Association between Late-Onset Ménière’s Disease and the Risk of Incident All-Cause Dementia
Abstract
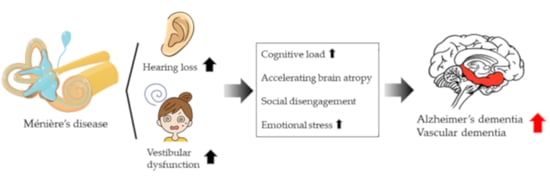
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Study Population
2.3. Study Variables
2.4. Outcome Measures
2.5. Sensitivity Test
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allan, L.M.; Ballard, C.; Rowan, E.N.; Kenny, R.A. Incidence and prediction of falls in dementia: A prospective study in older people. PLoS ONE 2009, 4, e5521. [Google Scholar] [CrossRef]
- Bowling, A.; Rowe, G.; Adams, S.; Sands, P.; Samsi, K.; Crane, M.; Joly, L.; Manthorpe, J. Quality of life in dementia: A systematically conducted narrative review of dementia-specific measurement scales. Aging Ment. Health 2015, 19, 13–31. [Google Scholar] [CrossRef]
- Cipriani, G.; Lucetti, C.; Nuti, A.; Danti, S. Wandering and dementia. Psychogeriatrics 2014, 14, 135–142. [Google Scholar] [CrossRef]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Norton, S.; Matthews, F.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef] [Green Version]
- Chern, A.; Sharma, R.K.; Golub, J.S. Hearing loss and incident dementia: Claims data from the New York SPARCS database. Otol. Neurotol. 2021, 43, 36–41. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Rah, Y.C.; Lee, M.K.; Park, S.; Kim, B.; Han, K.; Choi, J. Association between the severity of hearing loss and the risk of dementia within the 2010–2017 national insurance service survey in South Korea. Sci. Rep. 2020, 10, 20679. [Google Scholar] [CrossRef]
- Wei, E.X.; Oh, E.S.; Harun, A.; Ehrenburg, M.; Agrawal, Y. Vestibular loss predicts poorer spatial cognition in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 61, 995–1003. [Google Scholar] [CrossRef]
- Harun, A.; Oh, E.S.; Bigelow, R.T.; Studenski, S.; Agrawal, Y. Vestibular impairment in dementia. Otol. Neurotol. 2016, 37, 1137–1142. [Google Scholar] [CrossRef] [Green Version]
- Minor, L.B.; Schessel, D.A.; Carey, J.P. Ménière’s Disease. Curr. Opin. Neurol. 2004, 17, 9–16. [Google Scholar] [CrossRef]
- Van Cruijsen, N.; Jaspers, J.P.; van de Wiel, H.B.; Wit, H.P.; Albers, F.W. Psychological assessment of patients with Menière’s disease. Int. J. Audiol. 2006, 45, 496–502. [Google Scholar] [CrossRef] [Green Version]
- Van Cruijsen, N.; Hiemstra, W.M.; Meiners, L.C.; Wit, H.P.; Albers, F.W. Hippocampal volume measurement in patients with Ménière’s Disease: A pilot study. Acta Otolaryngol. 2007, 127, 1018–1023. [Google Scholar] [CrossRef]
- Seo, Y.J.; Kim, J.; Kim, S.H. The change of hippocampal volume and its relevance with inner ear function in Meniere’s disease patients. Auris Nasus Larynx 2016, 43, 620–625. [Google Scholar] [CrossRef]
- Previc, F.H. Vestibular loss as a contributor to Alzheimer’s disease. Med. Hypotheses 2013, 80, 360–367. [Google Scholar] [CrossRef]
- Lin, F.R.; Metter, E.J.; O’Brien, R.J.; Resnick, S.M.; Zonderman, A.B.; Ferrucci, L. Hearing loss and incident dementia. Arch. Neurol. 2011, 68, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.; Gibbons, L.E.; Gates, G.; Anderson, M.L.; McCurry, S.M.; McCormick, W.; Bowen, J.D.; Grabowski, T.J.; Crane, P.K.; Larson, E.B. Association of performance on dichotic auditory tests with risk for incident dementia and Alzheimer dementia. JAMA Otolaryngol. Neck Surg. 2021. [Google Scholar] [CrossRef]
- Caamano-Isorna, F.; Corral, M.; Montes-Martínez, A.; Takkouche, B. Education and dementia: A meta-analytic study. Neuroepidemiology 2006, 26, 226–232. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Lee, C.T.-C.; Lin, T.-Y.; Liu, C.-M. Exploring prior diseases associated with incident late-onset Alzheimer’s disease dementia. PLoS ONE 2020, 15, e0228172. [Google Scholar] [CrossRef]
- Panza, F.; Solfrizzi, V.; Seripa, D.; Imbimbo, B.P.; Capozzo, R.; Quaranta, N.; Pilotto, A.; Logroscino, G. Age-related hearing im-pairment and frailty in Alzheimer’s disease: Interconnected associations and mechanisms. front. Aging Neurosci. 2015, 7, 113. [Google Scholar] [CrossRef]
- Lin, F.; Ferrucci, L.; An, Y.; Goh, J.; Doshi, J.; Metter, E.; Davatzikos, C.; Kraut, M.; Resnick, S. Association of hearing impairment with brain volume changes in older adults. NeuroImage 2014, 90, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Mick, P.; Kawachi, I.; Lin, F.R. The Association between hearing loss and social isolation in older adults. Otolaryngol. Neck Surg. 2014, 150, 378–384. [Google Scholar] [CrossRef]
- Brandt, T.; Schautzer, F.; Hamilton, D.A.; Brüning, R.; Markowitsch, H.J.; Kalla, R.; Darlington, C.; Smith, P.; Strupp, M. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain 2005, 128, 2732–2741. [Google Scholar] [CrossRef]
- Dickerson, S.S.; Gruenewald, T.L.; Kemeny, M.E. When the social self is threatened: Shame, physiology, and health. J. Pers. 2004, 72, 1191–1216. [Google Scholar] [CrossRef]
- Lupien, S.J.; de Leon, M.; De Santi, S.; Convit, A.; Tarshish, C.Y.; Nair, N.P.V.; Thakur, M.; McEwen, B.S.; Hauger, R.L.; Meaney, M.J. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat. Neurosci. 1998, 1, 69–73. [Google Scholar] [CrossRef]
- Collip, D.; Habets, P.; Marcelis, M.; Gronenschild, E.; Lataster, T.; Lardinois, M.; Nicolson, N.A.; Myin-Germeys, I. Hippocampal volume as marker of daily life stress sensitivity in psychosis. Psychol. Med. 2013, 43, 1377–1387. [Google Scholar] [CrossRef]
- Ishiyama, G.; Lopez, I.A.; Ishiyama, P.; Vinters, H.V.; Ishiyama, A. The blood labyrinthine barrier in the human normal and Meniere’s disease macula utricle. Sci. Rep. 2017, 7, 253. [Google Scholar] [CrossRef]
- Attanasio, G.; Cagnoni, L.; Masci, E.; Ciciarello, F.; Diaferia, F.; Bruno, A.; Greco, A.; De Vincentiis, M. Chronic cerebrospinal venous insufficiency as a cause of inner ear diseases. Acta Oto Laryngol. 2017, 137, 460–463. [Google Scholar] [CrossRef]
- Ishiyama, G.; Wester, J.; Lopez, I.; Beltran-Parrazal, L.; Ishiyama, A. Oxidative stress in the blood labyrinthine barrier in the macula utricle of meniere’s disease patients. Front. Physiol. 2018, 9, 1068. [Google Scholar] [CrossRef]
- Pimentel-Coelho, P.M.; Michaud, J.-P.; Rivest, S. Effects of mild chronic cerebral hypoperfusion and early amyloid pathology on spatial learning and the cellular innate immune response in mice. Neurobiol. Aging 2013, 34, 679–693. [Google Scholar] [CrossRef]
- Ni, J.; Wu, Z.; Stoka, V.; Meng, J.; Hayashi, Y.; Peters, C.; Qing, H.; Turk, V.; Nakanishi, H. Increased expression and altered subcellular distribution of cathepsin B in microglia induce cognitive impairment through oxidative stress and inflammatory response in mice. Aging Cell 2019, 18, e12856. [Google Scholar] [CrossRef] [Green Version]
- Rim, T.H.; Kim, D.W.; Han, J.S.; Chung, E.J. Retinal vein occlusion and the risk of stroke development: A 9-year nationwide population-based study. Ophthalmology 2015, 122, 1187–1194. [Google Scholar] [CrossRef]



| The Number of Dementia Event | |
|---|---|
| Event | 270 |
| Comparison | 212 |
| Ménière’s disease | 58 |
| Total censored (No event) | 2210 |
| Comparison | 1772 |
| Ménière’s disease | 438 |
| Termination of study | 1830 |
| Comparison | 1440 |
| Ménière’s disease | 390 |
| Loss to follow up/Drop-out | 380 |
| Comparison | 332 |
| Ménière’s disease | 48 |
| Variables | Comparison (n = 18,706) | Dementia (n = 9353) | p Value |
|---|---|---|---|
| Sex | 0.929 | ||
| Male | 5239 (28%) | 2614 (27.9%) | |
| Female | 13,467 (72%) | 6739 (72.1%) | |
| Ages (years) | 1.000 | ||
| 65-74 | 4260 (22.8%) | 2130 (22.8%) | |
| 75-84 | 9310 (49.8%) | 4655 (49.8%) | |
| ≥85 | 5136 (27.5%) | 2568 (27.5%) | |
| Residence | 0.588 | ||
| Seoul | 2562 (13.7%) | 1281 (13.7%) | |
| Second area | 4506 (24.1%) | 2304 (24.6%) | |
| Third area | 11,638 (62.2%) | 5768 (61.7%) | |
| Household income | 0.990 | ||
| Low (0–30%) | 6268 (33.5%) | 3140 (33.6%) | |
| Middle (30–70%) | 4140 (22.1%) | 2064 (22.1%) | |
| High (70–100%) | 8298 (44.4%) | 4149 (44.4%) | |
| Comorbidities | 0.696 | ||
| No | 2678 (14.3%) | 1356 (14.5%) | |
| Yes | 16,028 (85.7%) | 7997 (85.5%) |
| Variables | Comparison (n = 1984) | Ménière’s Disease (n = 496) | p Value |
|---|---|---|---|
| Sex | 1.000 | ||
| Male | 592 (29.8%) | 148 (29.8%) | |
| Female | 1392 (70.2%) | 348 (70.2%) | |
| Ages (years) | 1.000 | ||
| 55-64 | 908 (45.8%) | 227 (45.8%) | |
| 65-74 | 812 (40.9%) | 203 (40.9%) | |
| ≥75 | 264 (13.3%) | 66 (13.3%) | |
| Residence | 1.000 | ||
| Seoul | 272 (13.7%) | 68 (13.7%) | |
| Second area | 680 (34.3%) | 170 (34.3%) | |
| Third area | 1032 (52%) | 258 (52%) | |
| Household income | 1.000 | ||
| Low (0–30%) | 376 (19%) | 94 (19%) | |
| Middle (30–70%) | 716 (36.1%) | 179 (36.1%) | |
| High (70–100%) | 89–2 (45%) | 223 (45%) | |
| Comorbidities | 1.000 | ||
| No | 268 (13.5%) | 67 (13.5%) | |
| Yes | 1716 (86.5%) | 429 (86.5%) |
| Variables | n | Case | Incidence | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|
| Group | |||||
| Comparison | 1984 | 212 | 11.3 | 1.00 (ref) | 1.00 (ref) |
| Ménière’sdisease | 496 | 58 | 14.3 | 1.55 (1.15–2.08) ** | 1.57 (1.17–2.12) ** |
| Sex | |||||
| Male | 740 | 60 | 9.1 | 1.00 (ref) | 1.00 (ref) |
| Female | 1740 | 210 | 13.0 | 1.40 (1.05–1.86) * | 1.37 (1.03–1.83) * |
| Ages (years) | |||||
| 55−64 | 1135 | 39 | 3.5 | 1.00 (ref) | 1.00 (ref) |
| 65−74 | 1015 | 154 | 16.3 | 4.68 (3.29–6.65) *** | 4.58 (3.21–6.54) *** |
| ≥75 | 330 | 77 | 34.8 | 12.16 (8.27–17.90) *** | 12.35 (8.37–18.22) *** |
| Residence | |||||
| Seoul | 340 | 31 | 9.8 | 1.00 (ref) | 1.00 (ref) |
| Second area | 850 | 101 | 13.0 | 1.34 (0.89–2.00) | 1.20 (0.80–1.81) |
| Third area | 1290 | 138 | 11.7 | 1.20 (0.81–1.77) | 0.96 (0.65–1.42) |
| Household income | |||||
| Low (0–30%) | 470 | 49 | 11.4 | 1.00 (ref) | 1.00 (ref) |
| Middle (30–70%) | 895 | 86 | 10.5 | 0.91 (0.64–1.29) | 1.02 (0.72–1.45) |
| High (70–100%) | 1115 | 135 | 13.1 | 1.13 (0.81–1.56) | 1.09 (0.78–1.52) |
| Comorbidities | |||||
| No | 335 | 27 | 9.0 | 1.00 (ref) | 1.00 (ref) |
| Yes | 2145 | 243 | 12.3 | 1.35 (0.91–2.01) | 1.14 (0.76–1.70) |
| Variables | n | Case | Incidence | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|
| Alzheimer’s disease | |||||
| Comparison | 1984 | 158 | 8.4 | 1.00 (ref) | 1.00 (ref) |
| Ménière’sdisease | 496 | 44 | 10.6 | 1.67 (1.19–2.35) ** | 1.69 (1.20–2.37) ** |
| Vascular dementia | |||||
| Comparison | 1984 | 42 | 2.2 | 1.00 (ref) | 1.00 (ref) |
| Ménière’sdisease | 496 | 16 | 3.8 | 2.02 (1.1–-3.63) * | 1.99 (1.10–3.57) * |
| Variables | Comparison (n = 18,706) | Dementia (n = 9353) | p value |
|---|---|---|---|
| Sex | 0.929 | ||
| Male | 5239 (28%) | 2614 (27.9%) | |
| Female | 13,467 (72%) | 6739 (72.1%) | |
| Ages (years) | 1.000 | ||
| 65-74 | 4260 (22.8%) | 2130 (22.8%) | |
| 75-84 | 9310 (49.8%) | 4655 (49.8%) | |
| ≥85 | 5136 (27.5%) | 2568 (27.5%) | |
| Residence | 0.588 | ||
| Seoul | 2562 (13.7%) | 1281 (13.7%) | |
| Second area | 4506 (24.1%) | 2304 (24.6%) | |
| Third area | 11,638 (62.2%) | 5768 (61.7%) | |
| Household income | 0.990 | ||
| Low (0–30%) | 6268 (33.5%) | 3140 (33.6%) | |
| Middle (30–70%) | 4140 (22.1%) | 2064 (22.1%) | |
| High (70–100%) | 8298 (44.4%) | 4149 (44.4%) | |
| Comorbidities | 0.696 | ||
| No | 2678 (14.3%) | 1356 (14.5%) | |
| Yes | 16,028 (85.7%) | 7997 (85.5%) |
| Non-dementia (n = 18,706) | Dementia (n = 9353) | |
|---|---|---|
| Ménière’sdisease | ||
| 0 | 18,233 (97.5%) | 9046 (96.7%) |
| 1 | 252 (1.3%) | 153 (1.6%) |
| 2 | 74 (0.4%) | 69 (0.7%) |
| 3 | 47 (0.3%) | 25 (0.3%) |
| 4 | 24 (0.1%) | 9 (0.1%) |
| 5 | 19 (0.1%) | 9 (0.1%) |
| 6 | 14 (0.1%) | 12 (0.1%) |
| 7 | 8 (0%) | 4 (0%) |
| 8 | 6 (0%) | 2 (0%) |
| 9 | 7 (0%) | 1 (0%) |
| 10 | 2 (0%) | 5 (0.1%) |
| 11 | 2 (0%) | 1 (0%) |
| 12 | 0 (0%) | 3 (0%) |
| 13 | 4 (0%) | 3 (0%) |
| 14 | 3 (0%) | 1 (0%) |
| 15 | 2 (0%) | 3 (0%) |
| 16 | 1 (0%) | 0 (0%) |
| 17 | 1 (0%) | 0 (0%) |
| 19 | 0 (0%) | 1 (0%) |
| 21 | 0 (0%) | 1 (0%) |
| 22 | 1 (0%) | 0 (0%) |
| 23 | 1 (0%) | 0 (0%) |
| 24 | 0 (0%) | 1 (0%) |
| 25 | 0 (0%) | 1 (0%) |
| 26 | 1 (0%) | 0 (0%) |
| 27 | 1 (0%) | 1 (0%) |
| 28 | 1 (0%) | 0 (0%) |
| 30 | 0 (0%) | 1 (0%) |
| 34 | 0 (0%) | 1 (0%) |
| 35 | 1 (0%) | 0 (0%) |
| 36 | 1 (0%) | 0 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.H.; Yu, H.; Ha, S.-S.; Son, G.M.; Park, K.J.; Lee, J.J.; Kim, D.-K. Association between Late-Onset Ménière’s Disease and the Risk of Incident All-Cause Dementia. J. Pers. Med. 2022, 12, 19. https://doi.org/10.3390/jpm12010019
Lee IH, Yu H, Ha S-S, Son GM, Park KJ, Lee JJ, Kim D-K. Association between Late-Onset Ménière’s Disease and the Risk of Incident All-Cause Dementia. Journal of Personalized Medicine. 2022; 12(1):19. https://doi.org/10.3390/jpm12010019
Chicago/Turabian StyleLee, Il Hwan, Hyunjae Yu, Seung-Su Ha, Gil Myeong Son, Ki Joon Park, Jae Jun Lee, and Dong-Kyu Kim. 2022. "Association between Late-Onset Ménière’s Disease and the Risk of Incident All-Cause Dementia" Journal of Personalized Medicine 12, no. 1: 19. https://doi.org/10.3390/jpm12010019
APA StyleLee, I. H., Yu, H., Ha, S.-S., Son, G. M., Park, K. J., Lee, J. J., & Kim, D.-K. (2022). Association between Late-Onset Ménière’s Disease and the Risk of Incident All-Cause Dementia. Journal of Personalized Medicine, 12(1), 19. https://doi.org/10.3390/jpm12010019