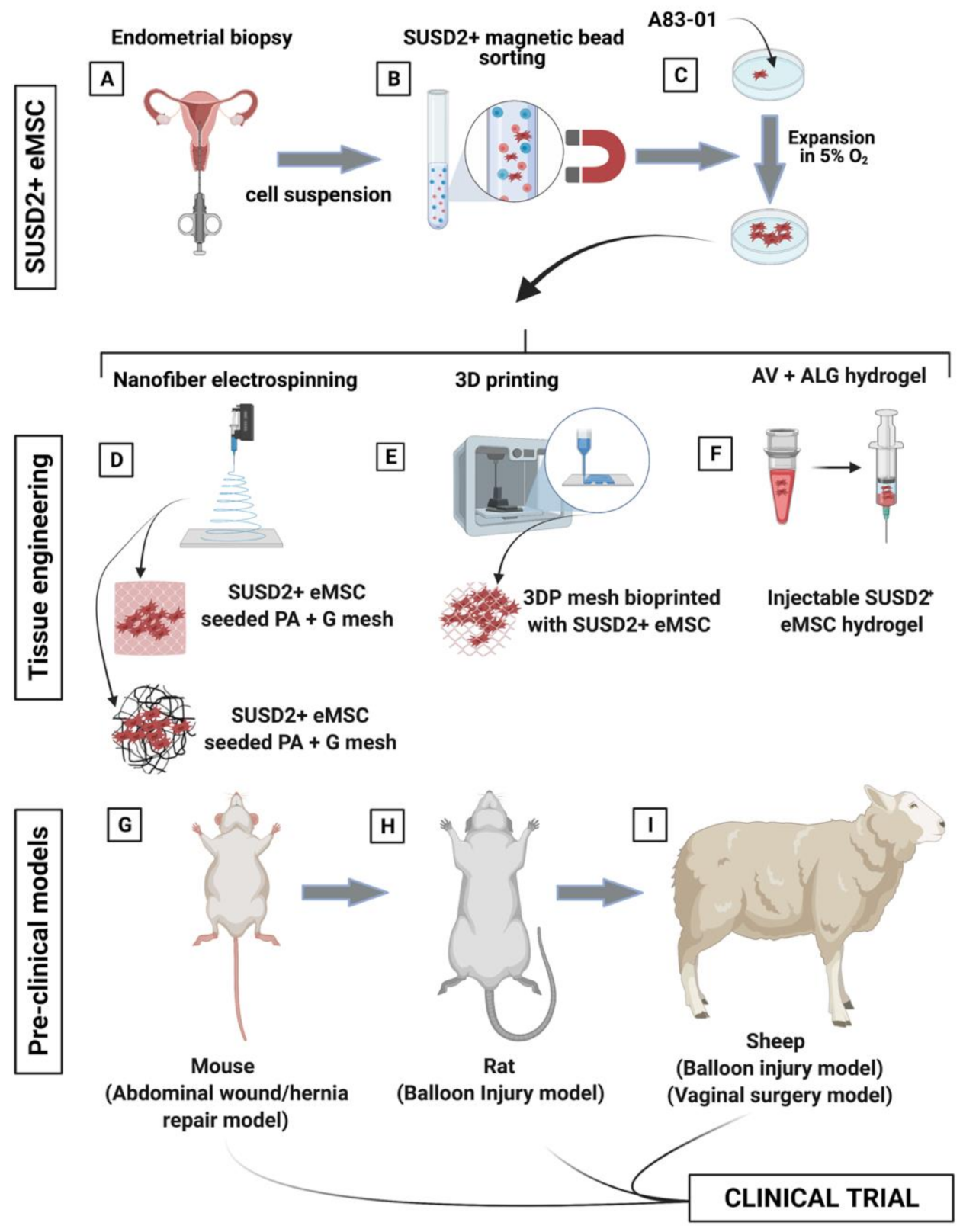
Figure 9.
Schematic showing the process of creating tissue engineering constructs comprising eMSC- seeded degradable poly(L-lactic acid)-co-poly(e-caprolactone (PLACL) nanofiber electrospun mesh. (A) eMSC are obtained from endometrial biopsy specimens in an office-based procedure, (B) purified by SUSD2 magnetic bead sorting; (C) and expanded in serum free media containing A83-01 in 5% O2. (D–F) Generation of biomimetic tissue engineered constructs included nanofiber electrospinning, 3D printing, and an aloe vera–alginate (AV-ALG) injectable hydrogel; and (G–I) pre-clinical models including a murine wound or abdominal defect repaired with mesh, rat balloon injury for eMSC hydrogel injection, and a sheep balloon injury and vaginal surgery model for eMSC hydrogel injection and bioengineered nanomesh insertion, respectively.
5. Potential Clinical Applications of eMSC
Various MSC-based therapies have performed poorly in clinical translation due to challenges in long-term engraftment of allogeneic MSC. Since perivascular eMSC and menstrual blood MSC derive from cyclically regenerating tissue, they present potential applications for tissue repair and possibly regeneration [40]. The ability to purify and characterise these cells through relatively non-invasive procedures has encouraged a separate investigation into their potential therapeutic applications in various clinical conditions with significant burden of disease [60]. As described, the restorative and regenerative capacity of autologous and heterologous perivascular eMSC is under investigation for applications in POP [8,11,19,39,44,72,73,74,76,79,80,81]. In particular, autologous ovine eMSC have persisted 30 days in a non-degradable PA mesh seeded with eMSC in situ in a transvaginal POP surgery model [72], xenogenic human eMSC for 1–2 weeks when implanted in a non-degradable PA+G composite mesh in immunocompromised rodent models of subcutaneous wound repair, and for 3 days in immunocompetent mice [70]. The eMSC exert strong paracrine effects which promotes early neovascularization, deposition of new collagen, and an improved anti-inflammatory response which is correlated with a reduction in stiffness of the mesh/tissue complex at 90 days [11]. In studies on PLCL/gelatin and 3D printed PCL constructs, eMSC also delayed mesh degradation, reduced the foreign body response, and induced endogenous cell influx to promote tissue repair [73,74]. These bioengineered constructs augmented with perivascular eMSC have the potential to improve host tissue response and ultimately prevent mesh exposure [60].
Menstrual blood MSC (MenSC), comprising unpurified endometrial stromal fibroblasts and eMSC derived from the shedding endometrium, have also been investigated as an attractive cell source for regenerative medicine [60]. These studies have demonstrated regenerative capacity in a myocardial infarct nude rat model, where they differentiated into striated, troponin I-expressing cardiac myocytes and reduced the infarcted area more than bone marrow MSC, which translated to an improvement in echocardiographic parameters of myocardial function [94]. Similar findings were demonstrated when MenSC were directly injected into ischaemic zones of an immunocompetent rat model of myocardial infarction, resulting in improved preservation of cardiomyocytes in the infarct zone [95]. MenSC have also demonstrated hepato-reparative potential in murine models of hepatic failure through paracrine effects that modulate liver fibrosis and by potentially differentiating toward hepatic cells [96,97,98]. In rodent models of ovarian insufficiency, MenSCs reduced apoptosis and increased numbers of primordial follicles which correlated with increased levels of serum anti-Mullerian hormone (AMH), oestrogen, and progesterone [99,100]. In a recent clinical trial in 15 women, intraovarian injection of autologous MenSC in poor ovarian responders increased clinical pregnancy and live births [101]. MenSC have demonstrated potential reparative application in other animal models of lung injury [102], wound repair [103], stroke [104], and muscular dystrophy [105].
In summary, perivascular eMSC and MenSCs have demonstrated promise in regenerative medicine through paracrine immunomodulatory effects and by enhancing endogenous stem cell function. The therapeutic potential of eMSC is further benefited by their relative ease of sampling, potency in culture, and reduced rates of spontaneous differentiation to fibroblasts under defined culture conditions [61]. Though hundreds of preclinical and a smaller number of clinical trials are underway, there remains much to be discovered about how the therapeutic potential of eMSC and MenSC can be harnessed for advancements in tissue repair and regenerative medicine.
6. Limitations
Prior to translating the use of MSC to the clinic, their limitations and potential adverse effects must be thoroughly explored. Perivascular MSC are rare cells that are difficult to harvest from adult tissues, which necessitates substantial ex-vivo culture expansion in order to achieve sufficient numbers of potent cells. However, prolonged culture of MSC results in spontaneous differentiation to fibroblasts, an occurrence which significantly limits culture expansion. However, novel small molecule inhibitors can overcome this limitation, e.g., A83-01. Short-term engraftment of MSC demonstrated in multiple pre-clinical studies is particularly challenging, as their efficacy may be limited by the small proportion (2–10%) of therapeutic cells that are estimated to remain in vivo in the days following administration. The overactive host innate immune system in diseased or ischaemic tissue has been purported to rapidly remove allogenic MSC due to a loss of vascular niches that would otherwise aid tissue integration [106]. Despite these challenges, MSC exhibit important paracrine actions, appearing to reset the innate immune system and promote endogenous cellular repair despite their lack of tissue integration. Thus, a protective delivery system (e.g., hydrogel) that improves tissue retention of MSC to further augment therapeutic efficacy in tissues is imperative to ensure their success in clinical translation.
7. Summary and Future Perspectives
From a clinical perspective, there is an opportunity to explore personalised surgical approaches to POP where tissue engineered meshes are combined with therapeutic and potentially autologous eMSC. The severity and consequences of POP is highly related to individual patient’s anatomy, a variable that is often assessed pre-operatively with diagnostic radiology assessments through modalities such as endovaginal ultrasound (EVUS), 2D perineal pelvic floor ultrasound (pPFUS), transperineal ultrasound (TPUS), and translabial ultrasound (TLUS), and pelvic CT and MRI [107,108].
Pre-operative imaging studies can be integrated with computer-aided design (CAD) to produce a customised and patient-specific construct that is more likely to integrate with the unique dynamics of their anatomy and potential tissue defects. 3D bioprinting can thus be implemented to incorporate eMSC in a biocompatible hydrogel carrier that encapsulates cells and prints them layer by layer to construct a 3D functional living tissue, or artificial organ [109,110,111,112].
The key to the discovery of a safe and surgically efficacious tissue engineered mesh lies in a controlled design that highly considers the host tissue which it is intended. Tissue engineering joins the forces of nanotechnology, 3D printing, and eMSC to design a construct that addresses mesh–tissue mechanical mismatch, induces favourable tissue responses, and crucially mimics vaginal ECM topography. The highly regenerative human endometrium provides an exciting new, readily available, biologically active, and cost-effective source of MSC. Since their discovery, great advances have improved our ability to prospectively isolate these cells and maintain their clonogenicity during culture expansion, allowing their successfully implementation in a novel tissue engineering techniques. Thus, the use of eMSC in various homologous, autologous, and allogenic cellular therapies serve immense potential in advancing tissue engineering applications in POP, to alleviate a great clinical burden of disease.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jpm11090840/s1, Figure S1: (A) Modified Pelvic Organ Prolapse Quantification System (POP-Q): definition and locations in the ewe. Ap 3 cm above the introitus in the posterior vaginal wall (range −3 above to +3 cm). Aa 3 cm above the introitus on the anterior vaginal wall (range −3 above to +3 cm). Ba above the urethra (anterior wall) (−3 to total vaginal length). (B) Traction applied with tissue forceps at Ap, Ba and Aa, at points as marked. Ewes with displacement +1 were considered to have POP. Copyright (Young et al.), reproduced with permission [93]. GH, genital hiatus; PB, perineal body; TVL, total vaginal length.
Author Contributions
Conceptualization, D.M.Z.B.H., S.M., and C.E.G.; writing—original draft preparation, D.M.Z.B.H.; writing—review and editing, D.M.Z.B.H., S.M., J.A.W., and C.E.G.; supervision, A.R., S.M., C.E.G., and J.A.W.; funding acquisition, A.R., S.M., C.E.G., and J.A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Health and Medical Research Council (NHMRC) of Australia: Grant 1159677 (C.E.G., S.M., J.A.W., and A.R.), Grant 1184841 (S.M., J.A.W. and A.R.) and 1173882 Investigator (C.E.G.); J&J WiSTEM2D Program; Hudson Institute of Medical Research; Monash University and the Victorian Government’s Operational Infrastructure Support Program.
Acknowledgments
The authors acknowledge assistance from the Monash Histology Platform, and Animal Research Facility at Monash Health Translational Research Precinct. Figure 9 was created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dietz, H.P. Pelvic floor trauma in childbirth. Aust. N. Z. J. Obstet. Gynaecol. 2013, 53, 220–230. [Google Scholar] [CrossRef]
- Nygaard, I. Prevalence of Symptomatic Pelvic Floor Disorders in US Women. JAMA 2008, 300, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Zwain, O.; Aoun, J.; Eisenstein, D. Minimally invasive surgery in pelvic floor repair. Curr. Opin. Obstet. Gynecol. 2017, 29, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.J.; Holman, C.D.J.; Moorin, R.; Tsokos, N. Lifetime Risk of Undergoing Surgery for Pelvic Organ Prolapse. Obstet. Gynecol. 2010, 116, 1096–1100. [Google Scholar] [CrossRef]
- Wu, J.M.; Matthews, C.A.; Conover, M.; Pate, V.; Funk, M.J. Lifetime Risk of Stress Urinary Incontinence or Pelvic Organ Prolapse Surgery. Obstet. Gynecol. 2014, 123, 1201–1206. [Google Scholar] [CrossRef]
- Olsen, A.L.; Smith, V.J.; Bergstrom, J.O.; Colling, J.C.; Clark, A.L. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet. Gynecol. 1997, 89, 501–506. [Google Scholar] [CrossRef]
- Vinchant, M.; Bitumba, I.; Letouzey, V.; Fernandez, H.; de Tayrac, R.; Deffieux, X. Reoperation rate and outcomes following the placement of polypropylene mesh by the vaginal route for cystocele: Very long-term follow-up. Int. Urogynecol. J. 2021, 32, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.; Unger, C.A.; Evans, J.M.; Jallad, K.; Mishra, K.; Karram, M.M.; Iglesia, C.B.; Rardin, C.R.; Barber, M.D. Evaluation and management of complications from synthetic mesh after pelvic reconstructive surgery: A multicenter study. Am. J. Obstet. Gynecol. 2014, 210, 163.e1–163.e8. [Google Scholar] [CrossRef]
- Holt, E. US FDA rules manufacturers to stop selling mesh devices. Lancet 2019, 393, 1686. [Google Scholar] [CrossRef]
- MacCraith, E.; Cunnane, E.M.; Joyce, M.; Forde, J.C.; O’Brien, F.J.; Davis, N.F. Comparison of synthetic mesh erosion and chronic pain rates after surgery for pelvic organ prolapse and stress urinary incontinence: A systematic review. Int. Urogynecol. J. 2021, 32, 573–580. [Google Scholar] [CrossRef]
- Ulrich, D.; Edwards, S.L.; Su, K.; Tan, K.S.; White, J.; Ramshaw, J.A.; Lo, C.; Rosamilia, A.; Werkmeister, J.A.; Gargett, C.E. Human Endometrial Mesenchymal Stem Cells Modulate the Tissue Response and Mechanical Behavior of Polyamide Mesh Implants for Pelvic Organ Prolapse Repair. Tissue Eng. Part A 2013, 20, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Jelovsek, J.E.; Maher, C.; Barber, M.D. Pelvic organ prolapse. Lancet 2007, 369, 1027–1038. [Google Scholar] [CrossRef]
- Mothes, A.R.; Radosa, M.P.; Altendorf-Hofmann, A.; Runnebaum, I.B. Risk index for pelvic organ prolapse based on established individual risk factors. Arch. Gynecol. Obstet. 2016, 293, 617–624. [Google Scholar] [CrossRef]
- Chow, D.; Rodríguez, L.V. Epidemiology and prevalence of pelvic organ prolapse. Curr. Opin. Urol. 2013, 23, 293–298. [Google Scholar] [CrossRef]
- Saunders, K. Recent Advances in Understanding Pelvic-Floor Tissue of Women with and Without Pelvic Organ Prolapse: Considerations for Physical Therapists. Phys. Ther. 2017, 97, 455–463. [Google Scholar] [CrossRef]
- Norton, P.A. Pelvic Floor Disorders: The Role of Fascia and Ligaments. Clin. Obstet. Gynecol. 1993, 36, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.T.; De Lancey, J.O.L. Functional Anatomy of the Pelvic Floor and Lower Urinary Tract. Clin. Obstet. Gynecol. 2004, 47, 3–17. [Google Scholar] [CrossRef]
- Memon, H.U.; Handa, V.L. Vaginal Childbirth and Pelvic Floor Disorders. Women’s Health 2013, 9, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Darzi, S.; Werkmeister, J.A.; Gargett, C.E.; Mukherjee, S. Emerging Nano/Micro-Structured Degradable Polymeric Meshes for Pelvic Floor Reconstruction. Nanomaterials 2020, 10, 1120. [Google Scholar] [CrossRef]
- Rubod, C.; Lecomte-Grosbras, P.; Brieu, M.; Giraudet, G.; Betrouni, N.; Cosson, M. 3D simulation of pelvic system numerical simulation for a better understanding of the contribution of the uterine ligaments. Int. Urogynecol. J. 2013, 24, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Brownridge, P. The nature and consequences of childbirth pain. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 59, S9–S15. [Google Scholar] [CrossRef]
- Lien, K.-C.; Mooney, B.; DeLancey, J.O.L.; Ashton-Miller, J.A. Levator Ani Muscle Stretch Induced by Simulated Vaginal Birth. Obstet. Gynecol. 2004, 103, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Lamblin, G.; Delorme, E.; Cosson, M.; Rubod, C. Cystocele and functional anatomy of the pelvic floor: Review and update of the various theories. Int. Urogynecol. J. 2016, 27, 1297–1305. [Google Scholar] [CrossRef]
- Stuepp, L.; Resende, A.P.M.; Oliveira, E.; Castro, R.; Girão, M.J.B.C.; Sartori, M. Pelvic floor muscle training for treatment of pelvic organ prolapse: An assessor-blinded randomized controlled trial. Int. Urogynecol. J. 2011, 22, 1233–1239. [Google Scholar] [CrossRef]
- Siddiqui, N.; Edenfield, A. Clinical challenges in the management of vaginal prolapse. Int. J. Women’s Health 2014, 6, 83–94. [Google Scholar] [CrossRef]
- Mancuso, E.; Downey, C.; Doxford-Hook, E.; Bryant, M.G.; Culmer, P. The use of polymeric meshes for pelvic organ prolapse: Current concepts, challenges, and future perspectives. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Nolfi, A.L.; Brown, B.N.; Liang, R.; Palcsey, S.L.; Bonidie, M.J.; Abramowitch, S.D.; Moalli, P.A. Host response to synthetic mesh in women with mesh complications. Am. J. Obstet. Gynecol. 2016, 215, 206.e1–206.e8. [Google Scholar] [CrossRef] [PubMed]
- Baylón, K.; Rodríguez-Camarillo, P.; Elías-Zúñiga, A.; Díaz-Elizondo, J.A.; Gilkerson, R.; Lozano, K. Past, Present and Future of Surgical Meshes: A Review. Membranes 2017, 7, 47. [Google Scholar] [CrossRef]
- Barone, W.R.; Abramowitch, S.D.; Moalli, P.A. Host Response to Biomaterials; Host Response to Biomaterials for Pelvic Floor Reconstruction; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 375–423. [Google Scholar]
- Shepherd, J.P.; Feola, A.; Abramowitch, S.D.; Moalli, P.A. Uniaxial biomechanical properties of seven different vaginally implanted meshes for pelvic organ prolapse. Int. Urogynecol. J. 2011, 23, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Pott, P.P.; Schwarz, M.L.R.; Gundling, R.; Nowak, K.; Hohenberger, P.; Roessner, E.D. Mechanical Properties of Mesh Materials Used for Hernia Repair and Soft Tissue Augmentation. PLoS ONE 2012, 7, e46978. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.L.; Damoiseaux, A.; IntHout, J.; Kluivers, K.B.; Withagen, M.I.J. Long-term outcome of vaginal mesh or native tissue in recurrent prolapse: A randomized controlled trial. Int. Urogynecol. J. 2018, 29, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.E.; Hansen, B.L.; Egger, M.J.; Nygaard, I.; Sanchez-Birkhead, A.C.; Hsu, Y.; Clark, L. Changed women: The long-term impact of vaginal mesh complications. Female Pelvic. Med. Reconstr. Surg. 2014, 20, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Margulies, R.U.; Lewicky-Gaupp, C.; Fenner, D.E.; McGuire, E.J.; Clemens, J.Q.; DeLancey, J.O. Complications requiring reoperation following vaginal mesh kit procedures for prolapse. Am. J. Obstet. Gynecol. 2008, 199, 678.e1–678.e4. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, R.M.; Liang, R.; Knight, K.; Carter-Brooks, C.M.; Abramowitch, S.; Moalli, P.A. Impact of polypropylene prolapse mesh on vaginal smooth muscle in rhesus macaque. Am. J. Obstet. Gynecol. 2019, 221, 330.e1–330.e9. [Google Scholar] [CrossRef]
- Liang, R.; Knight, K.M.; Barone, W.; Powers, R.W.; Nolfi, A.; Palcsey, S.; Abramowitch, S.; Moalli, P.A. Extracellular matrix regenerative graft attenuates the negative impact of polypropylene prolapse mesh on vagina in rhesus macaque. Am. J. Obstet. Gynecol. 2017, 216, 153.e–153.e9. [Google Scholar] [CrossRef]
- Feola, A.; Abramowitch, S.; Jallah, Z.; Stein, S.; Barone, W.; Palcsey, S.; Moalli, P. Deterioration in biomechanical properties of the vagina following implantation of a high-stiffness prolapse mesh. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 224–232. [Google Scholar] [CrossRef]
- Gargett, C.E.; Gurung, S.; Darzi, S.; Werkmeister, J.A.; Mukherjee, S. Tissue engineering approaches for treating pelvic organ prolapse using a novel source of stem/stromal cells and new materials. Curr. Opin. Urol. 2019, 29, 450–457. [Google Scholar] [CrossRef]
- Darzi, S.; Werkmeister, J.A.; Deane, J.A.; Gargett, C.E. Identification and Characterization of Human Endometrial Mesenchymal Stem/Stromal Cells and Their Potential for Cellular Therapy. Stem Cells Transl. Med. 2016, 5, 1127–1132. [Google Scholar] [CrossRef]
- Heathman, T.R.; Nienow, A.W.; McCall, M.; Coopman, K.; Kara, B.; Hewitt, C. The translation of cell-based therapies: Clinical landscape and manufacturing challenges. Regen. Med. 2015, 10, 49–64. [Google Scholar] [CrossRef]
- Kode, J.A.; Mukherjee, S.; Joglekar, M.V.; Hardikar, A.A. Mesenchymal stem cells: Immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy 2009, 11, 377–391. [Google Scholar] [CrossRef]
- Cao, W.; Cao, K.; Cao, J.; Wang, Y.; Shi, Y. Mesenchymal stem cells and adaptive immune responses. Immunol. Lett. 2015, 168, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Davies, L. Mesenchymal stromal cells and the innate immune response. Immunol. Lett. 2015, 168, 140–146. [Google Scholar] [CrossRef]
- Mukherjee, S.; Darzi, S.; Paul, K.; Werkmeister, J.A.; Gargett, C.E. Mesenchymal stem cell-based bioengineered constructs: Foreign body response, cross-talk with macrophages and impact of biomaterial design strategies for pelvic floor disorders. Interface Focus 2019, 9, 20180089. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Pagliara, D.; Locatelli, F. Mesenchymal stromal cell therapy: A revolution in Regenerative Medicine? Bone Marrow Transplant. 2011, 47, 164–171. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef]
- Ulrich, D.; Muralitharan, R.R.; Gargett, C.E. Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin. Biol. Ther. 2013, 13, 1387–1400. [Google Scholar] [CrossRef]
- Jabbour, H.N.; Kelly, R.W.; Fraser, H.M.; Critchley, H.O.D. Endocrine Regulation of Menstruation. Endocr. Rev. 2006, 27, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Nguyen, H.P.T.; Ye, L. Endometrial regeneration and endometrial stem/progenitor cells. Rev. Endocr. Metab. Disord. 2012, 13, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Henriet, P.; Chevronnay, H.P.G.; Marbaix, E. The endocrine and paracrine control of menstruation. Mol. Cell. Endocrinol. 2012, 358, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Cervelló, I.; Martínez-Conejero, J.A.; Horcajadas, J.A.; Pellicer, A.; Simón, C. Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum. Reprod. 2007, 22, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Schwab, K.E.; Gargett, C.E. Clonogenicity of Human Endometrial Epithelial and Stromal Cells1. Biol. Reprod. 2004, 70, 1738–1750. [Google Scholar] [CrossRef]
- Schwab, K.E.; Chan, R.; Gargett, C.E. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil. Steril. 2005, 84, 1124–1130. [Google Scholar] [CrossRef]
- Ulrich, D.; Tan, K.S.; Deane, J.; Schwab, K.; Cheong, A.; Rosamilia, A.; Gargett, C.E. Mesenchymal stem/stromal cells in post-menopausal endometrium. Hum. Reprod. 2014, 29, 1895–1905. [Google Scholar] [CrossRef]
- Schwab, K.E.; Gargett, C.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod. 2007, 22, 2903–2911. [Google Scholar] [CrossRef]
- Gargett, C.E.; Gurung, S. Endometrial Mesenchymal Stem/Stromal Cells, Their Fibroblast Progeny in Endometriosis, and More. Biol. Reprod. 2016, 94, 129. [Google Scholar] [CrossRef]
- Spitzer, T.L.; Rojas, A.; Zelenko, Z.; Aghajanova, L.; Erikson, D.W.; Barragan, F.; Meyer, M.; Tamaresis, J.S.; Hamilton, A.E.; Irwin, J.C.; et al. Perivascular Human Endometrial Mesenchymal Stem Cells Express Pathways Relevant to Self-Renewal, Lineage Specification, and Functional Phenotype1. Biol. Reprod. 2012, 86, 58. [Google Scholar] [CrossRef]
- Masuda, H.; Anwar, S.S.; Bühring, H.-J.; Rao, J.R.; Gargett, C.E. A Novel Marker of Human Endometrial Mesenchymal Stem-Like Cells. Cell Transplant. 2012, 21, 2201–2214. [Google Scholar] [CrossRef]
- Sivasubramaniyan, K.; Harichandan, A.; Schumann, S.; Sobiesiak, M.; Lengerke, C.; Maurer, A.; Kalbacher, H.; Bühring, H.-J. Prospective Isolation of Mesenchymal Stem Cells from Human Bone Marrow Using Novel Antibodies Directed Against Sushi Domain Containing 2. Stem Cells Dev. 2013, 22, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Bozorgmehr, M.; Gurung, S.; Darzi, S.; Nikoo, S.; Kazemnejad, S.; Zarnani, A.-H.; Gargett, C.E. Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front. Cell Dev. Biol. 2020, 8, 497. [Google Scholar] [CrossRef]
- Gurung, S.; Werkmeister, J.A.; Gargett, C.E. Inhibition of Transforming Growth Factor-β Receptor signaling promotes culture expansion of undifferentiated human Endometrial Mesenchymal Stem/stromal Cells. Sci. Rep. 2015, 5, 15042. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Williams, S.; Deane, J.; Werkmeister, J.A.; Gargett, C.E. The Transcriptome of Human Endometrial Mesenchymal Stem Cells Under TGFβR Inhibition Reveals Improved Potential for Cell-Based Therapies. Front. Cell Dev. Biol. 2018, 6, 164. [Google Scholar] [CrossRef] [PubMed]
- Lucciola, R.; Vrljicak, P.; Gurung, S.; Filby, C.; Darzi, S.; Muter, J.; Ott, S.; Brosens, J.J.; Gargett, C.E. Impact of Sustained Transforming Growth Factor-β Receptor Inhibition on Chromatin Accessibility and Gene Expression in Cultured Human Endometrial MSC. Front. Cell Dev. Biol. 2020, 8, 567610. [Google Scholar] [CrossRef]
- Samsonraj, R.; Rai, B.; Sathiyanathan, P.; Puan, K.J.; Rötzschke, O.; Hui, J.H.; Raghunath, M.; Stanton, L.W.; Nurcombe, V.; Cool, S.M. Establishing Criteria for Human Mesenchymal Stem Cell Potency. Stem Cells 2015, 33, 1878–1891. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Masuda, H. Adult stem cells in the endometrium. Mol. Hum. Reprod. 2010, 16, 818–834. [Google Scholar] [CrossRef]
- Mukherjee, S.; Darzi, S.; Paul, K.; Cousins, F.; Werkmeister, J.A.; Gargett, C.E. Electrospun Nanofiber Meshes With Endometrial MSCs Modulate Foreign Body Response by Increased Angiogenesis, Matrix Synthesis, and Anti-Inflammatory Gene Expression in Mice: Implication in Pelvic Floor. Front. Pharmacol. 2020, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Edwards, S.L.; Tan, K.S.; White, J.; Kandel, S.; Ramshaw, J.A.; Gargett, C.E.; Werkmeister, J.A. Induction of endometrial mesenchymal stem cells into tissue-forming cells suitable for fascial repair. Acta Biomater. 2014, 10, 5012–5020. [Google Scholar] [CrossRef]
- Edwards, S.; Ulrich, D.; White, J.; Su, K.; Rosamilia, A.; Ramshaw, J.; Gargett, C.; Werkmeister, J. Temporal changes in the biomechanical properties of endometrial mesenchymal stem cell seeded scaffolds in a rat model. Acta Biomater. 2015, 13, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Letouzey, V.; Tan, K.S.; Deane, J.; Ulrich, D.; Gurung, S.; Ong, Y.R.; Gargett, C.E. Isolation and Characterisation of Mesenchymal Stem/Stromal Cells in the Ovine Endometrium. PLoS ONE 2015, 10, e0127531. [Google Scholar] [CrossRef]
- Darzi, S.; Deane, J.; Nold, C.A.; Edwards, S.E.; Gough, D.; Mukherjee, S.; Gurung, S.; Tan, K.S.; Vashi, A.V.; Werkmeister, J.A.; et al. Endometrial Mesenchymal Stem/Stromal Cells Modulate the Macrophage Response to Implanted Polyamide/Gelatin Composite Mesh in Immunocompromised and Immunocompetent Mice. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Gurung, S.; Deane, J.; Darzi, S.; Werkmeister, J.A.; Gargett, C.E. In Vivo Survival of Human Endometrial Mesenchymal Stem Cells Transplanted Under the Kidney Capsule of Immunocompromised Mice. Stem Cells Dev. 2018, 27, 35–43. [Google Scholar] [CrossRef]
- Emmerson, S.; Mukherjee, S.; Melendez-Munoz, J.; Cousins, F.; Edwards, S.; Karjalainen, P.; Ng, M.; Tan, K.; Darzi, S.; Bhakoo, K.; et al. Composite mesh design for delivery of autologous mesenchymal stem cells influences mesh integration, exposure and biocompatibility in an ovine model of pelvic organ prolapse. Biomaterials 2019, 225, 119495. [Google Scholar] [CrossRef]
- Mukherjee, S.; Darzi, S.; Rosamilia, A.; Kadam, V.; Truong, Y.; Werkmeister, J.A.; Gargett, C.E. Blended Nanostructured Degradable Mesh with Endometrial Mesenchymal Stem Cells Promotes Tissue Integration and Anti-Inflammatory Response in Vivo for Pelvic Floor Application. Biomacromolecules 2019, 20, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Darzi, S.; McPhee, G.; Del Borgo, M.P.; Werkmeister, J.A.; Gargett, C.E.; Mukherjee, S. 3D bioprinted endometrial stem cells on melt electrospun poly ε-caprolactone mesh for pelvic floor application promote anti-inflammatory responses in mice. Acta Biomater. 2019, 97, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Ulrich, D.; Sturm, M.; Rosamilia, A.; Werkmeister, J.A.; Gargett, C.E. Comparing the Effect of TGF-β Receptor Inhibition on Human Perivascular Mesenchymal Stromal Cells Derived from Endometrium, Bone Marrow and Adipose Tissues. J. Pers. Med. 2020, 10, 261. [Google Scholar] [CrossRef]
- Paul, K.; Darzi, S.; Del Borgo, M.P.; Cousins, F.L.; Werkmeister, J.A.; Gargett, C.E.; Mukherjee, S. Vaginal delivery of tissue engineered endometrial mesenchymal stem/stromal cells in an aloe vera-alginate hydrogel alleviates maternal simulated birth injury. Appl. Mater. Today 2021, 22, 100890. [Google Scholar] [CrossRef]
- Han, X.; Meng, X.; Yin, Z.; Rogers, A.; Zhong, J.; Rillema, P.; Jackson, J.A.; Ichim, T.E.; Minev, B.; Carrier, E.; et al. Inhibition of intracranial glioma growth by endometrial regenerative cells. Cell Cycle 2009, 8, 606–610. [Google Scholar] [CrossRef] [PubMed][Green Version]
- MacNeil, S.; Mangir, N.; Roman, S.; Mironska, E. Tissue engineering for the pelvic floor. Curr. Opin. Urol. 2019, 29, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D.; Edwards, S.L.; White, J.F.; Supit, T.; Ramshaw, J.A.M.; Lo, C.; Rosamilia, A.; Werkmeister, J.A.; Gargett, C.E. A Preclinical Evaluation of Alternative Synthetic Biomaterials for Fascial Defect Repair Using a Rat Abdominal Hernia Model. PLoS ONE 2012, 7, e50044. [Google Scholar] [CrossRef] [PubMed]
- Vashaghian, M.; Zaat, S.J.; Smit, T.H.; Roovers, J.-P. Biomimetic implants for pelvic floor repair. Neurourol. Urodyn. 2018, 37, 566–580. [Google Scholar] [CrossRef]
- Mukherjee, S.; Venugopal, J.R.; Ravichandran, R.; Ramalingam, M.; Raghunath, M.; Ramakrishna, S. Nanofiber Technology for Controlling Stem Cell Functions and Tissue Engineering. Micro Nanotechnol. Eng. Stem Cells Tissues 2013, 1, 27–51. [Google Scholar] [CrossRef]
- He, H.; Wu, X.; Wang, Y.; Zhu, C.; Tong, X.; Yang, M.; Yang, L.; Huang, W.; Wu, F.; Zong, H.; et al. Preclinical animal study and human clinical trial data of co-electrospun poly(L-lactide-co-caprolactone) and fibrinogen mesh for anterior pelvic floor reconstruction. Int. J. Nanomed. 2016, 11, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Regueros, S.R.; Albersen, M.; Manodoro, S.; Zia, S.; Osman, N.I.; Bullock, A.; Chapple, C.; Deprest, J.; MacNeil, S. AcuteIn VivoResponse to an Alternative Implant for Urogynecology. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Shores, L.S.; Votaw, N.L.; Collier, J.H. Biomaterial strategies for generating therapeutic immune responses. Adv. Drug Deliv. Rev. 2017, 114, 3–18. [Google Scholar] [CrossRef]
- Paul, N.E.; Skazik, C.; Harwardt, M.; Bartneck, M.; Denecke, B.; Klee, D.; Salber, J.; Zwadlo-Klarwasser, G. Topographical control of human macrophages by a regularly microstructured polyvinylidene fluoride surface. Biomaterials 2008, 29, 4056–4064. [Google Scholar] [CrossRef]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef]
- Sridharan, I.; Ma, Y.; Kim, T.; Kobak, W.; Rotmensch, J.; Wang, R. Structural and mechanical profiles of native collagen fibers in vaginal wall connective tissues. Biomaterials 2012, 33, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Couri, B.M.; Lenis, A.T.; Borazjani, A.; Paraiso, M.F.R.; Damaser, M.S. Animal models of female pelvic organ prolapse: Lessons learned. Expert Rev. Obstet. Gynecol. 2012, 7, 249–260. [Google Scholar] [CrossRef]
- Emmerson, S.; Young, N.; Rosamilia, A.; Parkinson, L.; Edwards, S.L.; Vashi, A.V.; Davies-Tuck, M.; White, J.; Elgass, K.; Lo, C.; et al. Ovine multiparity is associated with diminished vaginal muscularis, increased elastic fibres and vaginal wall weakness: Implication for pelvic organ prolapse. Sci. Rep. 2017, 7, srep45709. [Google Scholar] [CrossRef]
- Mori da Cunha, M.; Mackova, K.; Hympanova, L.; Bortolini, M.; Deprest, J. Animal models for pelvic organ pro-lapse: Systematic review. Int. Urogynecol. J. 2021, 32, 1331–1344. [Google Scholar] [CrossRef]
- Ashton-Miller, J.A.; DeLancey, J.O.L. Functional Anatomy of the Female Pelvic Floor. Ann. N. Y. Acad. Sci. 2007, 1101, 266–296. [Google Scholar] [CrossRef]
- Ulrich, D.; Edwards, S.L.; Letouzey, V.; Su, K.; White, J.F.; Rosamilia, A.; Gargett, C.E.; Werkmeister, J.A. Regional Variation in Tissue Composition and Biomechanical Properties of Postmenopausal Ovine and Human Vagina. PLoS ONE 2014, 9, e104972. [Google Scholar] [CrossRef] [PubMed]
- Young, N.; Rosamilia, A.; Arkwright, J.; Lee, J.; Davies-Tuck, M.; Melendez, J.; Werkmeister, J.; Gargett, C.E. Vaginal wall weakness in parous ewes: A potential preclinical model of pelvic organ prolapse. Int. Urogynecol. J. 2016, 28, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Hida, N.; Nishiyama, N.; Miyoshi, S.; Kira, S.; Segawa, K.; Uyama, T.; Mori, T.; Miyado, K.; Ikegami, Y.; Cui, C.; et al. Novel Cardiac Precursor-Like Cells from Human Menstrual Blood-Derived Mesenchymal Cells. Stem Cells 2008, 26, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Hu, X.; Yu, H.; Xu, Y.; Wang, L.; Chen, H.; Chen, H.; Wu, R.; Zhang, Z.; Xiang, C.; et al. Human endometrial stem cells confer enhanced myocardial salvage and regeneration by paracrine mechanisms. J. Cell. Mol. Med. 2013, 17, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Khanjani, S.; Khanmohammadi, M.; Zarnani, A.H.; Talebi, S.; Edalatkhah, H.; Eghtesad, S.; Nikokar, I.; Kazemnejad, S. Efficient generation of functional hepatocyte-like cells from menstrual blood-derived stem cells. J. Tissue Eng. Regen. Med. 2015, 9, E124–E134. [Google Scholar] [CrossRef]
- Fathi-Kazerooni, M.; Tavoosidana, G.; Taghizadeh-Jahed, M.; Khanjani, S.; Golshahi, H.; Gargett, C.E.; Edalatkhah, H.; Kazemnejad, S. Comparative restoration of acute liver failure by menstrual blood stem cells compared with bone marrow stem cells in mice model. Cytotherapy 2017, 19, 1474–1490. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, C.; Chen, L.; Wang, X.; Xiang, B.; Wu, X.; Guo, Y.; Mou, X.; Yuan, L.; Chen, B.; et al. Human Menstrual Blood-Derived Stem Cells Ameliorate Liver Fibrosis in Mice by Targeting Hepatic Stellate Cells via Paracrine Mediators. Stem Cells Transl. Med. 2017, 6, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Li, P.; Tan, J. Human Menstrual Blood-Derived Stromal Cells Promote Recovery of Premature Ovarian Insufficiency Via Regulating the ECM-Dependent FAK/AKT Signaling. Stem Cell Rev. Rep. 2018, 15, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Manshadi, M.D.; Navid, S.; Hoshino, Y.; Daneshi, E.; Noory, P.; Abbasi, M. The effects of human menstrual blood stem cells-derived granulosa cells on ovarian follicle formation in a rat model of premature ovarian failure. Microsc. Res. Tech. 2019, 82, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Zafardoust, S.; Kazemnejad, S.; Darzi, M.; Fathi-Kazerooni, M.; Rastegari, H.; Mohammadzadeh, A. Improvement of Pregnancy Rate and Live Birth Rate in Poor Ovarian Responders by Intraovarian Administration of Autologous Menstrual Blood Derived- Mesenchymal Stromal Cells: Phase I/II Clinical Trial. Stem Cell Rev. Rep. 2020, 16, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Chen, L.; Wang, X.; Zhao, Y.; Wang, Y.; Xiang, C. Transplantation of Menstrual Blood-Derived Mesenchymal Stem Cells Promotes the Repair of LPS-Induced Acute Lung Injury. Int. J. Mol. Sci. 2017, 18, 689. [Google Scholar] [CrossRef] [PubMed]
- Farzamfar, S.; Salehi, M.; Ehterami, A.; Naseri-Nosar, M.; Vaez, A.; Zarnani, A.H.; Sahrapeyma, H.; Shokri, M.-R.; Aleahmad, M. Promotion of excisional wound repair by a menstrual blood-derived stem cell-seeded decellularized human amniotic membrane. Biomed. Eng. Lett. 2018, 8, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V.; Kaneko, Y.; Maki, M.; Yu, S.-J.; Ali, M.; Allickson, J.G.; Sanberg, C.D.; Kuzmin-Nichols, N.; Sanberg, P.R. Menstrual Blood Cells Display Stem Cell–Like Phenotypic Markers and Exert Neuroprotection Following Transplantation in Experimental Stroke. Stem Cells Dev. 2010, 19, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.H.; Uyama, T.; Miyado, K.; Terai, M.; Kyo, S.; Kiyono, T. Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol. Biol. Cell 2007, 18, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. MSCs: The Sentinel and Safe-Guards of Injury. J. Cell. Physiol. 2016, 231, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, Z.; Yang, Y.; Zhang, M.; Wu, J.; Miao, Y. Diagnostic value of pelvic floor ultrasonography for diagnosis of pelvic organ prolapse: A systematic review. Int. Urogynecol. J. 2019, 31, 15–33. [Google Scholar] [CrossRef]
- Eisenberg, V.H.; Steinberg, M.; Weiner, Z.; Alcalay, M.; Itskovitz-Eldor, J.; Schiff, E.; Lowenstein, L. Three-dimensional transperineal ultrasound for imaging mesh implants following sacrocolpopexy. Ultrasound Obstet. Gynecol. 2014, 43, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Truby, R.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161. [Google Scholar] [CrossRef]
- Mironov, V.; Kasyanov, V.; Markwald, R.R. Organ printing: From bioprinter to organ biofabrication line. Curr. Opin. Biotechnol. 2011, 22, 667–673. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).