Incidence and Predictors of Infections and All-Cause Death in Patients with Cardiac Implantable Electronic Devices: The Italian Nationwide RI-AIAC Registry
Abstract
:1. Introduction
2. Methods
2.1. Outcomes
2.2. Statistical Analysis
2.3. External Validation
3. Results
3.1. Follow-Up and Incidence of Adverse Outcomes
3.2. Clinical Factors Associated to Outcomes Occurrence
3.3. Association and Performance of Clinical Risk Scores
3.4. Predictive Ability of Clinical Scores
3.5. External Validation Analysis
4. Discussion
Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2015, 36, 2793–2867. Available online: https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehv316 (accessed on 26 August 2021).
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. Available online: http://www.ncbi.nlm.nih.gov/pubmed/34455430 (accessed on 7 November 2021). [CrossRef]
- Raatikainen, M.J.P.; Arnar, D.O.; Merkely, B.; Nielsen, J.C.; Hindricks, G.; Heidbuchel, H.; Camm, J. A Decade of Information on the Use of Cardiac Implantable Electronic Devices and Interventional Electrophysiological Procedures in the European Society of Cardiology Countries: 2017 Report from the European Heart Rhythm Association. Europace 2017, 19, ii1–ii90. Available online: http://academic.oup.com/europace/article/19/suppl_2/ii1/4100657/A-Decade-of-Information-on-the-Use-of-Cardiac (accessed on 26 August 2021). [CrossRef] [PubMed]
- Zecchin, M.; Torre, M.; Carrani, E.; Sampaolo, L.; Ciminello, E.; Ortis, B.; Ricci, R.; Proclemer, A.; Sinagra, G. Seventeen-year trend (2001–2017) in pacemaker and implantable cardioverter-defibrillator utilization based on hospital discharge database data: An analysis by age groups. Eur. J. Intern. Med. 2021, 84, 38–45. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32933841 (accessed on 7 November 2021). [CrossRef]
- Birnie, D.H.; Wang, J.; Alings, M.; Philippon, F.; Parkash, R.; Manlucu, J.; Angaran, P.; Rinne, P.; Coutu, B.; Low, R.A.; et al. Risk Factors for Infections Involving Cardiac Implanted Electronic Devices. J. Am. Coll. Cardiol. 2019, 74, 2845–2854. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0735109719379331 (accessed on 7 November 2021). [CrossRef] [PubMed]
- Diemberger, I.; Lorenzetti, S.; Vitolo, M.; Boriani, G. Infective endocarditis in patients with cardiac implantable electronic devices: Impact of comorbidities on outcome. Eur. J. Intern. Med. 2019, 66, e9–e10. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31280907 (accessed on 7 November 2021). [CrossRef]
- Diemberger, I.; Bonfiglioli, R.; Martignani, C.; Graziosi, M.; Biffi, M.; Lorenzetti, S.; Ziacchi, M.; Nanni, C.; Fanti, S.; Boriani, G. Contribution of PET imaging to mortality risk stratification in candidates to lead extraction for pacemaker or defibrillator infection: A prospective single center study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 194–205. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30196365 (accessed on 7 November 2021). [CrossRef]
- Diemberger, I.; Biffi, M.; Martignani, C.; Boriani, G. From lead management to implanted patient management: Indications to lead extraction in pacemaker and cardioverter-defibrillator systems. Expert Rev. Med. Devices 2011, 8, 235–255. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21381913 (accessed on 7 November 2021). [CrossRef]
- Sgreccia, D.; Vitolo, M.; Valenti, A.C.; Manicardi, M.; Boriani, G. Burden of disease and costs of infections associated with cardiac implantable electronic devices. Expert Rev. Pharm. Outcomes Res. 2021, 1–10. Available online: http://www.ncbi.nlm.nih.gov/pubmed/34517745 (accessed on 8 November 2021). [CrossRef]
- Polyzos, K.A.; Konstantelias, A.A.; Falagas, M.E. Risk factors for cardiac implantable electronic device infection: A systematic review and meta-analysis. Europace 2015, 17, 767–777. Available online: https://academic.oup.com/europace/article-lookup/doi/10.1093/europace/euv053 (accessed on 8 November 2021). [CrossRef]
- Han, H.-C.; Hawkins, N.M.; Pearman, C.M.; Birnie, D.H.; Krahn, A.D. Epidemiology of cardiac implantable electronic device infections: Incidence and risk factors. Europace 2021, 23, iv3–iv10. Available online: http://www.ncbi.nlm.nih.gov/pubmed/34051086 (accessed on 8 November 2021). [CrossRef]
- Traykov, V.; Bongiorni, M.G.; Boriani, G.; Burri, H.; Costa, R.; Dagres, N.; Deharo, J.C.; Epstein, L.M.; Erba, P.A.; Snygg-Martin, U.; et al. Clinical practice and implementation of guidelines for the prevention, diagnosis and management of cardiac implantable electronic device infections: Results of a worldwide survey under the auspices of the European Heart Rhythm Association. Europace 2019, 21, 1270–1279. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31209483 (accessed on 8 November 2021). [CrossRef]
- Diemberger, I.; Massaro, G.; Rossillo, A.; Chieffo, E.; Dugo, D.; Guarracini, F.; Pellegrino, P.L.; Perna, F.; Landolina, M.; De Ponti, R.; et al. Temporary transvenous cardiac pacing: A survey on current practice. J. Cardiovasc. Med. 2020, 21, 420–427. Available online: http://journals.lww.com/10.2459/JCM.0000000000000959 (accessed on 8 November 2021). [CrossRef]
- Rubini, G.; Ferrari, C.; Carretta, D.; Santacroce, L.; Ruta, R.; Iuele, F.; Lavelli, V.; Merenda, N.; D’Agostino, C.; Sardaro, A.; et al. Usefulness of 18F-FDG PET/CT in patients with cardiac implantable electronic device suspected of late infection. J. Clin. Med. 2020, 9, 2246. Available online: https://www.mdpi.com/2077-0383/9/7/2246 (accessed on 8 November 2021). [CrossRef] [PubMed]
- Galea, N.; Bandera, F.; Lauri, C.; Autore, C.; Laghi, A.; Erba, P.A. Multimodality imaging in the diagnostic work-up of endocarditis and cardiac implantable electronic device (CIED) infection. J. Clin. Med. 2020, 9, 2237. Available online: https://www.mdpi.com/2077-0383/9/7/2237 (accessed on 8 November 2021). [CrossRef]
- Malagù, M.; Vitali, F.; Brieda, A.; Cimaglia, P.; De Raffaele, M.; Tazzari, E.; Musolino, C.; Balla, C.; Serenelli, M.; Cultrera, R.; et al. Antibiotic prophylaxis based on individual infective risk stratification in cardiac implantable electronic device: The PRACTICE study. Europace 2021. Available online: https://academic.oup.com/europace/advance-article/doi/10.1093/europace/euab222/6364843 (accessed on 8 November 2021). [CrossRef]
- Balla, C.; Brieda, A.; Righetto, A.; Vitali, F.; Malagù, M.; Cultrera, R.; Bertini, M. Predictors of infection after “de novo” cardiac electronic device implantation. Eur. J. Intern. Med. 2020, 77, 73–78. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0953620520300844 (accessed on 8 November 2021). [CrossRef] [PubMed]
- Bahamondez-Canas, T.F.; Heersema, L.A.; Smyth, H.D.C. Current status of in vitro models and assays for susceptibility testing for wound biofilm infections. Biomedicines 2019, 7, 34. Available online: https://www.mdpi.com/2227-9059/7/2/34 (accessed on 8 November 2021). [CrossRef] [Green Version]
- Shariff, N.; Eby, E.; Adelstein, E.; Jan, S.; Shalaby, A.; Saba, S.; Wang, N.C.; Schwartzman, D. Health and Economic Outcomes Associated with Use of an Antimicrobial Envelope as a Standard of Care for Cardiac Implantable Electronic Device Implantation. J. Cardiovasc. Electrophysiol. 2015, 26, 783–789. Available online: http://doi.wiley.com/10.1111/jce.12684 (accessed on 8 November 2021). [CrossRef]
- Kolek, M.J.; Dresen, W.F.; Wells, Q.S.; Ellis, C.R. Use of an antibacterial envelope is associated with reduced cardiac implantable electronic device infections in high-risk patients. PACE-Pacing Clin. Electrophysiol. 2013, 36, 354–361. Available online: http://doi.wiley.com/10.1111/pace.12063 (accessed on 9 November 2021). [CrossRef]
- Kolek, M.J.; Patel, N.J.; Clair, W.K.; Whalen, S.P.; Rottman, J.N.; Kanagasundram, A.; Shen, S.T.; Saavedra, P.J.; Estrada, J.C.; Abraham, R.L.; et al. Efficacy of a Bio-Absorbable Antibacterial Envelope to Prevent Cardiac Implantable Electronic Device Infections in High-Risk Subjects. J. Cardiovasc. Electrophysiol. 2015, 26, 1111–1116. Available online: http://doi.wiley.com/10.1111/jce.12768 (accessed on 9 November 2021). [CrossRef] [Green Version]
- Tarakji, K.G.; Mittal, S.; Kennergren, C.; Corey, R.; Poole, J.E.; Schloss, E.; Gallastegui, J.; Pickett, R.A.; Evonich, R.; Philippon, F.; et al. Antibacterial Envelope to Prevent Cardiac Implantable Device Infection. N. Engl. J. Med. 2019, 380, 1895–1905. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30883056 (accessed on 9 November 2021). [CrossRef]
- Henrikson, C.A.; Sohail, M.R.; Acosta, H.; Johnson, E.E.; Rosenthal, L.; Pachulski, R.; Dan, D.; Paladino, W.; Khairallah, F.S.; Gleed, K.; et al. Antibacterial Envelope Is Associated With Low Infection Rates After Implantable Cardioverter-Defibrillator and Cardiac Resynchronization Therapy Device Replacement: Results of the Citadel and Centurion Studies. JACC Clin. Electrophysiol. 2017, 3, 1158–1167. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29759500 (accessed on 9 November 2021). [CrossRef] [PubMed]
- Essebag, V.; Verma, A.; Healey, J.S.; Krahn, A.D.; Kalfon, E.; Coutu, B.; Ayala-Paredes, F.; Tang, A.S.; Sapp, J.; Sturmer, M.; et al. Clinically significant pocket hematoma increases long-term risk of device infection: BRUISE CONTROL INFECTION study. J. Am. Coll. Cardiol. 2016, 67, 1300–1308. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26988951 (accessed on 10 November 2021). [CrossRef]
- Boriani, G.; Vitolo, M.; Wright, D.J.; Biffi, M.; Brown, B.; Tarakji, K.G.; Wilkoff, B.L. Infections associated with cardiac electronic implantable devices: Economic perspectives and impact of the TYRX™ antibacterial envelope. Europace 2021, 23 (Suppl. 4), iv33–iv44. Available online: https://pubmed.ncbi.nlm.nih.gov/34160600/ (accessed on 9 November 2021). [CrossRef]
- Daneman, N.; Homenauth, E.; Saskin, R.; Ng, R.; Ha, A.; Wijeysundera, H.C. The predictors and economic burden of early-, mid- and late-onset cardiac implantable electronic device infections: A retrospective cohort study in Ontario, Canada. Clin. Microbiol. Infect. 2020, 26, 255.e1–255.e6. [Google Scholar] [CrossRef]
- Sohail, M.R.; Corey, G.R.; Wilkoff, B.L.; Poole, J.E.; Mittal, S.; Kennergren, C.; Greenspon, A.J.; Cheng, A.; Lande, J.D.; Lexcen, D.R.; et al. Clinical Presentation, Timing, and Microbiology of CIED Infections: An Analysis of the WRAP-IT Trial. JACC Clin. Electrophysiol. 2021, 7, 50–61. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33478712 (accessed on 10 November 2021). [CrossRef]
- Greenspon, A.J.; Eby, E.L.; Petrilla, A.A.; Sohail, M.R. Treatment patterns, costs, and mortality among Medicare beneficiaries with CIED infection. PACE-Pacing Clin. Electrophysiol. 2018, 41, 495–503. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29411401 (accessed on 10 November 2021). [CrossRef] [PubMed] [Green Version]
- Tarakji, K.G.; Wazni, O.M.; Harb, S.; Hsu, A.; Saliba, W.; Wilkoff, B.L. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: The impact of the infection type and the presence of vegetation on survival. Europace 2014, 16, 1490–1495. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25087154 (accessed on 11 November 2021). [CrossRef]
- Brunner, M.; Olschewski, M.; Geibeli, A.; Bode, C.; Zehender, M. Long-term survival after pacemaker implantation: Prognostic importance of gender and baseline patient characteristics. Eur. Heart J. 2004, 25, 88–95. Available online: http://www.ncbi.nlm.nih.gov/pubmed/14683747 (accessed on 11 November 2021). [CrossRef] [PubMed] [Green Version]
- Boriani, G.; Berti, E.; Belotti, L.M.B.; Biffi, M.; De Palma, R.; Malavasi, V.L.; Bottoni, N.; Rossi, L.; De Maria, E.; Mantovan, R.; et al. Cardiac device therapy in patients with left ventricular dysfunction and heart failure: ‘real-world’ data on long-term outcomes (mortality, hospitalizations, days alive and out of hospital). Eur. J. Heart Fail. 2016, 18, 693–702. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27060289 (accessed on 11 November 2021). [CrossRef] [PubMed] [Green Version]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Beswick, A.D. Systematic review of risk prediction scores for surgical site infection or periprosthetic joint infection following joint arthroplasty. Epidemiol. Infect. 2017, 145, 1738–1749. Available online: https://www.cambridge.org/core/product/identifier/S0950268817000486/type/journal_article (accessed on 12 November 2021). [CrossRef] [PubMed]
- Proietti, M.; Mujovic, N.; Potpara, T.S. Optimizing Stroke and Bleeding Risk Assessment in Patients with Atrial Fibrillation: A Balance of Evidence, Practicality and Precision. Thromb. Haemost. 2018, 118, 2014–2017. Available online: http://www.thieme-connect.de/DOI/DOI?10.1055/s-0038-1676074 (accessed on 7 December 2018). [CrossRef] [Green Version]
- Mull, H.J.; Stolzmann, K.L.; Shin, M.H.; Kalver, E.; Schweizer, M.L.; Branch-Elliman, W. Novel Method to Flag Cardiac Implantable Device Infections by Integrating Text Mining With Structured Data in the Veterans Health Administration’s Electronic Medical Record. JAMA Netw Open. 2020, 3, e2012264. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32955571 (accessed on 12 November 2021). [CrossRef]
- Palmisano, P.; Ziacchi, M.; Belotti, G.; Rapacciuolo, A.; Santini, L.; Stabile, G.; Zoni Berisso, M.; De Ponti, R.; Landolina, M.; Ricci, R.P.; et al. Clinical and organizational management of cardiac implantable electronic device replacements: An Italian Survey promoted by AIAC (Italian Association of Arrhythmology and Cardiac Pacing). J. Cardiovasc. Med. 2019, 20, 531–541. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31259858 (accessed on 12 November 2021). [CrossRef]
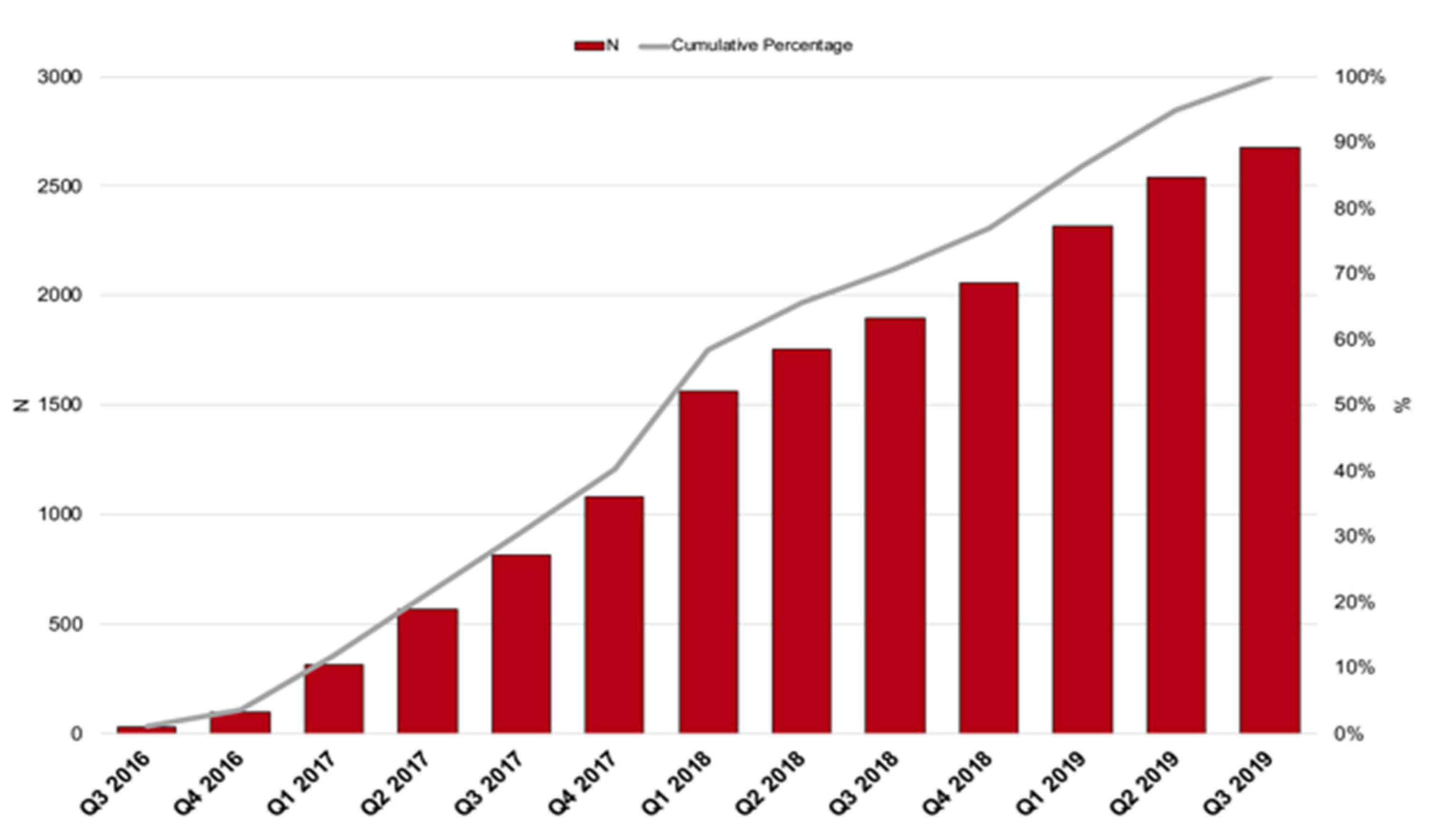

| N = 2675 | |
|---|---|
| Age, years median (IQR) | 78 (70–84) |
| Age Classes, n (%) <65 years 65–74 years ≥75 years | 422 (15.8) 630 (23.6) 1623 (60.7) |
| Male Sex, n (%) | 1720 (64.3) |
| Admission, n (%) Ward Daily Service | 2263 (84.6) 412 (15.4) |
| Procedure, n (%) First Implantation Replacement Further Replacement Contralateral Implantation Upgrading Revision Other | 1874 (70.1) 448 (16.7) 205 (7.7) 17 (0.6) 72 (2.7) 51 (1.9) 8 (0.3) |
| CIED Type, n (%) Pacemaker ICD CRT-P CRT-D Other | 1785 (66.7) 450 (16.8) 106 (4.0) 329 (12.3) 5 (0.2) |
| >2 Leads, n (%) | 194 (7.3) |
| Early Revision, n (%) | 38 (1.4) |
| Prolonged Temporary Pacing, n (%) | 73 (2.7) |
| eGFR <60 mL/min, n (%) | 622 (23.3) |
| Pre-Dialysis/Dialysis, n (%) | 41 (1.5) |
| Diabetes Mellitus, n (%) | 550 (20.6) |
| Heart Failure, n (%) | 740 (27.7) |
| Use of Oral Corticosteroids, n (%) | 72 (2.7) |
| Use of Oral Anticoagulants, n (%) | 821 (30.7) |
| Use of Immunosuppressive Therapy, n (%) | 18 (0.7) |
| 24 h Pre-Implantation Fever, n (%) | 12 (0.4) |
| Hospital-Acquired Infection, n (%) | 44 (1.6) |
| Antibiotic Prophylaxis, n (%) | 2636 (98.5) |
| Antibiotic Type, n (%) 2636 Cephalosporins Clindamycin Penicillin Other | 2186 (82.9) 70 (2.6) 250 (9.3) 130 (4.9) |
| Overall Infection Risk Factors, n median [IQR] | 2 (1,2) |
| PADIT Score, median [IQR] | 1 (0–4) |
| Kolek Score, median [IQR] | 1 (0–2) |
| Shariff Score, median [IQR] | 1 (0–2) |
| No Infection N= 2495 | Infection N= 28 | p | |
|---|---|---|---|
| Age, years median (IQR) | 77 (69–83) | 74 (66–77) | 0.041 |
| Age Classes, n (%) <65 years 65–74 years ≥75 years | 411 (16.5) 597 (23.9) 1487 (59.6) | 5 (17.9) 9 (32.1) 14 (50) | 0.538 |
| Male Sex, n (%) | 1601 (64.2) | 19 (67.9) | 0.686 |
| Admission, n (%) Ward Daily Service | 2112 (84.6) 383 (15.4) | 23 (82.1) 5 (17.9) | 0.715 |
| Procedure, n (%) First Implantation Replacement Further Replacement Contralateral Implantation Upgrading Revision Other | 1745 (69.9) 428 (17.2) 186 (7.5) 14 (0.6) 67 (2.7) 48 (1.9) 7 (0.3) | 15 (53.6) 2 (7.1) 4 (14.3) 3 (10.7) 2 (7.1) 1 (3.6) 1 (3.6) | <0.001 |
| CIED Type, n (%) Pacemaker ICD CRT-P CRT-D Other | 1654 (66.3) 426 (17.1) 98 (3.9) 312 (12.5) 5 (0.2) | 13 (46.5) 8 (28.6) 3 (10.7) 4 (14.3) 0 | 0.131 |
| CIED Type Recode, n (%) Pacemaker Any Other CIED | 1654 (66.3) 841 (33.7) | 13 (46.4) 15 (53.6) | 0.027 |
| >2 Leads, n (%) | 184 (7.4) | 3 (10.7) | 0.502 |
| Early Revision, n (%) | 34 (1.4) | 2 (7.1) | 0.010 |
| Prolonged Temporary Pacing, n (%) | 62 (2.5) | 1 (3.6) | 0.714 |
| eGFR <60 mL/min, n (%) | 553 (22.2) | 6 (21.4) | 0.926 |
| Pre-Dialysis/Dialysis, n (%) | 32 (1.3) | 2 (7.1) | 0.007 |
| Diabetes Mellitus, n (%) | 499 (20.0) | 11 (39.3) | 0.012 |
| Heart Failure, n (%) | 689 (27.6) | 11 (39.3) | 0.170 |
| Use of Oral Corticosteroids, n (%) | 62 (2.5) | 1 (3.6) | 0.714 |
| Use of Oral Anticoagulants, n (%) | 759 (30.4) | 9 (32.1) | 0.844 |
| Use of Immunosuppressive Therapy, n (%) | 17 (0.7) | 0 | 1.000 |
| 24 h Pre-Implantation Fever, n (%) | 10 (0.4) | 0 | 1.000 |
| Hospital-Acquired Infection, n (%) | 37 (1.5) | 2 (7.1) | 0.016 |
| Antibiotic Prophylaxis, n (%) | 2455 (98.4) | 28 (100) | 0.499 |
| Antibiotic Type, n (%) 2636 Cephalosporins Clindamycin Penicillin Other | 2055 (83.7) 61 (2.5) 224 (9.1) 116 (4.7) | 19 (67.9) 2 (7.1) 5 (17.9) 2 (7.1) | 0.120 |
| Overall Infection Risk Factors, n median [IQR] | 2 (1–2) | 2 (1–3) | 0.086 |
| PADIT Score, median [IQR] | 1 (0–4) | 4 (1–5) | 0.008 |
| Kolek Score, median [IQR] | 1 (0–2) | 1 (0–2) | 0.136 |
| Shariff Score, median [IQR] | 1 (0–2) | 2 (1–3) | 0.080 |
| Univariate Analysis | Multivariate Analysis | Score Point | |||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Age Classes <65 years (ref.) 65–74 years ≥75 years | - 1.24 (0.41–3.72) 0.77 (0.28–2.16) | - 0.702 0.625 | - - - | - - - | - - - |
| Procedure First Implantation Any Replacement Revision/Upgrading/Reimplantation | - 1.14 (0.44–2.94) 5.99 (2.4–14.94) | - 0.792 <0.001 | - 1.21 (0.46–3.18) 4.08 (1.38–12.08) | - 0.698 0.011 | 0 1 2 |
| CIED Pacemaker (ref.) Any Other CIED | - 2.27 (1.08–4.79) | - 0.032 | - 1.66 (0.75–3.68) | - 0.209 | - - |
| Early Revision | 5.57 (1.27–24.40) | 0.023 | 1.68 (0.30–8.87) | 0.579 | - |
| Pre-Dialysis/Dialysis | 5.92 (1.35–26) | 0.019 | 3.44 (0.74–15.94) | 0.116 | - |
| Diabetes Mellitus | 2.59 (1.21–5.56) | 0.015 | 2.22 (1.02–4.84) | 0.045 | 1 |
| Hospital-Acquired Infection | 5.11 (1.17–22.32) | 0.030 | 3.96 (0.85–18.57) | 0.080 | 1 |
| Univariate Analysis | Multivariate Analysis | Score Points | |||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Age Classes <65 years (ref.) 65-74 years ≥75 years | - 2.32 (1.05–5.17) 4.04 (1.96–8.34) | - 0.039 <0.001 | - 2.07 (0.92–4.65) 3.10 (1.45–6.64) | - 0.079 0.006 | 0 1 2 |
| CIED Pacemaker (ref) Any Other CIED | - 0.7 (0.49–1.02) | - 0.062 | - 0.95 (0.64–1.41) | - 0.798 | |
| Prolonged Temporary Pacing | 3.06 (1.58–5.94) | 0.001 | 2.9 (1.45–5.77) | 0.002 | 1 |
| eGFR <60 mL/min | 2.55 (1.83–3.57) | <0.001 | 2.03 (1.43–2.88) | <0.001 | 1 |
| Pre-Dialysis/Dialysis | 1.62 (1.25–2.11) | <0.001 | |||
| Diabetes Mellitus | 1.58 (1.09–2.28) | 0.015 | 1.43 (0.98–2.08) | 0.063 | 1 |
| Use of Oral Corticosteroids | 2.47 (1.2–5.08) | 0.014 | 2.60 (1.25–5.40) | 0.010 | 1 |
| Hospital-Acquired Infection | 3.21 (1.41–7.32) | 0.006 | 3.29 (1.29–8.35) | 0.012 | 1 |
| Infection | Clinical Events | |||
|---|---|---|---|---|
| C-Index (95% CI) | p | C-Index (95% CI) | p | |
| PADIT Score | 0.64 (0.53–0.76) | 0.010 | 0.51 (0.47–0.56) | 0.612 |
| Kolek Score | 0.56 (0.46–0.66) | 0.261 | 0.63 (0.59–0.68) | <0.001 |
| Shariff Score | 0.58 (0.47–0.68) | 0.159 | 0.62 (0.58–0.67) | <0.001 |
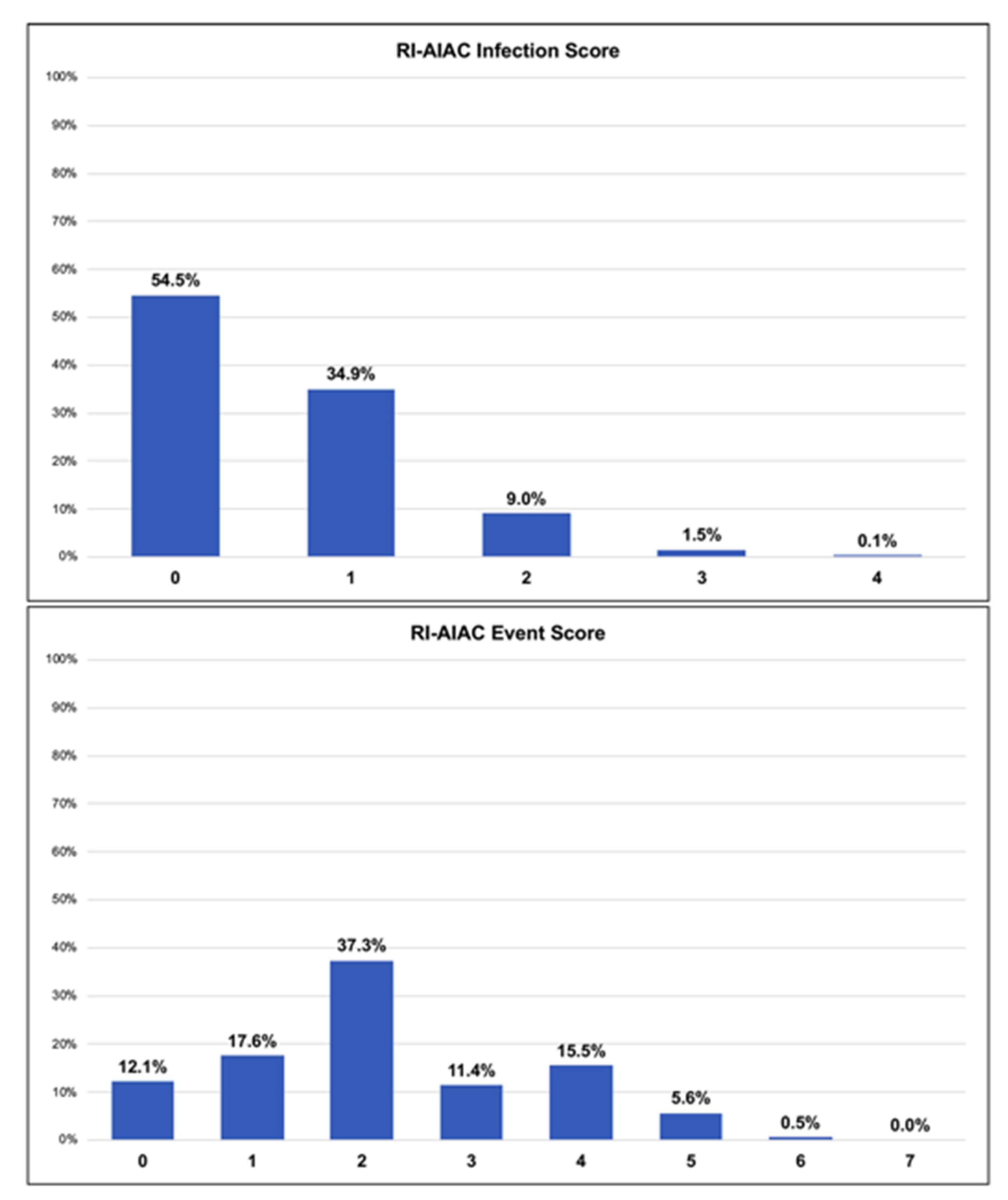
| RI-AIAC Infection | 0.64 (0.52–0.75) | 0.015 | - | - |
| RI-AIAC Event * | - | - | 0.67 (0.63–0.71) | <0.001 |
| Infection | Clinical Events | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| PADIT Score * | 1.28 (1.1–1.50) | 0.002 | 1.01 (0.94–1.09) | 0.748 |
| Kolek Score † | 1.55 (0.73–3.27) | 0.251 | 1.36 (1.2–1.55) | <0.001 |
| Shariff Score † | 1.69 (0.89–3.21) | 0.112 | 1.32 (1.17–1.47) | <0.001 |
| RI-AIAC Infection † | 2.38 (1.6–3.55) | <0.001 | - | - |
| RI-AIAC Event * | - | - | 1.56 (1.38-1.75) | <0.001 |
| Infection | Clinical Events | SE | SP | |||
|---|---|---|---|---|---|---|
| C-Index (95% CI) | p | C-Index (95% CI) | p | |||
| PADIT Score | 0.53 (0.38–0.67) | 0.746 | 0.49 (0.44–0.53) | 0.600 | - | - |
| Kolek Score | 0.64 (0.5–0.79) | 0.065 | 0.65 (0.6–0.7) * | <0.001 | 70.3% | 51.2% |
| Shariff Score | 0.62 (0.46–0.77) | 0.131 | 0.56 (0.51–0.62) † | 0.025 | - | - |
| RI-AIAC Infection | 0.58 (0.42–0.74) | 0.292 | - | - | - | - |
| RI-AIAC Event | - | - | 0.68 (0.63–0.72) | <0.001 | 95.9% | 16.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boriani, G.; Proietti, M.; Bertini, M.; Diemberger, I.; Palmisano, P.; Baccarini, S.; Biscione, F.; Bottoni, N.; Ciccaglioni, A.; Dal Monte, A.; et al. Incidence and Predictors of Infections and All-Cause Death in Patients with Cardiac Implantable Electronic Devices: The Italian Nationwide RI-AIAC Registry. J. Pers. Med. 2022, 12, 91. https://doi.org/10.3390/jpm12010091
Boriani G, Proietti M, Bertini M, Diemberger I, Palmisano P, Baccarini S, Biscione F, Bottoni N, Ciccaglioni A, Dal Monte A, et al. Incidence and Predictors of Infections and All-Cause Death in Patients with Cardiac Implantable Electronic Devices: The Italian Nationwide RI-AIAC Registry. Journal of Personalized Medicine. 2022; 12(1):91. https://doi.org/10.3390/jpm12010091
Chicago/Turabian StyleBoriani, Giuseppe, Marco Proietti, Matteo Bertini, Igor Diemberger, Pietro Palmisano, Stefano Baccarini, Francesco Biscione, Nicola Bottoni, Antonio Ciccaglioni, Alessandro Dal Monte, and et al. 2022. "Incidence and Predictors of Infections and All-Cause Death in Patients with Cardiac Implantable Electronic Devices: The Italian Nationwide RI-AIAC Registry" Journal of Personalized Medicine 12, no. 1: 91. https://doi.org/10.3390/jpm12010091
APA StyleBoriani, G., Proietti, M., Bertini, M., Diemberger, I., Palmisano, P., Baccarini, S., Biscione, F., Bottoni, N., Ciccaglioni, A., Dal Monte, A., Ferrari, F. A., Iacopino, S., Piacenti, M., Porcelli, D., Sangiorgio, S., Santini, L., Malagù, M., Stabile, G., Imberti, J. F., ... on behalf of RI-AIAC Registry Investigators. (2022). Incidence and Predictors of Infections and All-Cause Death in Patients with Cardiac Implantable Electronic Devices: The Italian Nationwide RI-AIAC Registry. Journal of Personalized Medicine, 12(1), 91. https://doi.org/10.3390/jpm12010091














