Plasma Levels of Mid-Regional Proadrenomedullin Accurately Identify H1N1pdm09 Influenza Virus Patients with Risk of Intensive Care Admission and Mortality in the Emergency Department
Abstract
1. Introduction
2. Materials and Methods
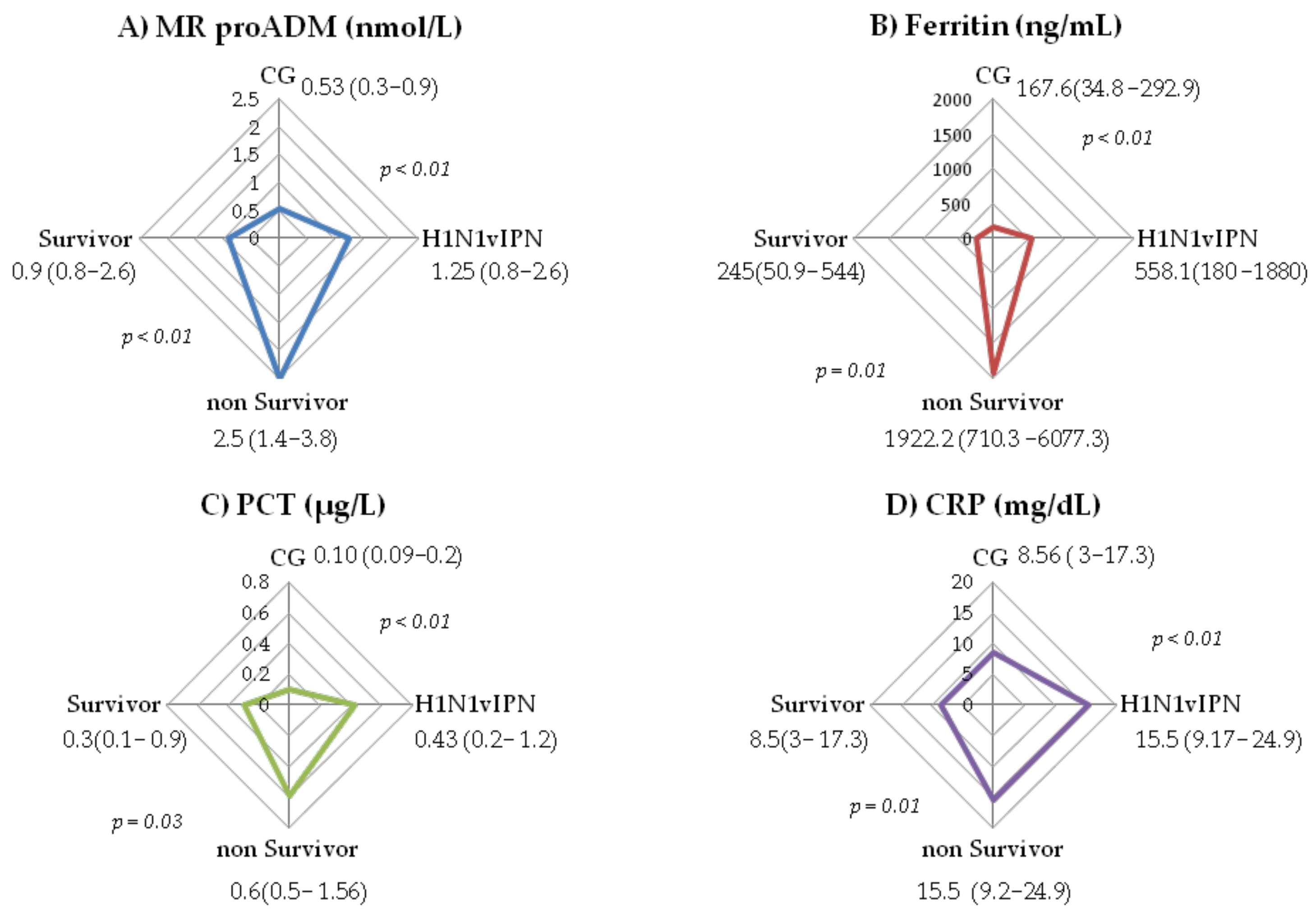
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Morens, D.M.; Taubenberger, J.K.; Harvey, H.A.; Memoli, M.J. The 1918 influenza pandemic: Lessons for 2009 and the future. Crit. Care Med. 2010, 38, e10–e20. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Pop-Vicas, A. Clinical review: Primary influenza viral pneumonia. Crit. Care 2009, 13, 235. [Google Scholar] [CrossRef]
- Rello, J.; Rodríguez, A.; Ibañez, P.; Socias, L.; Cebrian, J.; Marques, A.; Guerrero, J.; Ruiz-Santana, S.; Marquez, E.; Del Nogal-Saez, F.; et al. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit. Care 2009, 13, R148. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, N.; Woodhead, M. Predicting the unpredictable: Is it possible clinically to separate H1N1 from non-H1N1 community-acquired pneumonia? Thorax 2011, 66, 187–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruuskanen, O.; Lahti, E.; Jennings, L.C.; Murdoch, D.R. Viral pneumonia. Lancet 2011, 377, 1264–1275. [Google Scholar] [CrossRef]
- Shahpori, R.; Stelfox, H.T.; Doig, C.J.; Boiteau, P.J.E.; Zygun, D.A. Sequential Organ Failure Assessment in H1N1 pandemic planning. Crit. Care Med. 2011, 39, 827–832. [Google Scholar] [CrossRef]
- Pereira, J.M.; Moreno, R.P.; Matos, R.; Rhodes, A.; Martin-Loeches, I.; Cecconi, M.; Lisboa, T.; Rello, J. Severity assessment tools in ICU patients with 2009 Influenza A (H1N1) pneumonia. Clin. Microbiol. Infect. 2012, 18, 1040–1048. [Google Scholar] [CrossRef]
- Minne, L.; Abu-Hanna, A.; De Jonge, E. Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit. Care 2009, 12, R161. [Google Scholar] [CrossRef]
- Méndez, R.; Aldás, I.; Menéndez, R. Biomarkers in Community-Acquired Pneumonia (Cardiac and Non-Cardiac). J. Clin. Med. 2020, 9, 549. [Google Scholar] [CrossRef] [PubMed]
- Ingram, P.R.; Inglis, T.; Moxon, D.; Speers, D. Procalcitonin and C-reactive protein in severe 2009 H1N1 influenza infection. Intensive Care Med. 2010, 36, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, G.; Knaak, C.; Vorderwülbecke, G.; La Rosée, P.; Balzer, F.; Schenk, T.; Schuster, F.S.; Nyvlt, P.; Janka, G.; Brunkhorst, F.M.; et al. Hyperferritinemia in Critically Ill Patients. Crit. Care Med. 2020, 48, 459–465. [Google Scholar] [CrossRef]
- Eto, T. A review of the biological properties and clinical implications of adrenomedullin and proadrenomedullin N-terminal 20 peptide (PAMP), hypotensive and vasodilating peptides. Peptides 2001, 22, 1693–1711. [Google Scholar] [CrossRef]
- Valenzuela-Sánchez, F.; Valenzuela-Méndez, B.; Rodríguez-Gutiérrez, J.F.; Estella, A.; González-García, M. New role of biomarkers: Mid-regional pro-adrenomedullin, the biomarker of organ failure. Ann. Transl. Med. 2016, 4, 329. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, S.; Rollas, K.; Alagoz, A.; Seğmen, F.; Sipit, T. Performance evaluation of MR-proadrenomedullin and other scoring systems in severe sepsis with pneumonia. J. Thorac. Dis. 2014, 6, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Saeed, K.; Wilson, D.C.; Bloos, F.; Schuetz, P.; van der Does, Y.; Melander, O.; Hausfater, P.; Legramante, J.M.; Claessens, Y.-E.; Amin, D.; et al. The early identification of disease progression in patients with suspected infection presenting to the emergency department: A multi-centre derivation and validation study. Crit. Care 2019, 23, 255. [Google Scholar] [CrossRef]
- Gonzalez del Castillo, J.; Clemente-Callejo, C.; Llopis, F.; Irimia, A.; Oltra-Hostalet, F.; Rechner, C.; Schwabe, A.; Fernandez-Rodriguez, V.; Sánchez-Mora, C.; Giol-Amich, J.; et al. Midregional proadrenomedullin safely reduces hospitalization in a low severity cohort with infections in the ED: A randomized controlled multi-centre interventional pilot study. Eur. J. Intern. Med. 2021, 88, 104–113. [Google Scholar] [CrossRef]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care 2019, 23, 258. [Google Scholar] [CrossRef]
- Benatti, M.N.; Fabro, A.T.; Miranda, C.H. Endothelial glycocalyx shedding in the acute respiratory distress syndrome after flu syndrome. J. Intensive Care 2020, 8, 72. [Google Scholar] [CrossRef]
- Salgado, D.R.; Ortiz, J.A.; Favory, R.; Creteur, J.; Vincent, J.-L.; De Backer, D. Microcirculatory abnormalities in patients with severe influenza A (H1N1) infection. Can. J. Anesth. 2010, 57, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin. Infect. Dis. 2007, 44, S27–S72. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Rodriguez, A.; Lisboa, T.; Gallego, M.; Lujan, M.; Wunderink, R. PIRO score for community-acquired pneumonia: A new prediction rule for assessment of severity in intensive care unit patients with community-acquired pneumonia. Crit. Care Med. 2009, 37, 456–462. [Google Scholar] [CrossRef]
- Caruhel, P.; Mazier, C.; Kunde, J.; Morgenthaler, N.G.; Darbouret, B. Homogeneous time-resolved fluoroimmunoassay for the measurement of midregionalproadrenomedullin in plasma on the fully automated system B.R.A.H.M.S KRYPTOR®. Clin. Biochem. 2009, 42, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Carrasco, M.; Lagunes, L.; Antón, A.; Gattarello, S.; Laborda, C.; Pumarola, T.; Rello, J. Influenza infection in the intensive care unit: Four years after the 2009 pandemic. Enferm. Infecc. Microbiol. Clín. 2016, 34, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Satterwhite, L.; Mehta, A.; Martin, G.S. Novel findings from the second wave of adult pH1N1 in the United States. Crit. Care Med. 2010, 38, 2059–2061. [Google Scholar] [CrossRef] [PubMed]
- Riera, M.; Payeras-Cifre, A.; A Marcos, M.; Viasus, D.; Farinas, M.C.; Segura, F.; Torre-Cisneros, J.; Martin-Quiros, A.; Rodríguez-Baño, J.; Vila, J.; et al. Clinical presentation and prognosis of the 2009 H1N1 influenza A infection in HIV-1-infected patients: A Spanish multicenter study. AIDS 2010, 24, 2461–2467. [Google Scholar] [CrossRef]
- Valenzuela-Sanchez, F.; Valenzuela-Mendez, B.; Rodriguez-Gutierrez, J.; Bohollo De Austria, R.; Rubio-Quiñones, J.; Puget-Martínez, L.; Valiente Alemán, I.; Angel Estella-García, A. Initial levels of mr-proadrenomedullin: A predictor of severity in patients with influenza a virus pneumonia. Intensive Care Med. Exp. 2015, 3, A832. [Google Scholar] [CrossRef]
- Valenzuela-Sánchez, F.; Valenzuela-Méndez, B.; Rodríguez-Gutiérrez, J.F.; Rello, J. Personalized medicine in severe influenza. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 893–897. [Google Scholar] [CrossRef]
- Andaluz-Ojeda, D.; Nguyen, H.B.; Meunier-Beillard, N.; Cicuéndez, R.; Quenot, J.-P.; Calvo, D.; Dargent, A.; Zarca, E.; Andrés, C.; Nogales, L.; et al. Superior accuracy of mid-regional proadrenomedullin for mortality prediction in sepsis with varying levels of illness severity. Ann. Intensive Care 2017, 7, 15. [Google Scholar] [CrossRef]
- Sega, F.V.D.; Fortini, F.; Spadaro, S.; Ronzoni, L.; Zucchetti, O.; Manfrini, M.; Mikus, E.; Fogagnolo, A.; Torsani, F.; Pavasini, R.; et al. Time course of endothelial dysfunction markers and mortality in COVID-19 patients: A pilot study. Clin. Transl. Med. 2021, 11, e283. [Google Scholar] [CrossRef]
- Nägele, M.P.; Haubner, B.; Tanner, F.C.; Ruschitzka, F.; Flammer, A.J. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis 2020, 314, 58–62. [Google Scholar] [CrossRef]
- Potere, N.; Valeriani, E.; Candeloro, M.; Tana, M.; Porreca, E.; Abbate, A.; Spoto, S.; Rutjes, A.W.S.; Di Nisio, M. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: A systematic review and meta-analysis. Crit. Care 2020, 24, 389. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Van Lier, D.; Kox, M.; Pickkers, P. Promotion of vascular integrity in sepsis through modulation of bioactive adrenomedullin and dipeptidyl peptidase. J. Intern. Med. 2020, 289, 792–806. [Google Scholar] [CrossRef]
- Gregoriano, C.; Koch, D.; Kutz, A.; Haubitz, S.; Conen, A.; Bernasconi, L.; Hammerer-Lercher, A.; Saeed, K.; Mueller, B.; Schuetz, P. The vasoactive peptide MR-pro-adrenomedullin in COVID-19 patients: An observational study. Clin. Chem. Lab. Med. 2021, 59, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- De Guadiana-Romualdo, L.G.; Martínez, M.M.; Mulero, M.D.R.; Esteban-Torrella, P.; Olivo, M.H.; García, M.J.A.; Campos-Rodríguez, V.; Sancho-Rodríguez, N.; Martínez, M.G.; Alcaraz, A.; et al. Circulating MR-proADM levels, as an indicator of endothelial dysfunction, for early risk stratification of mid-term mortality in COVID-19 patients. Int. J. Infect. Dis. 2021, 111, 211–218. [Google Scholar] [CrossRef]
- Sasso, B.L.; Gambino, C.M.; Scichilone, N.; Giglio, R.V.; Bivona, G.; Scazzone, C.; Muratore, R.; Milano, S.; Barbagallo, M.; Agnello, L.; et al. Clinical Utility of MidregionalProadrenomedullin in Patients with COVID-19. Lab. Med. 2021, 52, 493–498. [Google Scholar] [CrossRef]
- Montrucchio, G.; Sales, G.; Rumbolo, F.; Palmesino, F.; Fanelli, V.; Urbino, R.; Filippini, C.; Mengozzi, G.; Brazzi, L. Effectiveness of mid-regional pro-adrenomedullin (MR-proADM) as prognostic marker in COVID-19 critically ill patients: An observational prospective study. PLoS ONE 2021, 16, e0246771. [Google Scholar] [CrossRef] [PubMed]
- Spoto, S.; Agrò, F.E.; Sambuco, F.; Travaglino, F.; Valeriani, E.; Fogolari, M.; Mangiacapra, F.; Costantino, S.; Ciccozzi, M.; Angeletti, S. High value of mid-regional proadrenomedullin in COVID-19: A marker of widespread endothelial damage, disease severity, and mortality. J. Med. Virol. 2020, 93, 2820–2827. [Google Scholar] [CrossRef] [PubMed]
- Roedl, K.; Jarczak, D.; Fischer, M.; Haddad, M.; Boenisch, O.; de Heer, G.; Burdelski, C.; Frings, D.; Sensen, B.; Karakas, M.; et al. MR-proAdrenomedullin as a predictor of renal replacement therapy in a cohort of critically ill patients with COVID-19. Biomarkers 2021, 26, 417–424. [Google Scholar] [CrossRef]
- Lippi, G.; Henry, B.M. Pooled analysis of mid-regional pro-adrenomedullin values in COVID-19 patients with critical illness. Intern. Emerg. Med. 2021, 16, 1723–1725. [Google Scholar] [CrossRef]
- Saeed, K.; Legramante, J.M.; Angeletti, S.; Curcio, F.; Miguens, I.; Poole, S.; Tascini, C.; Sozio, E.; Del Castillo, J.G. Mid-regional pro-adrenomedullin as a supplementary tool to clinical parameters in cases of suspicion of infection in the emergency department. Expert Rev. Mol. Diagn. 2021, 21, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Zaninotto, M.; Mion, M.M.; Marchioro, L.; Padoan, A.; Plebani, M. Endothelial dysfunction and Mid-Regional proAdrenomedullin: What role in SARS-CoV-2 infected Patients? Clin. Chim. Acta 2021, 523, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, D.; Badawi, A. C-reactive protein as a biomarker of severe H1N1 influenza. Inflamm. Res. 2018, 68, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Cuquemelle, E.; Soulis, F.; Villers, D.; Roche-Campo, F.; Somohano, C.A.; Fartoukh, M.; Kouatchet, A.; Mourvillier, B.; Dellamonica, J.; Picard, W.; et al. Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicentre study. Intensive Care Med. 2011, 37, 796–800. [Google Scholar] [CrossRef]
- Pfister, R.; Kochanek, M.; Leygeber, T.; Brun-Buisson, C.; Cuquemelle, E.; Machado, M.B.P.; Piacentini, E.; E Hammond, N.; Ingram, P.R.; Michels, G. Procalcitonin for diagnosis of bacterial pneumonia in critically ill patients during 2009 H1N1 influenza pandemic: A prospective cohort study, systematic review and individual patient data meta-analysis. Crit. Care 2014, 18, R44. [Google Scholar] [CrossRef]
- Bobbio-Pallavicini, F.; Verde, G.; Spriano, P.; Losi, R.; Bosatra, M.G.; Braschi, A.; Iotti, G.A.; Chiaranda, M.; Villa, S. Body iron status in critically ill patients: Significance of serum ferritin. Intensive Care Med. 1989, 15, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.C.R.; Longhi, F.; Branco, R.G.; Piva, J.P.; Lacks, D.; Tasker, R. Ferritin levels in children with severe sepsis and septic shock. Acta Paediatr. 2007, 96, 1829–1831. [Google Scholar] [CrossRef]



| Variable | H1N1vIPN n = 75 | H1N1vIPN Survivors; n = 53; (70.67%) | H1N1vIPN Non-Survivors; n = 22; (29.33%) |
|---|---|---|---|
| Age median (IQR) | 53 (44–64) | 52 (42.7–64) | 55 (47–70) |
| Men (%) | 49.3% | 49.1% | 50% |
| Women (%) | 50.7% | 50.9% | 50% * |
| BMI median (IQR) | 27 (23–32) | 27 (24–33.3) | 27 (23–30.3) |
| Obesity (BMI > 30) (%) | 31% | 33.3% | 26.3% |
| Smoker/COPD (%) | 32.4% | 34.6% | 27.3% |
| Diabetes Mellitus (%) | 21.3% | 20.8% | 22.7% |
| Immunosuppression (%) | 28% | 30.2% | 22.7% |
| Hematologic malignancy(%) | 6.6% | 5.6 % | 9% |
| Apache II median (IQR) | 17 (12–23) | 15 (12–21.7) | 21 (18–25) * |
| SAPS II median (IQR) | 45 (27–56) | 37 (27–51) | 53.5 (45–60) * |
| SOFA median (IQR) | 8 (8.5–10.7) | 6 (4–9) | 11 (8–12) * |
| Lactic acid (mmol/L) median (IQR) | 1.43 (0.98–1.8) | 6 (4–9) | 1.6 (1–2.5) |
| Stay in ICU median (IQR) | 13 (7–23) | 11 (7–23) | 14.5 (7–26) |
| Mechanical ventilation (%) | 85.3% | 75% | 100% * |
| days in MV median (IQR) | 7.5 (4–17) | 7 (3–16.2) | 12 (5.7–22.5) * |
| PaO2/FiO2 median (IQR) | 90 (60–146) | 107 (72–178) | 55 (46–90) * |
| Prone position (%) | 60.3% | 48.7% | 84.2% * |
| Recruitment maneuvers (%) | 27.6% | 20.5% | 42.1% |
| Septic shock (%) | 56% | 45.3% | 81.8% * |
| Corticosteroids (%) | 33.3% | 45.3% | 31.8% |
| Diuretics (%) | 34.5% | 33.3% | 36.8% |
| Continuous renal replacement therapy (CRRT) (%) | 10.7% | 5.7% | 22.7% * |
| Coinfection and Superinfection in Patients with Influenza A H1N1 Admitted to the ICU | |||
|---|---|---|---|
| COINFECTION | SUPERINFECTION | ||
| MICROORGANISMS | n. isolated cases | MICROORGANISMS | n. isolated cases |
| Streptococcus pneumoniae | 6 | Krebsiella pneumoniae | 4 |
| Staphylococcus epidermidis | 2 | Staphylococcus epidermidis | 3 |
| Krebsiella pneumoniae | 2 | Enterococcus faecium | 3 |
| Candida albicans | 2 | Candida albicans | 2 |
| Staphylococcus aureus | 1 | Acinetobacter baumanii | 2 |
| Pseudomonas aeruginosa | 2 | ||
| Serratia marcescens | 1 | ||
| Total | 13 | Total | 17 |
| Gram+ | 9 | Gram+ | 6 |
| Gram− | 2 | Gram− | 9 |
| Yeast | 2 | Yeast | 2 |
| AUC | Sensitivity | Specificity | PPV | NPV | Criterion | |
|---|---|---|---|---|---|---|
| MR pro ADM | 0.832 | 55% | 90% | 91.5% | 50.7% | >1.1 |
| Ferritin | 0.743 | 65.5% | 84.2% | 85.4% | 62.7% | >325.2 |
| PCT | 0.775 | 80% | 71.05% | 84.5% | 64.3% | >0.20 |
| CRP | 0.645 | 66.7% | 68.42% | 80.6% | 51% | >12 |
| AUC | Sensitivity | Specificity | PPV | NPV | Criterion | |
|---|---|---|---|---|---|---|
| MR-proADM | 0.853 | 95.45% | 75.82% | 48.8% | 98.6% | >1.2 |
| Ferritin | 0.799 | 78.95% | 77.92% | 46.9% | 93.7% | >627.2 |
| PCT | 0.786 | 95.45% | 60.44% | 36.8% | 98.2% | >0.275 |
| CRP | 0.645 | 86.36% | 52.75% | 30.6% | 94.1% | >12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela-Méndez, B.; Valenzuela-Sánchez, F.; Rodríguez-Gutiérrez, J.F.; Bohollo-de-Austria, R.; Estella, Á.; Martínez-García, P.; González-García, M.Á.; Rello, J. Plasma Levels of Mid-Regional Proadrenomedullin Accurately Identify H1N1pdm09 Influenza Virus Patients with Risk of Intensive Care Admission and Mortality in the Emergency Department. J. Pers. Med. 2022, 12, 84. https://doi.org/10.3390/jpm12010084
Valenzuela-Méndez B, Valenzuela-Sánchez F, Rodríguez-Gutiérrez JF, Bohollo-de-Austria R, Estella Á, Martínez-García P, González-García MÁ, Rello J. Plasma Levels of Mid-Regional Proadrenomedullin Accurately Identify H1N1pdm09 Influenza Virus Patients with Risk of Intensive Care Admission and Mortality in the Emergency Department. Journal of Personalized Medicine. 2022; 12(1):84. https://doi.org/10.3390/jpm12010084
Chicago/Turabian StyleValenzuela-Méndez, Blanca, Francisco Valenzuela-Sánchez, Juan Francisco Rodríguez-Gutiérrez, Rafael Bohollo-de-Austria, Ángel Estella, Pilar Martínez-García, María Ángela González-García, and Jordi Rello. 2022. "Plasma Levels of Mid-Regional Proadrenomedullin Accurately Identify H1N1pdm09 Influenza Virus Patients with Risk of Intensive Care Admission and Mortality in the Emergency Department" Journal of Personalized Medicine 12, no. 1: 84. https://doi.org/10.3390/jpm12010084
APA StyleValenzuela-Méndez, B., Valenzuela-Sánchez, F., Rodríguez-Gutiérrez, J. F., Bohollo-de-Austria, R., Estella, Á., Martínez-García, P., González-García, M. Á., & Rello, J. (2022). Plasma Levels of Mid-Regional Proadrenomedullin Accurately Identify H1N1pdm09 Influenza Virus Patients with Risk of Intensive Care Admission and Mortality in the Emergency Department. Journal of Personalized Medicine, 12(1), 84. https://doi.org/10.3390/jpm12010084






