Grey and White Matter Volume Changes after Preterm Birth: A Meta-Analytic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection Criteria
2.3. Data Collection Process and Data Extraction
2.4. Data on Full-Term Controls
2.5. Statistical Analysis
3. Results
3.1. Study Selection and Characteristics
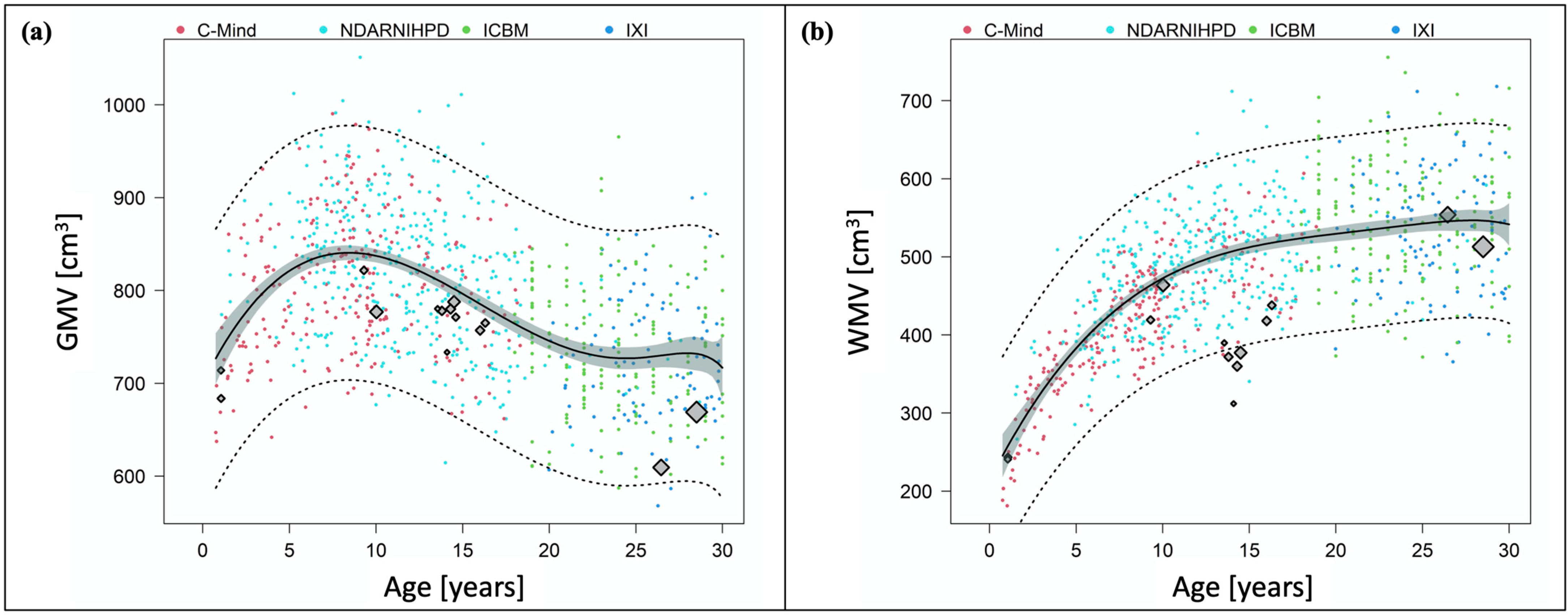
3.2. Development of Grey Matter Volume after Preterm Birth
3.3. Development of White Matter Volume after Preterm Birth
3.4. Total Intracranial Volume after Preterm Birth
4. Discussion
4.1. Changes of Grey and White Matter Volume after Preterm Birth across the First Half of Lifespan
4.2. What Is Needed in the Future?
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [Green Version]
- Nosarti, C.; Al-Asady, M.H.S.; Frangou, S.; Stewart, A.L.; Rifkin, L.; Murray, R.M. Adolescents who were born very preterm have decreased brain volumes. Brain 2002, 125, 1616–1623. [Google Scholar] [CrossRef] [Green Version]
- Nosarti, C.; Nam, K.-W.; Walshe, M.; Murray, R.M.; Cuddy, M.; Rifkin, L.; Allin, M.P.G. Preterm birth and structural brain alterations in early adulthood. NeuroImage Clin. 2014, 6, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Inder, T.E.; Warfield, S.K.; Wang, H.; Hüppi, P.S.; Volpe, J.J. Abnormal cerebral structure is present at term in premature infants. Pediatrics 2005, 115, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soria-Pastor, S.; Padilla, N.; Zubiaurre-Elorza, L.; Ibarretxe-Bilbao, N.; Botet, F.; Costas-Moragas, C.; Falcon, C.; Bargallo, N.; Mercader, J.M.; Junque, C. Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics 2009, 124, e1161–e1170. [Google Scholar] [CrossRef]
- Volpe, J.J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef] [Green Version]
- Meng, C.; Bäuml, J.G.; Daamen, M.; Jaekel, J.; Neitzel, J.; Scheef, L.; Busch, B.; Baumann, N.; Boecker, H.; Zimmer, C.; et al. Extensive and interrelated subcortical white and gray matter alterations in preterm-born adults. Brain Struct. Funct. 2016, 221, 2109–2121. [Google Scholar] [CrossRef]
- Hedman, A.M.; van Haren, N.E.M.; Schnack, H.G.; Kahn, R.S.; Hulshoff Pol, H.E. Human brain changes across the life span: A review of 56 longitudinal magnetic resonance imaging studies. Hum. Brain Mapp. 2012, 33, 1987–2002. [Google Scholar] [CrossRef]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Coupé, P.; Catheline, G.; Lanuza, E.; Manjón, J.V. Alzheimer’s Disease Neuroimaging Initiative Towards a unified analysis of brain maturation and aging across the entire lifespan: A MRI analysis. Hum. Brain Mapp. 2017, 38, 5501–5518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coupé, P.; Manjón, J.V.; Lanuza, E.; Catheline, G. Lifespan changes of the human brain in Alzheimer’s Disease. Sci. Rep. 2019, 9, 3998. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6. [Google Scholar] [CrossRef] [Green Version]
- Dobbing, J.; Sands, J. Quantitative growth and development of human brain. Arch. Dis. Child. 1973, 48, 757–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020.
- Elzhov, T.V.; Mullen, K.M.; Spiess, A.-N.; Bolker, B. minpack.lm: R Interface to the Levenberg-Marquardt Nonlinear Least-Squares Algorithm Found in MINPACK, Plus Support for Bounds. 2016. Available online: https://CRAN.R-project.org/package=minpack.lm (accessed on 16 April 2021).
- Spiess, A.-N. Propagate: Propagation of Uncertainty. 2018. Available online: https://cran.r-project.org/web/packages/propagate/propagate.pdf (accessed on 16 April 2021).
- Pascoe, M.J.; Melzer, T.R.; Horwood, L.J.; Woodward, L.J.; Darlow, B.A. Altered grey matter volume, perfusion and white matter integrity in very low birthweight adults. NeuroImage Clin. 2019, 22, 101780. [Google Scholar] [CrossRef]
- Lemola, S.; Oser, N.; Urfer-Maurer, N.; Brand, S.; Holsboer-Trachsler, E.; Bechtel, N.; Grob, A.; Weber, P.; Datta, A.N. Effects of gestational age on brain volume and cognitive functions in generally healthy very preterm born children during school-age: A voxel-based morphometry study. PLoS ONE 2017, 12, e0183519. [Google Scholar] [CrossRef] [PubMed]
- Northam, G.B.; Liégeois, F.; Chong, W.K.; Wyatt, J.S.; Baldeweg, T. Total brain white matter is a major determinant of IQ in adolescents born preterm. Ann. Neurol. 2011, 69, 702–711. [Google Scholar] [CrossRef]
- Padilla, N.; Falcón, C.; Sanz-Cortés, M.; Figueras, F.; Bargallo, N.; Crispi, F.; Eixarch, E.; Arranz, A.; Botet, F.; Gratacós, E. Differential effects of intrauterine growth restriction on brain structure and development in preterm infants: A magnetic resonance imaging study. Brain Res. 2011, 1382, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Narberhaus, A.; Segarra, D.; Caldú, X.; Giménez, M.; Junqué, C.; Pueyo, R.; Botet, F. Gestational age at preterm birth in relation to corpus callosum and general cognitive outcome in adolescents. J. Child Neurol. 2007, 22, 761–765. [Google Scholar] [CrossRef]
- Giménez, M.; Junqué, C.; Narberhaus, A.; Botet, F.; Bargalló, N.; Mercader, J.M. Correlations of thalamic reductions with verbal fluency impairment in those born prematurely. Neuroreport 2006, 17, 463–466. [Google Scholar] [CrossRef]
- Giménez, M.; Junqué, C.; Narberhaus, A.; Bargalló, N.; Botet, F.; Mercader, J.M. White matter volume and concentration reductions in adolescents with history of very preterm birth: A voxel-based morphometry study. Neuroimage 2006, 32, 1485–1498. [Google Scholar] [CrossRef]
- Thompson, D.K.; Matthews, L.G.; Alexander, B.; Lee, K.J.; Kelly, C.E.; Adamson, C.L.; Hunt, R.W.; Cheong, J.L.Y.; Spencer-Smith, M.; Neil, J.J.; et al. Tracking regional brain growth up to age 13 in children born term and very preterm. Nat. Commun. 2020, 11, 696. [Google Scholar] [CrossRef]
- Peterson, B.S.; Vohr, B.; Staib, L.H.; Cannistraci, C.J.; Dolberg, A.; Schneider, K.C.; Katz, K.H.; Westerveld, M.; Sparrow, S.; Anderson, A.W.; et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. J. Am. Med. Assoc. 2000, 284, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Ment, L.R.; Kesler, S.; Vohr, B.; Katz, K.H.; Baumgartner, H.; Schneider, K.C.; Delancy, S.; Silbereis, J.; Duncan, C.C.; Constable, R.T.; et al. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics 2009, 123, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, J.; Mitchell, A.; Kalpakidou, A.; Walshe, M.; Jung, H.-Y.; Nosarti, C.; Santosh, P.; Rifkin, L.; Wyatt, J.; Murray, R.M.; et al. Cerebellar growth and behavioural & neuropsychological outcome in preterm adolescents. Brain 2008, 131, 1344–1351. [Google Scholar] [CrossRef] [Green Version]
- Karolis, V.R.; Froudist-Walsh, S.; Kroll, J.; Brittain, P.J.; Tseng, C.E.J.; Nam, K.W.; Reinders, A.A.T.S.; Murray, R.M.; Williams, S.C.R.; Thompson, P.M.; et al. Volumetric grey matter alterations in adolescents and adults born very preterm suggest accelerated brain maturation. Neuroimage 2017, 163, 379–389. [Google Scholar] [CrossRef] [Green Version]
- de Kieviet, J.F.; Zoetebier, L.; van Elburg, R.M.; Vermeulen, R.J.; Oosterlaan, J. Brain development of very preterm and very low-birthweight children in childhood and adolescence: A meta-analysis. Dev. Med. Child Neurol. 2012, 54, 313–323. [Google Scholar] [CrossRef]
- Volpe, J.J. Dysmaturation of premature brain: Importance, cellular mechanisms, and potential interventions. Pediatr. Neurol. 2019, 95, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Han, B.H.; Luo, N.L.; Chricton, C.A.; Xanthoudakis, S.; Tam, J.; Arvin, K.L.; Holtzman, D.M. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J. Neurosci. 2002, 22, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Segovia, K.N.; McClure, M.; Moravec, M.; Luo, N.L.; Wan, Y.; Gong, X.; Riddle, A.; Craig, A.; Struve, J.; Sherman, L.S.; et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann. Neurol. 2008, 63, 520–530. [Google Scholar] [CrossRef] [Green Version]
- Nosarti, C.; Giouroukou, E.; Healy, E.; Rifkin, L.; Walshe, M.; Reichenberg, A.; Chitnis, X.; Williams, S.C.R.; Murray, R.M. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain 2008, 131, 205–217. [Google Scholar] [CrossRef] [Green Version]
- Morsing, E.; Malova, M.; Kahn, A.; Lätt, J.; Björkman-Burtscher, I.M.; Maršál, K.; Ley, D. Brain volumes and developmental outcome in childhood following fetal growth restriction leading to very preterm birth. Front. Physiol. 2018, 9, 1583. [Google Scholar] [CrossRef] [PubMed]
- Manjón, J.V.; Coupé, P. volBrain: An online MRI brain volumetry system. Front. Neuroinform. 2016, 10, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Author (Year) | Group | Sample (n) | Age at Scan (Years) | TIV Mean (cm3) | TIV SD (cm3) | GMV Mean (cm3) | GMV SD (cm3) | WMV Mean (cm3) | WMV SD (cm3) | GA (Weeks) | BW (g) | Male (%) | Year of Birth | Country of Origin | Methodology of Brain Volume Estimation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pascoe (2019) [17] | 150 | 28.5 | 1504 | 140 | 669 | 61 | 513 | 61 | 28.8 | 1077 | 41.3 | 1986 | New Zealand | CAT12 toolbox (SPM12) | |
| Lemola (2017) [18] | 57 | 10.0 | 1397 | 148 | 777 | 70 | 464 | 67 | 29.7 | 1447 | 65.3 | 1998–2006 | Switzerland | New segment toolbox (SPM8) | |
| Meng (2016) [7] | 85 | 26.5 | 1385 | 609.4 | 554 | 30.67 | 1356 | 55.3 | 1985–1986 | Germany | VBM8 toolbox (SPM8) | ||||
| Northam (2011) [19] | positive cUS | 27 | 16 | 1601 | 222 | 757 | 57 | 418 | 46 | 27.1 | 1081 | 44.4 | 1989–1994 | United Kingdom | VBM5 toolbox (SPM5) |
| Northam (2011) [19] | normal cUS | 22 | 16.3 | 1480 | 193 | 765 | 65 | 438 | 49 | 28.1 | 1098 | 31.8 | 1989–1994 | United Kingdom | VBM5 toolbox (SPM5) |
| Padilla (2011) [20] | IUGR | 18 | 1.1 * | 969.6 | 101.8 | 683.7 | 64.5 | 243.5 | 36.9 | 32.1 | 1060 | 38.9 | 2006–2007 | Spain | VBM5 toolbox (SPM5) |
| Padilla (2011) [20] | AGA | 15 | 1.1 * | 1001.1 | 95.4 | 714.0 | 57.0 | 240.7 | 38.0 | 31 | 1580 | 73.3 | 2006–2007 | Spain | VBM5 toolbox (SPM5) |
| Soria-Pastor (2009) [5] | 20 | 9.3 | 1641.2 | 172.6 | 821.7 | 84.9 | 419.2 | 53.8 | 32.5 | 1754 | 55 | 1996–1998 | Spain | SPM5 | |
| Narberhaus (2007) [21] | GA ≤ 27 | 9 | 14.1 | 1354.8 | 174.1 | 733.4 | 54.7 | 312.1 | 57.9 | 26.4 | 899 | 77.8 | 1983–1994 | Spain | SPM2 |
| Narberhaus (2007) [21] | GA 28–30 | 19 | 14.6 | 1488.5 | 164.9 | 771.3 | 133.2 | 377.2 | 57.9 | 29 | 1140 | 42.1 | 1983–1994 | Spain | SPM2 |
| Narberhaus (2007) [21] | GA 31–33 | 25 | 13.8 | 1445.3 | 146.4 | 778.1 | 72.1 | 372.0 | 52.1 | 31.7 | 1534 | 44 | 1983–1994 | Spain | SPM2 |
| Narberhaus (2007) [21] | GA 34–36 | 11 | 13.55 | 1473.3 | 148.4 | 780.3 | 69.9 | 389.8 | 45.1 | 34.6 | 2446 | 63.6 | 1983–1994 | Spain | SPM2 |
| Gimenez (2006b) [23] | 50 | 14.5 | 1488.8 | 148.8 | 787.8 | 80.8 | 377.2 | 47.4 | 29.9 | 1327 | 48 | 1982–1994 | Spain | SPM2 | |
| Gimenez (2006a) [22] | 30 | 14.3 | 1460 | 140 | 780 | 70 | 360 | 50 | 29.1 | 1108 | n.a. | n.a. | Spain | SPM2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitz-Koep, B.; Haller, B.; Coupé, P.; Menegaux, A.; Gaser, C.; Zimmer, C.; Wolke, D.; Bartmann, P.; Sorg, C.; Hedderich, D.M. Grey and White Matter Volume Changes after Preterm Birth: A Meta-Analytic Approach. J. Pers. Med. 2021, 11, 868. https://doi.org/10.3390/jpm11090868
Schmitz-Koep B, Haller B, Coupé P, Menegaux A, Gaser C, Zimmer C, Wolke D, Bartmann P, Sorg C, Hedderich DM. Grey and White Matter Volume Changes after Preterm Birth: A Meta-Analytic Approach. Journal of Personalized Medicine. 2021; 11(9):868. https://doi.org/10.3390/jpm11090868
Chicago/Turabian StyleSchmitz-Koep, Benita, Bernhard Haller, Pierrick Coupé, Aurore Menegaux, Christian Gaser, Claus Zimmer, Dieter Wolke, Peter Bartmann, Christian Sorg, and Dennis M. Hedderich. 2021. "Grey and White Matter Volume Changes after Preterm Birth: A Meta-Analytic Approach" Journal of Personalized Medicine 11, no. 9: 868. https://doi.org/10.3390/jpm11090868






