Nonwearable Sensor-Based In-Home Assessment of Subtle Daily Behavioral Changes as a Candidate Biomarker for Mild Cognitive Impairment
Abstract
:1. Introduction
2. Changes in Daily Behavioral Patterns in MCI and AD Using Performance- and Questionnaire-Based Assessments
2.1. Definition of Daily Bahavior and Traditional Assessment Methods to Evaluate Daily Bahavioral Changes
2.2. Changes in Daily Bahavioral Patterns Observed in MCI and AD Based on Performance- and Questionnarie-Based Assessments
3. Changes in Daily Behavioral Patterns in MCI and AD Based on Nonwearable Sensor-Based In-Home Assessment
3.1. Digital Technologies for Monitoring of Daily Behavioral Patterns
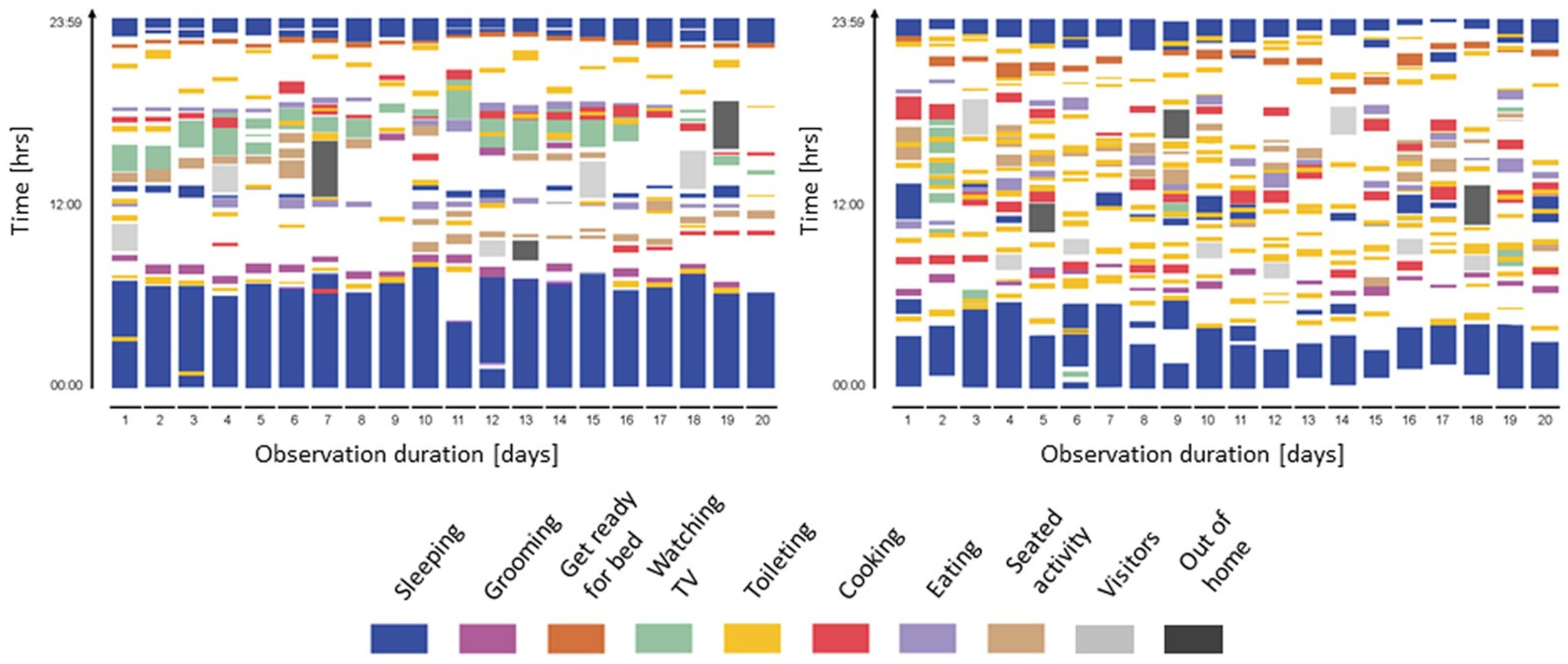
3.2. Changes in Daily Bahavioral Patterns Observed in MCI and AD Based on Nonwearable Sensor-Based In-Home Assessment
3.3. Machine Learning–Based Prediction Model for Detecting Individuals with MCI
| References | Participants and Study Protocol (1. Study Design; 2. Participants; 3. Sensor Type; 4. Duration; 5. Machine Learning Technique) | Main Findings |
|---|---|---|
| Hayes et al. [26] |
| - Walking speed was more variable in patients with MCI. - Day-to-day pattern of activities was more variable in patients with MCI. |
| Dodge et al. [27] |
| - Daily walking speeds and their variability were associated with non-amnestic MCI. |
| Hayes et al. [28] |
| - Patients with amnestic MCI showed less sleep disturbance than both those with non-amnestic MCI and healthy elderly. |
| Petersen et al. [29] |
| - Patients with MCI spent an average 1.67 h more inside the home than healthy elderly. |
| Urwyler et al. [30] |
| - Patients with dementia showed unorganized behavior patterns. |
| Rawtaer et al. [31] |
| - Patients with MCI were less active than healthy subjects and had more sleep interruptions per night. - Patients with MCI had forgotten their medications more times per month than healthy subjects. |
| Akl et al. [34] |
| - Variabilities in weekly walking speed, morning and evening walking speeds, and subjects’ age and gender were the most important for the process of detecting MCI. - This study autonomously detected MCI with receiver operating characteristic curve (0.97) and precision–recall curve (0.93) using a time windows of 24 weeks. |
| Akl et al. [35] |
| - This study automatically detected MCI (F0.5 score, 0.856) and non-amnestic MCI (F0.5 score, 0.958). |
| Alberdi et al. [36] |
| - Sleep and overnight patterns along with daily routine features contributed to the prediction of several health assessments. - All algorithms could build statistically significant prediction models. |
| Nakaoku et al. [37] |
| - Three independent power monitoring parameters (air conditioner, microwave oven, and induction heater) representing activity behavior were associated with cognitive impairment. - The prediction model with power monitoring data had better predictive ability (accuracy, 0.82; sensitivity, 0.48; and specificity, 0.96). |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Dementia. Available online: https://www.who.int/en/news-room/fact-sheets/detail/dementia (accessed on 13 November 2021).
- Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Tobimatsu, S. Electrophysiological biomarkers for improved etiological diagnosis of cognitive impairment. Curr. Biomark. Find. 2014, 4, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Jekel, K.; Damian, M.; Wattmo, C.; Hausner, L.; Bullock, R.; Connelly, P.J.; Dubois, B.; Eriksdotter, M.; Ewers, M.; Grassel, E.; et al. Mild cognitive impairment and deficits in instrumental activities of daily living: A systematic review. Alzheimer’s Res. Ther. 2015, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabira, T.; Hotta, M.; Murata, M.; Yoshiura, K.; Han, G.; Ishikawa, T.; Koyama, A.; Ogawa, N.; Maruta, M.; Ikeda, Y.; et al. Age-related changes in instrumental and basic activities of daily living impairment in older adults with very mild Alzheimer’s disease. Dement. Geriatr. Cogn. Dis. Extra 2020, 10, 27–37. [Google Scholar] [CrossRef]
- Bruderer-Hofstetter, M.; Sikkes, S.A.M.; Münzer, T.; Niedermann, K. Development of a model on factors affecting instrumental activities of daily living in people with mild cognitive impairment—A Delphi study. BMC Neurol. 2020, 20, 264. [Google Scholar] [CrossRef]
- Ahn, I.S.; Kim, J.H.; Kim, S.; Chung, J.W.; Kim, H.; Kang, H.S.; Kim, D.K. Impairment of instrumental activities of daily living in patients with mild cognitive impairment. Psychiatry Investig. 2009, 6, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Piau, A.; Wild, K.; Mattek, N.; Kaye, J. Current state of digital biomarker technologies for real-life, home-based monitoring of cognitive function for mild cognitive impairment to mild Alzheimer disease and implications for clinical care: Systematic review. J. Med. Internet Res. 2019, 21, e12785. [Google Scholar] [CrossRef]
- Narasimhan, R.; Muthukumaran, G.; McGlade, C. Current state of non-wearable sensor technologies for monitoring activity patterns to detect symptoms of mild cognitive impairment to Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2021, 2021, 2679398. [Google Scholar] [CrossRef]
- Stavropoulos, T.G.; Papastergiou, A.; Mpaltadoros, L.; Nikolopoulos, S.; Kompatsiaris, I. IoT wearable sensors and devices in elderly care: A literature review. Sensors 2020, 20, 2826. [Google Scholar] [CrossRef]
- Debes, C.; Merentitis, A.; Sukhanov, S.; Niessen, M.; Frangiadakis, N.; Bauer, A. Monitoring activities of daily living in smart homes: Understanding human behavior. IEEE Signal. Process. Mag. 2016, 33, 81–94. [Google Scholar] [CrossRef]
- Kourtis, L.C.; Regele, O.B.; Wright, J.M.; Jones, G.B. Digital biomarkers for Alzheimer’s disease: The mobile/wearable devices opportunity. Npj Digit. Med. 2019, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Mlinac, M.E.; Feng, M.C. Assessment of activities of daily living, self-care, and independence. Arch. Clin. Neuropsychol. 2016, 31, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Tago, M.; Katsuki, N.E.; Yaita, S.; Nakatani, E.; Yamashita, S.; Oda, Y.; Yamashita, S.I. High inter-rater reliability of Japanese bedriddenness ranks and cognitive function scores: A hospital-based prospective observational study. BMC Geriatr. 2021, 21, 168. [Google Scholar] [CrossRef]
- Sikkes, S.A.M.; de Lange-de Klerk, E.S.M.; Pijnenburg, Y.A.L.; Gillissen, F.; Romkes, R.; Knol, D.L.; Uitdehaag, B.M.J.; Scheltens, P. A new informant-based questionnaire for instrumental activities of daily living in dementia. Alzheimer’s Dement. 2012, 8, 536–543. [Google Scholar] [CrossRef] [Green Version]
- Gary, K.W. Direct Assessment of Functional Status. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Pereira, F.S.; Yassuda, M.S.; Oliveira, A.M.; Diniz, B.S.; Radanovic, M.; Talib, L.L.; Gattaz, W.F.; Forlenza, O.V. Profiles of functional deficits in mild cognitive impairment and dementia: Benefits from objective measurement. J. Int. Neuropsychol. Soc. 2010, 16, 297–305. [Google Scholar] [CrossRef]
- Alzheimer’s Disease Co-operative Study ADL Scale for Mild Cognitive Impairment (ADCS-ADL-MCI). Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Perneczky, R.; Pohl, C.; Sorg, C.; Hartmann, J.; Tosic, N.; Grimmer, T.; Heitele, S.; Kurz, A. Impairment of activities of daily living requiring memory or complex reasoning as part of the MCI syndrome. Int. J. Geriatr. Psychiatry 2006, 21, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Perneczky, R.; Pohl, C.; Sorg, C.; Hartmann, J.; Komossa, K.; Alexopoulos, P.; Wagenpfeil, S.; Kurz, A. Complex activities of daily living in mild cognitive impairment: Conceptual and diagnostic issues. Age Ageing 2006, 35, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.R.; Lee, K.S.; Cheong, H.K.; Eom, J.S.; Oh, B.H.; Hong, C.H. Characteristic profiles of instrumental activities of daily living in different subtypes of mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2009, 27, 278–285. [Google Scholar] [CrossRef]
- Pérès, K.; Chrysostome, V.; Fabrigoule, C.; Orgogozo, J.M.; Dartigues, J.F.; Barberger-Gateau, P. Restriction in complex activities of daily living in MCI: Impact on outcome. Neurology 2006, 67, 461–466. [Google Scholar] [CrossRef]
- Eisa, S.; Moreira, A. A behaviour monitoring system (BMS) for ambient assisted living. Sensors 2017, 17, 1946. [Google Scholar] [CrossRef] [Green Version]
- Hayes, T.L.; Abendroth, F.; Adami, A.; Pavel, M.; Zitzelberger, T.A.; Kaye, J.A. Unobtrusive assessment of activity patterns associated with mild cognitive impairment. Alzheimer’s Dement. 2008, 4, 395–405. [Google Scholar] [CrossRef]
- Dodge, H.H.; Mattek, N.C.; Austin, D.; Hayes, T.L.; Kaye, J.A. In-home walking speeds and variability trajectories associated with mild cognitive impairment. Neurology 2012, 78, 1946–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, T.L.; Riley, T.; Mattek, N.; Pavel, M.; Kaye, J.A. Sleep habits in mild cognitive impairment. Alzheimer Dis. Assoc. Disord. 2014, 28, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, J.; Austin, D.; Mattek, N.; Kaye, J. Time out-of-home and cognitive, physical, and emotional wellbeing of older adults: A longitudinal mixed effects model. PLoS ONE 2015, 10, e0139643. [Google Scholar] [CrossRef]
- Urwyler, P.; Stucki, R.; Rampa, L.; Müri, R.; Mosimann, U.P.; Nef, T. Cognitive impairment categorized in community-dwelling older adults with and without dementia using in-home sensors that recognise activities of daily living. Sci. Rep. 2017, 7, 42084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawtaer, I.; Mahendran, R.; Kua, E.H.; Tan, H.P.; Tan, H.X.; Lee, T.S.; Ng, T.P. Early detection of mild cognitive impairment with in-home sensors to monitor behavior patterns in community-dwelling senior citizens in Singapore: Cross-sectional feasibility study. J. Med. Internet Res. 2020, 22, e16854. [Google Scholar] [CrossRef] [PubMed]
- Sajda, P. Machine learning for detection and diagnosis of disease. Annu. Rev. Biomed. Eng. 2006, 8, 537–565. [Google Scholar] [CrossRef] [Green Version]
- Uddin, S.; Khan, A.; Hossain, M.E.; Moni, M.A. Comparing different supervised machine learning algorithms for disease prediction. BMC Med. Inform. Decis. Mak. 2019, 19, 281. [Google Scholar] [CrossRef]
- Akl, A.; Taati, B.; Mihailidis, A. Autonomous unobtrusive detection of mild cognitive impairment in older adults. IEEE Trans. Biomed. Eng. 2015, 62, 1383–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akl, A.; Chikhaoui, B.; Mattek, N.; Kaye, J.; Austin, D.; Mihailidis, A. Clustering home activity distributions for automatic detection of mild cognitive impairment in older adults. J. Ambient. Intell. Smart Environ. 2016, 8, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Alberdi, A.; Weakley, A.; Schmitter-Edgecombe, M.; Cook, D.J.; Aztiria, A.; Basarab, A.; Barrenechea, M. Smart home-based prediction of multi-domain symptoms related to Alzheimer’s disease. IEEE J. Biomed. Health Inform. 2018, 22, 1720–1731. [Google Scholar] [CrossRef] [Green Version]
- Nakaoku, Y.; Ogata, S.; Murata, S.; Nishimori, M.; Ihara, M.; Iihara, K.; Takegami, M.; Nishimura, K. AI-assisted in-house power monitoring for the detection of cognitive impairment in older adults. Sensors 2021, 21, 6249. [Google Scholar] [CrossRef] [PubMed]
- IoT x AI! Collaborative Development of a New Function of “Goo of Things Denkyu” that Estimates the Risk of Deterioration of Daily Living Functions of the Elderly. Available online: https://pr.goo.ne.jp/goo/2020/26289/ (accessed on 13 November 2021).
- Park, H.D. Current status of clinical application of point-of-care testing. Arch. Pathol. Lab. Med. 2021, 145, 168–175. [Google Scholar] [CrossRef]
- Nichols, J.H. Utilizing point-of-care testing to optimize patient care. EJIFCC 2021, 32, 140–144. [Google Scholar] [PubMed]
- Christodouleas, D.C.; Kaur, B.; Chorti, P. From point-of-care testing to eHealth diagnostic devices (eDiagnostics). ACS Cent. Sci. 2018, 4, 1600–1616. [Google Scholar] [CrossRef]
- Bodington, R.; Kassianides, X.; Bhandari, S. Point-of-care testing technologies for the home in chronic kidney disease: A narrative review. Clin. Kidney J. 2021, 14, 2316–2331. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.; Casteigne, B.; Chapman, W.D.; Creed, A.; Forrest, F.; Lapins, A.; Shatz, R.; Sawyer, R.P. Diagnostic biomarkers in Alzheimer’s disease. Biomark. Neuropsychiatr. 2021, 5, 100041. [Google Scholar] [CrossRef]
- Ausó, E.; Gómez-Vicente, V.; Esquiva, G. Biomarkers for Alzheimer’s disease early diagnosis. J. Pers. Med. 2020, 10, 114. [Google Scholar] [CrossRef]
- Yamasaki, T.; Muranaka, H.; Kaseda, Y.; Mimori, Y.; Tobimatsu, S. Understanding the pathophysiology of Alzheimer’s disease and mild cognitive impairment: A mini review on fMRI and ERP studies. Neurol. Res. Int. 2012, 2012, 719056. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Horie, S.; Muranaka, H.; Kaseda, Y.; Mimori, Y.; Tobimatsu, S. Relevance of in vivo neurophysiological biomarkers for mild cognitive impairment and Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 31, S137–S154. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T. Use of VEPs as electrodiagnistic biomarkers of mild cognitive impairment. Neurol. Clin. Neurosci. 2021, 9, 3–9. [Google Scholar] [CrossRef]
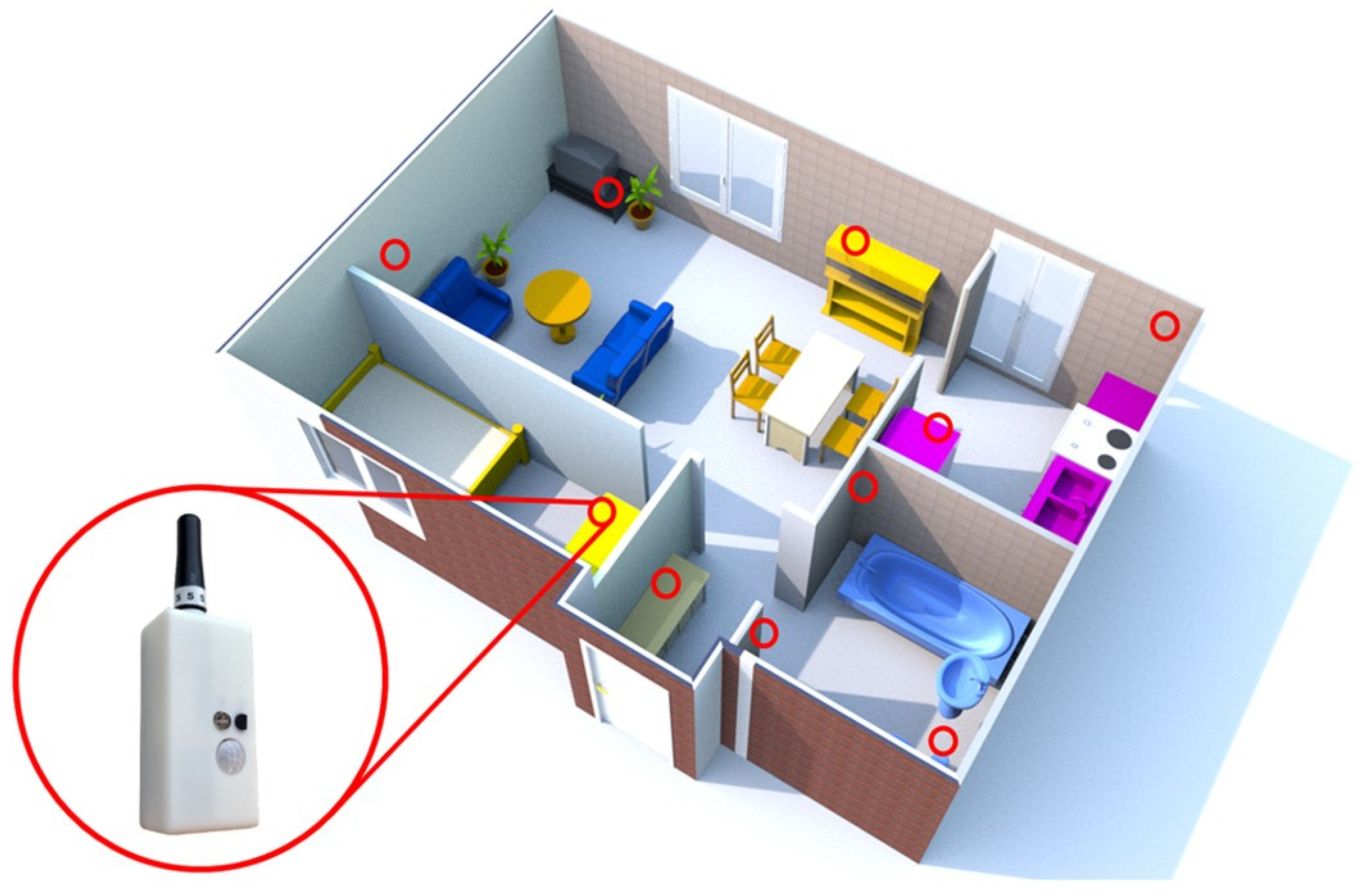
| Assessment Methods | Characteristics | Strengths | Weaknesses |
|---|---|---|---|
| Performance-based assessment | - Behavioral evaluation by a trained rater | - More objective than the questionnaire method | - Time consuming - Expensive - Only a limited number of activities can be evaluated - It does not always reflect the actual ADL at home |
| Informant-based questionnaire | - Questionnaire method completed by a suitable informant | - Easier than performance-based assessment - More objective than a self-assessment questionnaire | - The results are influenced by the person’s physical and mental conditions |
| Self-assessment questionnaire | - Questionnaire method completed by the patient himself/herself | - The easiest method | - Results are not always accurate because of cognitive decline |
| Nonwearable sensor-based in-home assessment | - Behavioral evaluation by various sensors installed at home | - More objective and quantitative than other methods | - Expensive - It takes time and effort to install sensors, monitor behavior, and analyze results |
| Sensor | Measurement Type | Characteristics |
|---|---|---|
| Infrared sensors | Motion | - Most frequently used nonwearable sensors - Discover human presence in a room - Detect motion in a specific area - Locate a human within a house |
| Ultrasonic sensors | Motion | - Person detection and localization by measuring distances to objects |
| Photoelectric sensors | Motion | - Detect a light source and output a signal |
| Vibration sensors | Vibration | - Detect a person falling, interaction with various objects, flushing toilets, and water flows |
| Pressure sensors | Pressure on object | - Detect the presence of a person, steps, and fall events - Deploy in the form of floor mats and smart tiles |
| Magnetic switches | Opening or closing | - Detect opening and closing of doors or cupboards - Provide information on users accessing particular rooms and opening dressers, refrigerators, or trash cans |
| Audio sensors | Activity-related sound | - Detect sounds in a house - Discriminate between different types of sounds |
| Wattmeter and other sensors | Consumption information | - Measure electricity consumption of domestic appliances and light |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamasaki, T.; Kumagai, S. Nonwearable Sensor-Based In-Home Assessment of Subtle Daily Behavioral Changes as a Candidate Biomarker for Mild Cognitive Impairment. J. Pers. Med. 2022, 12, 11. https://doi.org/10.3390/jpm12010011
Yamasaki T, Kumagai S. Nonwearable Sensor-Based In-Home Assessment of Subtle Daily Behavioral Changes as a Candidate Biomarker for Mild Cognitive Impairment. Journal of Personalized Medicine. 2022; 12(1):11. https://doi.org/10.3390/jpm12010011
Chicago/Turabian StyleYamasaki, Takao, and Shuzo Kumagai. 2022. "Nonwearable Sensor-Based In-Home Assessment of Subtle Daily Behavioral Changes as a Candidate Biomarker for Mild Cognitive Impairment" Journal of Personalized Medicine 12, no. 1: 11. https://doi.org/10.3390/jpm12010011
APA StyleYamasaki, T., & Kumagai, S. (2022). Nonwearable Sensor-Based In-Home Assessment of Subtle Daily Behavioral Changes as a Candidate Biomarker for Mild Cognitive Impairment. Journal of Personalized Medicine, 12(1), 11. https://doi.org/10.3390/jpm12010011







