Changes in Bihemispheric Structural Connectivity Following Middle Cerebral Artery Infarction
Abstract
:1. Introduction
2. Methods
2.1. Patient Groups
2.2. Healthy Control Group
2.3. MRI Acquisition and Analysis
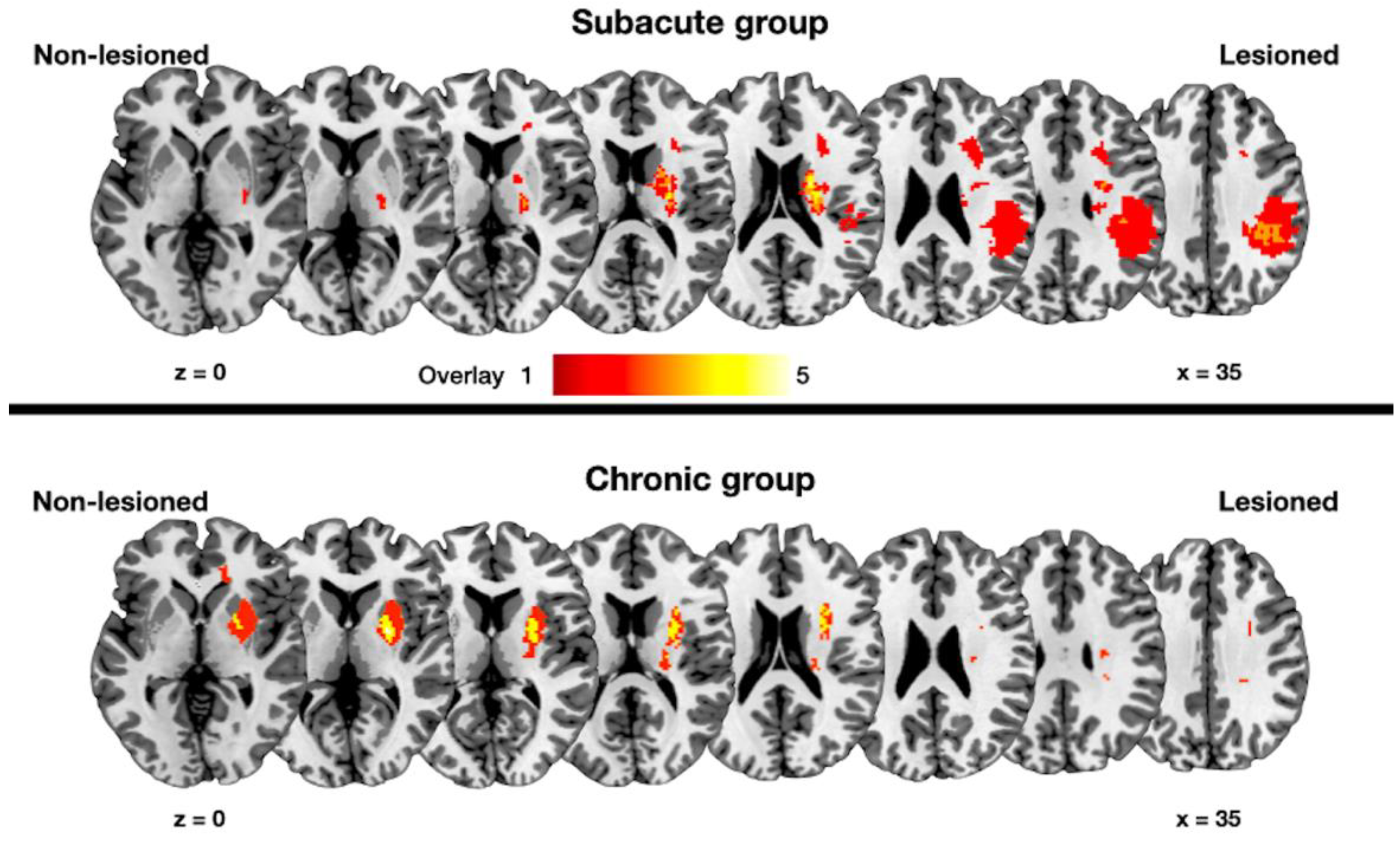
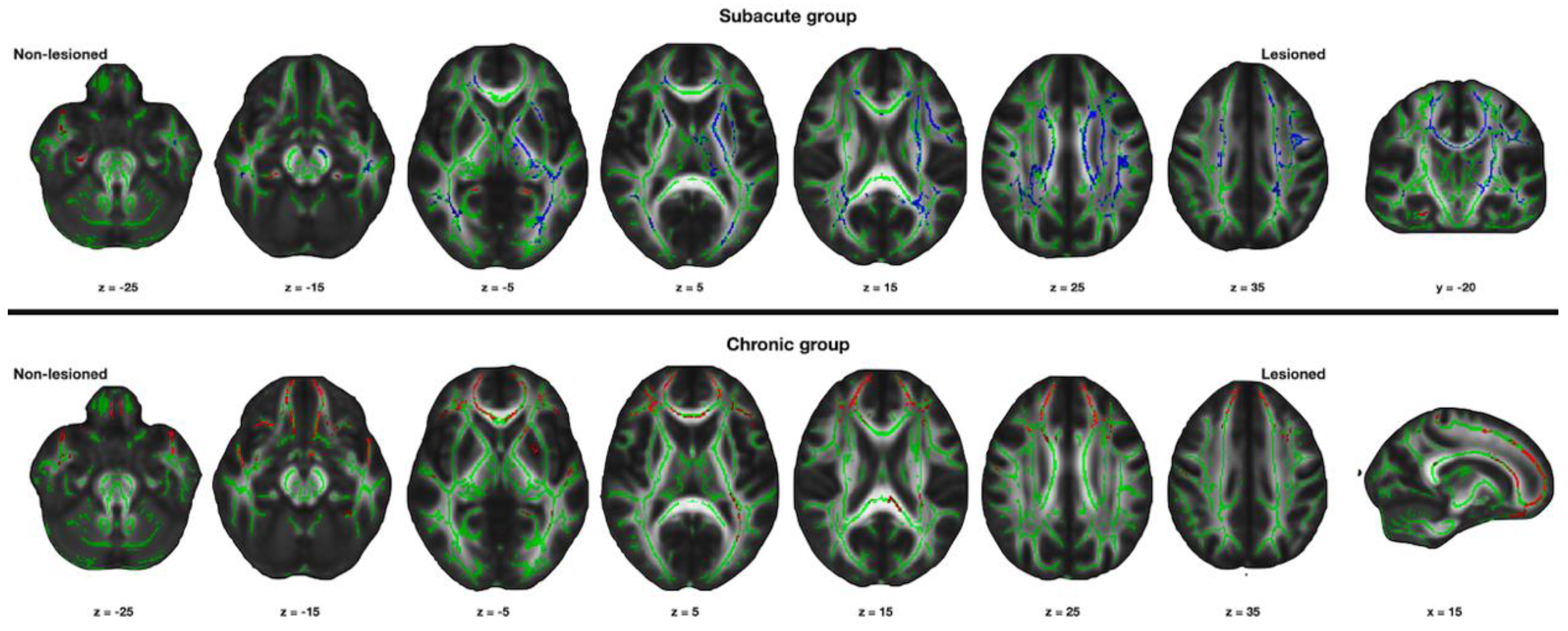
3. Results
3.1. Changes in the Structural Connectivity in the Subacute Group
3.2. Changes in the Structural Connectivity in the Chronic Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayo, N.E.; Wood-Dauphinee, S.; Ahmed, S.; Gordon, C.; Higgins, J.; McEwen, S.; Salbach, N. Disablement following stroke. Disabil. Rehabil. 1999, 21, 258–268. [Google Scholar] [CrossRef]
- Ward, N.S.; Brown, M.M.; Thompson, A.J.; Frackowiak, R.S. Neural correlates of outcome after stroke: A cross-sectional fmri study. Brain 2003, 126, 1430–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schallert, T.; Leasure, J.L.; Kolb, B. Experience-associated structural events, subependymal cellular proliferative activity, and functional recovery after injury to the central nervous system. J. Cereb. Blood Flow Metab. 2000, 20, 1513–1528. [Google Scholar] [CrossRef] [Green Version]
- Nudo, R.J.; Wise, B.M.; SiFuentes, F.; Milliken, G.W. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 1996, 272, 1791–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nudo, R.J. Postinfarct cortical plasticity and behavioral recovery. Stroke 2007, 38, 840–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmichael, S.T. Cellular and molecular mechanisms of neural repair after stroke: Making waves. Ann. Neurol. 2006, 59, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Seitz, R.J.; Höflich, P.; Binkofski, F.; Tellmann, L.; Herzog, H.; Freund, H.J. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch. Neurol. 1998, 55, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C.; Nelles, G.; Benson, R.R.; Kaplan, J.D.; Parker, R.A.; Kwong, K.K.; Kennedy, D.N.; Finklestein, S.P.; Rosen, B.R. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke 1997, 28, 2518–2527. [Google Scholar] [CrossRef]
- Weiller, C.; Ramsay, S.C.; Wise, R.J.; Friston, K.J.; Frackowiak, R.S. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann. Neurol. 1993, 33, 181–189. [Google Scholar] [CrossRef]
- Chollet, F.; DiPiero, V.; Wise, R.J.; Brooks, D.J.; Dolan, R.J.; Frackowiak, R.S. The functional anatomy of motor recovery after stroke in humans: A study with positron emission tomography. Ann. Neurol. 1991, 29, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Ward, N.S.; Brown, M.M.; Thompson, A.J.; Frackowiak, R.S. Neural correlates of motor recovery after stroke: A longitudinal fmri study. Brain 2003, 126, 2476–2496. [Google Scholar] [CrossRef]
- Woytowicz, E.J.; Rietschel, J.C.; Goodman, R.N.; Conroy, S.S.; Sorkin, J.D.; Whitall, J.; McCombe Waller, S. Determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer assessment of the upper extremity in chronic stroke. Arch. Phys. Med. Rehabil. 2017, 98, 456–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafto, M.A.; Tyler, L.K.; Dixon, M.; Taylor, J.R.; Rowe, J.B.; Cusack, R.; Calder, A.J.; Marslen-Wilson, W.D.; Duncan, J.; Dalgleish, T.; et al. The Cambridge Centre for Ageing and Neuroscience (cam-can) study protocol: A cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 2014, 14, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. Fsl. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assaf, Y.; Pasternak, O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J. Mol. Neurosci. MN 2008, 34, 51–61. [Google Scholar] [CrossRef]
- Kyeong, S.; Kang, H.; Kyeong, S.; Kim, D.H. Differences in brain areas affecting language function after stroke. Stroke 2019, 50, 2956–2959. [Google Scholar] [CrossRef]
- Wei, P.T.; Leong, D.; Calabrese, E.; White, L.; Pierce, T.; Platt, S.; Provenzale, J. Diffusion tensor imaging of neural tissue organization: Correlations between radiologic and histologic parameters. Neuroradiol. J. 2013, 26, 501–510. [Google Scholar] [CrossRef]
- Du, J.; Yang, F.; Zhang, Z.; Hu, J.; Xu, Q.; Hu, J.; Zeng, F.; Lu, G.; Liu, X. Early functional mri activation predicts motor outcome after ischemic stroke: A longitudinal, multimodal study. Brain Imaging Behav. 2018, 12, 1804–1813. [Google Scholar] [CrossRef]
- Grefkes, C.; Fink, G.R. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 2014, 13, 206–216. [Google Scholar] [CrossRef]
- Rehme, A.K.; Eickhoff, S.B.; Rottschy, C.; Fink, G.R.; Grefkes, C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. NeuroImage 2012, 59, 2771–2782. [Google Scholar] [CrossRef]
- Feng, W.; Wang, J.; Chhatbar, P.Y.; Doughty, C.; Landsittel, D.; Lioutas, V.A.; Kautz, S.A.; Schlaug, G. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann. Neurol. 2015, 78, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Park, C.H.; Boudrias, M.H.; Gerloff, C.; Hummel, F.C.; Ward, N.S. Assessing the integrity of corticospinal pathways from primary and secondary cortical motor areas after stroke. Stroke 2012, 43, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.C.; Nair, V.A.; Prabhakaran, V. Role of the contralesional vs. Ipsilesional hemisphere in stroke recovery. Front. Hum. Neurosci. 2017, 11, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seitz, R.J.; Azari, N.P.; Knorr, U.; Binkofski, F.; Herzog, H.; Freund, H.J. The role of diaschisis in stroke recovery. Stroke 1999, 30, 1844–1850. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, S.T.; Tatsukawa, K.; Katsman, D.; Tsuyuguchi, N.; Kornblum, H.I. Evolution of diaschisis in a focal stroke model. Stroke 2004, 35, 758–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feydy, A.; Carlier, R.; Roby-Brami, A.; Bussel, B.; Cazalis, F.; Pierot, L.; Burnod, Y.; Maier, M.A. Longitudinal study of motor recovery after stroke: Recruitment and focusing of brain activation. Stroke 2002, 33, 1610–1617. [Google Scholar] [CrossRef] [Green Version]
- Manganotti, P.; Patuzzo, S.; Cortese, F.; Palermo, A.; Smania, N.; Fiaschi, A. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clin. Neurophysiol. 2002, 113, 936–943. [Google Scholar] [CrossRef]
- Shimizu, T.; Hosaki, A.; Hino, T.; Sato, M.; Komori, T.; Hirai, S.; Rossini, P.M. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain 2002, 125, 1896–1907. [Google Scholar] [CrossRef]
- Ward, N.S.; Cohen, L.G. Mechanisms underlying recovery of motor function after stroke. Arch. Neurol. 2004, 61, 1844–1848. [Google Scholar] [CrossRef] [Green Version]
| Group | Age (Years) | Duration | FMUL | Lesion Volume (cm3) |
|---|---|---|---|---|
| Subacute | 75.8 | 12 | 48 | 6.0 |
| Subacute | 67.2 | 13 | 59 | 5.8 |
| Subacute | 75.6 | 16 | 64 | 1.8 |
| Subacute | 68.9 | 17 | 64 | 1.3 |
| Subacute | 69.7 | 17 | 47 | 6.1 |
| Subacute | 75.8 | 18 | 44 | 2.2 |
| Subacute | 70.2 | 19 | 66 | 153.2 |
| Subacute | 72.3 | 29 | 43 | 0.2 |
| Subacute | 70.8 | 32 | 63 | 15.1 |
| Subacute | 68.6 | 46 | 55 | 25.6 |
| Subacute | 73.3 | 52 | 57 | 3.5 |
| Subacute | 71.9 | 62 | 63 | 6.5 |
| Subacute | 71.4 | 62 | 47 | 2.5 |
| Subacute | 70.9 | 67 | 66 | 1.6 |
| Subacute | 81.5 | 72 | 52 | 5.2 |
| Subacute | 73.4 | 76 | 45 | 3.1 |
| Subacute | 64.0 | 78 | 57 | 3.7 |
| Subacute | 74.3 | 82 | 64 | 4.6 |
| Chronic | 74.4 | 383 | 60 | 9.9 |
| Chronic | 68.9 | 454 | 66 | 0.8 |
| Chronic | 66.8 | 558 | 63 | 57.4 |
| Chronic | 73.5 | 643 | 63 | 3.3 |
| Chronic | 94.3 | 1237 | 63 | 19.8 |
| Chronic | 72.8 | 1428 | 64 | 0.6 |
| Chronic | 77.1 | 2911 | 63 | 3.4 |
| Chronic | 86.2 | 3649 | 60 | 1.1 |
| Chronic | 75.9 | 8064 | 64 | 1.7 |
| Parameter | T1 | DTI |
|---|---|---|
| Matrix | 256 × 256 | 112 × 112 |
| Field of view (mm2) | 230 × 230 | 224 × 224 |
| Repetition time (ms) | 1900 | 9700 |
| Echo time (ms) | 2.57 | 92.00 |
| Slice thickness (mm) | 1 | 2 |
| Flip angle (°) | 9 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.H.; Kang, H. Changes in Bihemispheric Structural Connectivity Following Middle Cerebral Artery Infarction. J. Pers. Med. 2022, 12, 81. https://doi.org/10.3390/jpm12010081
Kim DH, Kang H. Changes in Bihemispheric Structural Connectivity Following Middle Cerebral Artery Infarction. Journal of Personalized Medicine. 2022; 12(1):81. https://doi.org/10.3390/jpm12010081
Chicago/Turabian StyleKim, Dae Hyun, and Hyunkoo Kang. 2022. "Changes in Bihemispheric Structural Connectivity Following Middle Cerebral Artery Infarction" Journal of Personalized Medicine 12, no. 1: 81. https://doi.org/10.3390/jpm12010081






