What Lies behind Paraneoplastic Hypercalcemia Secondary to Well-Differentiated Neuroendocrine Neoplasms? A Systematic Review of the Literature
Abstract
1. Introduction
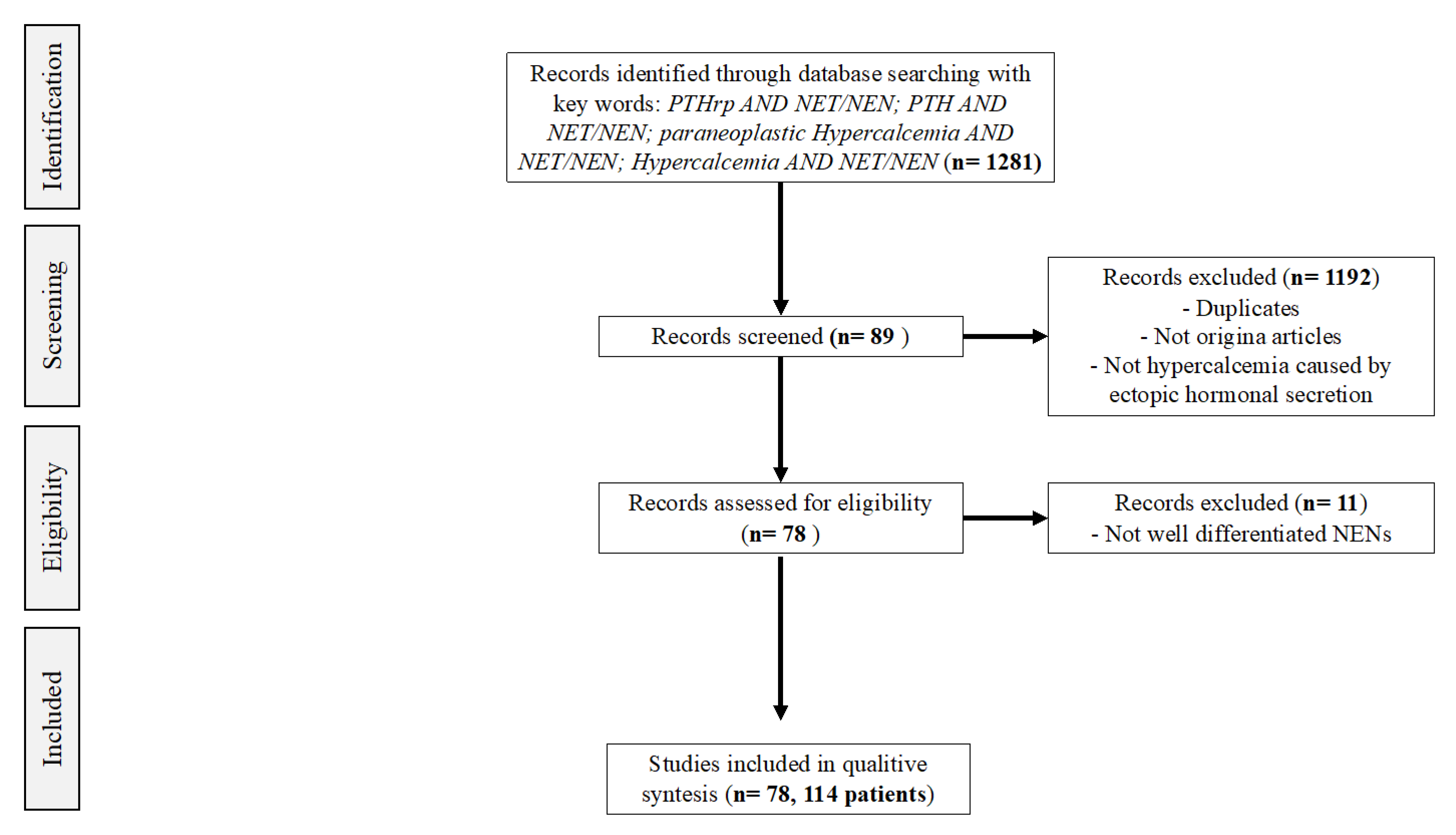
2. Materials and Methods
3. Results
3.1. Clinical Presentation
3.2. Symptomatology
3.3. Treatment Approach for Paraneoplastic Hypercalcemia
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Evans, D.B. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Zamponi, V.; La Salvia, A.; Tarsitano, M.G.; Mikovic, N.; Rinzivillo, M.; Panzuto, F.; Giannetta, E.; Faggiano, A.; Mazzilli, R. Effect of Neuroendocrine Neoplasm Treatment on Human Reproductive Health and Sexual Function. J. Clin. Med. 2022, 11, 3983. [Google Scholar] [CrossRef] [PubMed]
- Modica, R.; Scandurra, C.; Maldonato, N.M.; Dolce, P.; Dipietrangelo, G.G.; Centello, R.; Di Vito, V.; Giannetta, E.; Isidori, A.M.; Lenzi, A.; et al. Health-related quality of life in patients with neuroendocrine neoplasms: A two-wave longitudinal study. J. Endocrinol. Investig. 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scandurra, C.; Modica, R.; Maldonato, N.M.; Dolce, P.; Dipietrangelo, G.G.; Centello, R.; Di Vito, V.; Bottiglieri, F.; de Cicco, F.; Giannetta, E.; et al. Quality of Life in Patients with Neuroendocrine Neoplasms: The Role of Severity, Clinical Heterogeneity, and Resilience. J. Clin. Endocrinol. Metab. 2021, 106, e316–e327. [Google Scholar] [CrossRef] [PubMed]
- Gaudenzi, G.; Dicitore, A.; Carra, S.; Saronni, D.; Pozza, C.; Giannetta, E.; Persani, L.; Vitale, G. MANAGEMENT of ENDOCRINE DISEASE: Precision medicine in neuroendocrine neoplasms: An update on current management and future perspectives. Eur. J. Endocrinol. 2019, 181, R1–R10. [Google Scholar] [CrossRef]
- Kaltsas, G.; Androulakis, I.I.; de Herder, W.W.; Grossman, A.B. Paraneoplastic syndromes secondary to neuroendocrine tumours. Endocr. Relat. Cancer 2010, 17, R173–R193. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.K.; Angelousi, A.; Weickert, M.O.; Randeva, H.S.; Kaltsas, G.; Grossman, A. Paraneoplastic endocrine syndromes. Endocr. Relat. Cancer 2017, 24, R173–R190. [Google Scholar] [CrossRef]
- Asonitis, N.; Angelousi, A.; Zafeiris, C.; Lambrou, G.I.; Dontas, I.; Kassi, E. Diagnosis, Pathophysiology and Management of Hypercalcemia in Malignancy: A Review of the Literature. Horm. Metab. Res. 2019, 51, 770–778. [Google Scholar] [CrossRef]
- Stewart, A.F. Clinical practice. Hypercalcemia associated with cancer. New Engl. J. Med. 2005, 352, 373–379. [Google Scholar] [CrossRef]
- Rosner, M.H.; Dalkin, A.C. Onco-nephrology: The pathophysiology and treatment of malignancy-associated hypercalcemia. Clin. J. Am. Soc. Nephrol. 2012, 7, 1722–1729. [Google Scholar] [CrossRef]
- Tsoli, M.; Dimitriadis, G.K.; Androulakis, I.I.; Kaltsas, G.; Grossman, A. Paraneoplastic Syndromes Related to Neuroendocrine Tumors. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Faggiano, A.; Colao, A. Editorial-Special Issue: Foreword to the Special Issue on NIKE: Neuroendocrine Tumors, Innovation in Knowledge and Education. Front. Endocrinol. 2021, 12, 722145. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Giannetta, E.; Sesti, F.; Modica, R.; Grossrubatscher, E.M.; Guarnotta, V.; Ragni, A.; Zanata, I.; Colao, A.; Faggiano, A. Case Report: Unmasking Hypercalcemia in Patients With Neuroendocrine Neoplasms. Experience From Six Italian Referral Centers. Front. Endocrinol. 2021, 12, 665698. [Google Scholar] [CrossRef]
- Copur, M.S.; Vargas, L.; Wedel, W.; Merani, S.; Cushman-Vokoun, A.; Drincic, A. Pancreatic Neuroendocrine Tumor With Humoral Hypercalcemia and High Tumor PD-L1 Score. Oncology 2020, 34, 548–552. [Google Scholar] [CrossRef]
- Ataallah, B.; Buttar, B.S.; Kulina, G.; Kaell, A. Hypercalcemia in a Patient Diagnosed with a Vasoactive Intestinal Peptide Tumor. Cureus 2020, 12, e6882. [Google Scholar] [CrossRef]
- van Lierop, A.H.; Bisschop, P.H.; Boelen, A.; van Eeden, S.; Engelman, A.F.; van Dijkum, E.J.N.; Klümpen, H.-J. Hypercalcaemia due to a calcitriol-producing neuroendocrine tumour. J. Surg. Case Rep. 2019, 2019, rjz346. [Google Scholar] [CrossRef]
- Gild, M.L.; Tsang, V.; Samra, J.; Clifton-Bligh, R.J.; Tacon, L.; Gill, A.J. Hypercalcemia in Glucagon Cell Hyperplasia and Neoplasia (Mahvash Syndrome): A New Association. J. Clin. Endocrinol. Metab. 2018, 103, 3119–3123. [Google Scholar] [CrossRef]
- Daskalakis, K.; Chatzelis, E.; Tsoli, M.; Papadopoulou-Marketou, N.; Dimitriadis, G.K.; Tsolakis, A.V.; Kaltsas, G. Endocrine paraneoplastic syndromes in patients with neuroendocrine neoplasms. Endocrine 2019, 64, 384–392. [Google Scholar] [CrossRef]
- Symington, M.; Davies, L.; Kaltsas, G.; Weickert, M.O. Malignant hypercalcaemia related to parathyroid hormone-related peptide (PTHrP) secretion from a metastatic pancreatic neuroendocrine tumour (NET). BMJ Case Rep. 2017, 2017, bcr2017219692. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Z.; Wang, G.; Wang, X.; Liu, X. Superior mediastinal typical carcinoid detected by 99mTc-MIBI SPECT/CT imaging: A case report. Medicine 2017, 96, e9457. [Google Scholar] [CrossRef]
- Ranade, R.; Basu, S. Metabolic Bone Disease in the Context of Metastatic Neuroendocrine Tumor: Differentiation from Skeletal Metastasis, the Molecular PET-CT Imaging Features, and Exploring the Possible Etiopathologies Including Parathyroid Adenoma (MEN1) and Paraneoplastic Humoral Hypercalcemia of Malignancy Due to PTHrP Hypersecretion. World J. Nucl. Med. 2017, 16, 62–67. [Google Scholar] [PubMed]
- Valdes-Socin, H.; Almanza, M.R.; Fernández-Ladreda, M.T.; Van Daele, D.; Polus, M.; Chavez, M.; Beckers, A. Use of cinacalcet and sunitinib to treat hypercalcaemia due to a pancreatic neuroendocrine tumor. Arch. Endocrinol. Metab. 2017, 61, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Iliuta, I.A.; Beauregard, J.M.; Couture, F.; Douville, P.; Mac-Way, F. Reversal of Severe and Refractory Humoral Hypercalcemia With 177Lu-Octreotate Peptide Receptor Radionuclide Therapy for Neuroendocrine Tumor of the Pancreas. Clin. Nucl. Med. 2015, 40, e448–e450. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Abell, S.; Hicks, R.J.; Hofman, M.S.; Sachithanandan, N.; McKelvie, P.; MacIsaac, R.J. Protracted hypocalcaemia following a single dose of denosumab in humoral hypercalcaemia of malignancy due to PTHrP-secreting neuroendocrine tumour. Clin. Endocrinol. 2014, 81, 940–942. [Google Scholar] [CrossRef]
- Zhu, V.; de Las Morenas, A.; Janicek, M.; Hartshorn, K. Hypercalcemia from metastatic pancreatic neuroendocrine tumor secreting 1,25-dihydroxyvitamin D. J. Gastrointest. Oncol. 2014, 5, E84–E87. [Google Scholar]
- Kamp, K.; Feelders, R.A.; van Adrichem, R.C.S.; de Rijke, Y.B.; van Nederveen, F.H.; Kwekkeboom, D.J.; de Herder, W.W. Parathyroid hormone-related peptide (PTHrP) secretion by gastroenteropancreatic neuroendocrine tumors (GEP-NETs): Clinical features, diagnosis, management, and follow-up. J. Clin. Endocrinol. Metab. 2014, 99, 3060–3069. [Google Scholar] [CrossRef]
- Rossi, R.E.; Naik, K.; Navalkissoor, S.; Imber, C.; O’Beirne, J.; Toumpanakis, C.; Caplin, M.E. Case report of multimodality treatment for metastatic parathyroid hormone-related peptide-secreting pancreatic neuroendocrine tumour. Tumori 2014, 100, 153e–156e. [Google Scholar] [CrossRef]
- Milanesi, A.; Yu, R.; Wolin, E.M. Humoral hypercalcemia of malignancy caused by parathyroid hormone-related peptide-secreting neuroendocrine tumors. Report of six cases. Pancreatology 2013, 13, 324–326. [Google Scholar] [CrossRef]
- Shah, R.H.; Martinez, D. Pancreatic neuroendocrine tumor associated with humoral hypercalcemia of malignancy and carcinoid tumor: A case report and review of the literature. Pancreas 2013, 42, 549–551. [Google Scholar] [CrossRef]
- Kanakis, G.; Kaltsas, G.; Granberg, D.; Grimelius, L.; Papaioannou, D.; Tsolakis, A.V.; Öberg, K. Unusual complication of a pancreatic neuroendocrine tumor presenting with malignant hypercalcemia. J. Clin. Endocrinol. Metab. 2012, 97, E627–E631. [Google Scholar] [CrossRef]
- Kandil, E.; Noureldine, S.; Khalek, M.A.; Daroca, P.; Friedlander, P. Ectopic secretion of parathyroid hormone in a neuroendocrine tumor: A case report and review of the literature. Int. J. Clin. Exp. Med. 2011, 4, 234–240. [Google Scholar]
- Ghazi, A.A.; Boustani, I.; Amouzegar, A.; Attarian, H.; Pourafkari, M.; Gashti, H.N.; Sabetian, T.; Tirgari, F.; Ghazi, S.; Kovacs, K. Postpartum hypercalcemia secondary to a neuroendocrine tumor of pancreas; a case report and review of literature. Iran. J. Med. Sci. 2011, 36, 217–221. [Google Scholar]
- Shirai, K.; Inoue, I.; Kato, J.; Maeda, H.; Moribata, K.; Shingaki, N.; Ueda, K.; Deguchi, H.; Maekita, T.; Iguchi, M.; et al. A case of a giant glucagonoma with parathyroid hormone-related peptide secretion showing an inconsistent postsurgical endocrine status. Intern. Med. 2011, 50, 1689–1694. [Google Scholar] [CrossRef]
- Takeda, K.; Hara, N.; Kawaguchi, M.; Nishiyama, T.; Takahashi, K. Parathyroid hormone-related peptide-producing non-familial pheochromocytoma in a child. Int. J. Urol. 2010, 17, 673–676. [Google Scholar] [CrossRef]
- Morita, Y.; Suzuki, S.; Sakaguchi, T.; Oishi, K.; Suzuki, A.; Fukumoto, K.; Inaba, K.; Baba, S.; Takehara, Y.; Konno, H. Pancreatic neuroendocrine cell tumor secreting parathyroid hormone-related protein and gastrin: Report of a case. Surg. Today 2010, 40, 1192–1196. [Google Scholar] [CrossRef]
- Demura, M.; Yoneda, T.; Wang, F.; Zen, Y.; Karashima, S.; Zhu, A.; Cheng, Y.; Yamagishi, M.; Takeda, Y. Ectopic production of parathyroid hormone in a patient with sporadic medullary thyroid cancer. Endocr. J. 2010, 57, 161–170. [Google Scholar] [CrossRef]
- Srirajaskanthan, R.; McStay, M.; Toumpanakis, C.; Meyer, T.; Caplin, M.E. Parathyroid hormone-related peptide-secreting pancreatic neuroendocrine tumours: Case series and literature review. Neuroendocrinology 2009, 89, 48–55. [Google Scholar] [CrossRef]
- Brzozowska, M.M.; Wolmarans, L.; Conaglen, J.V. Hypercalcaemia caused by a carcinoid tumour. Intern. Med. J. 2009, 39, 415–418. [Google Scholar] [CrossRef]
- van den Eynden, G.G.; Neyret, A.; Fumey, G.; Rizk-Rabin, M.; Vermeulen, P.B.; Bouizar, Z.; Body, J.-J.; Dirix, L.Y. PTHrP, calcitonin and calcitriol in a case of severe, protracted and refractory hypercalcemia due to a pancreatic neuroendocrine tumor. Bone 2007, 40, 1166–1171. [Google Scholar] [CrossRef]
- Barakat, M.T.; Ashrafian, H.; Todd, J.F.; Meeran, K.; Williams, G.R. Severe hypercalcaemia from secretion of parathyroid hormone-related peptide. Lancet Oncol. 2004, 5, 633–635. [Google Scholar] [CrossRef]
- Mullerpatan, P.M.; Joshi, S.R.; Shah, R.C.; Tampi, C.S.; Doctor, V.M.; Jagannath, P.; Modlin, I. Calcitonin-secreting tumor of the pancreas. Dig. Surg. 2004, 21, 321–324. [Google Scholar] [CrossRef]
- Abraham, P.; Ralston, S.H.; Hewison, M.; Fraser, W.D.; Bevan, J.S. Presentation of a PTHrP-secreting pancreatic neuroendocrine tumour, with hypercalcaemic crisis, pre-eclampsia, and renal failure. Postgrad. Med. J. 2002, 78, 752–753. [Google Scholar] [CrossRef]
- Clemens, P.; Gregor, M.; Lamberts, R. Pancreatic neuroendocrine tumor with extensive vascularisation and parathyroid hormone-related protein (PTHrP)--associated hypercalcemia of malignancy. Exp. Clin. Endocrinol. Diabetes 2001, 109, 378–385. [Google Scholar] [CrossRef]
- Papazachariou, I.M.; Virlos, I.T.; Williamson, R.C. Parathyroid hormone-related peptide in pancreatic neuroendocrine tumours associated with hypercalcaemia. HPB 2001, 3, 221–225. [Google Scholar] [CrossRef]
- Loh, K.-C.; Matthay, K.K.; Hoover, M.; Bracho, F.A.; Cortez, A.B.; Conte, F.A.; Albanese, C.T.; Miller, T.R.; Price, D.C.; Flores, A.J.; et al. Hypercalcemia in malignant paraganglioma due to parathyroid hormone-related protein. Horm. Res. 1998, 50, 217–221. [Google Scholar] [CrossRef]
- van de Loosdrecht, A.A.; van Bodegraven, A.A.; Sepers, J.M.; Sindram, J.W. Long-term follow-up of two patients with metastatic neuroendocrine tumours treated with octreotide. Neth. J. Med. 1998, 53, 118–123. [Google Scholar] [CrossRef]
- Mantzoros, C.S.; Suva, L.J.; Moses, A.C.; Spark, R. Intractable hypercalcaemia due to parathyroid hormone-related peptide secretion by a carcinoid tumour. Clin. Endocrinol. 1997, 46, 373–375. [Google Scholar] [CrossRef]
- Wu, T.J.; Lin, C.L.; Taylor, R.L.; Kvols, L.K.; Kao, P.C. Increased parathyroid hormone-related peptide in patients with hypercalcemia associated with islet cell carcinoma. Mayo Clin. Proc. 1997, 72, 1111–1115. [Google Scholar] [CrossRef]
- Mao, C.; Carter, P.; Schaefer, P.; Zhu, L.; Dominguez, J.M.; Hanson, D.J.; Appert, H.E.; Kim, K.; Howard, J.M. Malignant islet cell tumor associated with hypercalcemia. Surgery 1995, 117, 37–40. [Google Scholar] [CrossRef]
- Anthony, L.B.; May, M.E.; Oates, J.A. Case report: Lanreotide in the management of hypercalcemia of malignancy. Am. J. Med. Sci. 1995, 309, 312–314. [Google Scholar] [CrossRef]
- Ratcliffe, W.A.; Bowden, S.J.; Dunnet, F.P.; Hughes, S.; Emly, J.F.; Baker, J.T.; Pye, J.K.; Williams, C.P. Expression and processing of parathyroid hormone-related protein in a pancreatic endocrine cell tumour associated with hypercalcaemia. Clin. Endocrinol. 1994, 40, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Noguchi, Y.; Matsukawa, H.; Kondo, J.; Matsumoto, A.; Nakatani, Y.; Kitamura, H.; Ito, T. Thymus carcinoid producing parathyroid hormone (PTH)-related protein: Report of a case. Surg. Today 1994, 24, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Mune, T.; Katakami, H.; Kato, Y.; Yasuda, K.; Matsukura, S.; Miura, K. Production and secretion of parathyroid hormone-related protein in pheochromocytoma: Participation of an alpha-adrenergic mechanism. J. Clin. Endocrinol. Metab. 1993, 76, 757–762. [Google Scholar] [PubMed]
- Williams, E.J.; Ratcliffe, W.A.; Stavri, G.T.; Stamatakis, J.D. Hypercalcaemia secondary to secretion of parathyroid hormone related protein from a somatostatinoma of the pancreas. Ann. Clin. Biochem. 1992, 29, 354–357. [Google Scholar] [CrossRef]
- Bridgewater, J.A.; Ratcliffe, W.A.; Bundred, N.J.; Owens, C.W. Malignant phaeochromocytoma and hypercalcaemia. Postgrad. Med. J. 1993, 69, 77–79. [Google Scholar] [CrossRef]
- Miraliakbari, B.A.; Asa, S.L.; Boudreau, S.F. Parathyroid hormone-like peptide in pancreatic endocrine carcinoma and adenocarcinoma associated with hypercalcemia. Hum. Pathol. 1992, 23, 884–887. [Google Scholar] [CrossRef]
- Tarver, D.S.; Birch, S.J. Case report: Life-threatening hypercalcaemia secondary to pancreatic tumour secreting parathyroid hormone-related protein—Successful control by hepatic arterial embolization. Clin. Radiol. 1992, 46, 204–205. [Google Scholar] [CrossRef]
- Mitlak, B.H.; Hutchison, J.S.; Kaufman, S.D.; Nussbaum, S.R. Parathyroid hormone-related peptide mediates hypercalcemia in an islet cell tumor of the pancreas. Horm. Metab. Res. 1991, 23, 344–346. [Google Scholar] [CrossRef]
- Bresler, L.; Boissel, P.; Conroy, T.; Grosdidier, J. Pancreatic islet cell carcinoma with hypercalcemia: Complete remission 5 years after surgical excision and chemotherapy. Am. J. Gastroenterol. 1991, 86, 635–638. [Google Scholar]
- Harrison, M.; James, N.; Broadley, K.; Bloom, S.R.; Armour, R.; Wimalawansa, S.; Heath, D.; Waxman, J. Somatostatin analogue treatment for malignant hypercalcaemia. BMJ 1990, 300, 1313–1314. [Google Scholar] [CrossRef][Green Version]
- Kimura, S.; Nishimura, Y.; Yamaguchi, K.; Nagasaki, K.; Shimada, K.; Uchida, H. A case of pheochromocytoma producing parathyroid hormone-related protein and presenting with hypercalcemia. J. Clin. Endocrinol. Metab. 1990, 70, 1559–1563. [Google Scholar] [CrossRef]
- Rizzoli, R.; Sappino, A.P.; Bonjour, J.P. Parathyroid hormone-related protein and hypercalcemia in pancreatic neuro-endocrine tumors. Int. J. Cancer 1990, 46, 394–398. [Google Scholar] [CrossRef]
- Dodwell, D.; Gurney, H.; Scarffe, H.; Abbas, S. Treatment of a pancreatic tumour secreting parathyroid hormone related protein. BMJ 1990, 300, 1653. [Google Scholar] [CrossRef][Green Version]
- Wynick, D.; Ratcliffe, W.A.; Heath, D.A.; Ball, S.; Barnard, M.; Bloom, S.R. Treatment of a malignant pancreatic endocrine tumour secreting parathyroid hormone related protein. BMJ 1990, 300, 1314–1315. [Google Scholar] [CrossRef][Green Version]
- Heitz, P.U.; Harder, F.; Haas, H.G.; Kloeppel, G. Tumor of the pancreas inducing hypercalcemia. Ultrastruct. Pathol. 1989, 13, 585–588. [Google Scholar] [CrossRef]
- Venkatesh, S.; Vassilopoulou-Sellin, R.; Samaan, N.A. Somatostatin analogue: Use in the treatment of vipoma with hypercalcemia. Am. J. Med. 1989, 87, 356–357. [Google Scholar] [CrossRef]
- Friesen, S.R. Update on the diagnosis and treatment of rare neuroendocrine tumors. Surg. Clin. North Am. 1987, 67, 379–393. [Google Scholar] [CrossRef]
- Sarfati, E.; Lavergne, A.; Gossot, D.; Fischer, D.; Dubost, C. Bronchial carcinoid tumor and hypercalcemia. Ann. Intern. Med. 1987, 106, 476–477. [Google Scholar] [CrossRef]
- Shetty, M.R. Pancreatic islet cell tumors. Surgery 1987, 101, 380. [Google Scholar]
- Vair, D.B.; Boudreau, S.F.; Reid, E.L. Pancreatic islet-cell neoplasia, with secretion of a parathormone-like substance and hypercalcemia. Can. J. Surg. 1987, 30, 108–110. [Google Scholar]
- Arps, H.; Dietel, M.; Schulz, A.; Janzarik, H.; Kloppel, G. Pancreatic endocrine carcinoma with ectopic PTH-production and paraneoplastic hypercalcaemia. Virchows Arch. A Pathol. Anat. Histopathol. 1986, 408, 497–503. [Google Scholar] [CrossRef]
- Grossman, E.; Knecht, A.; Holtzman, E.; Nussinovich, N.; Rosenthal, T. Uncommon presentation of pheochromocytoma: Case studies. Angiology 1985, 36, 759–765. [Google Scholar] [CrossRef]
- Baba, S.; Machida, K.; Ozaki, I.; Okushima, T.; Murabayashi, S.; Kamata, Y.; Imamura, K.; Takebe, K. A malignant pheochromocytoma with ileus, polyuria and hypercalcemia: A case of recurrence 17 years after the initial operation. Endocrinol. JPN 1985, 32, 337–345. [Google Scholar] [CrossRef]
- Loveridge, N.; Kent, G.N.; Heath, D.A.; Jones, E.L. Parathyroid hormone-like bioactivity in a patient with severe osteitis fibrosa cystica due to malignancy: Renotropic actions of a tumour extract as assessed by cytochemical bioassay. Clin. Endocrinol. 1985, 22, 135–146. [Google Scholar] [CrossRef]
- Shanberg, A.M.; Baghdassarian, R.; Tansey, L.A.; Bacon, D.; Greenberg, P.; Perley, M. Pheochromocytoma with hypercalcemia: Case report and review of literature. J. Urol. 1985, 133, 258–259. [Google Scholar] [CrossRef]
- Stewart, A.F.; Hoecker, J.L.; Mallette, L.E.; Segre, G.V.; Amatruda, T.T., Jr.; Vignery, A. Hypercalcemia in pheochromocytoma. Evidence for a novel mechanism. Ann. Intern. Med. 1985, 102, 776–779. [Google Scholar] [CrossRef]
- Rasbach, D.A.; Hammond, J.M. Pancreatic islet cell carcinoma with hypercalcemia. Primary hyperparathyroidism or humoral hypercalcemia of malignancy. Am. J. Med. 1985, 78, 337–342. [Google Scholar] [CrossRef]
- Fairhurst, B.J.; Shettar, S.P. Hypercalcaemia and phaeochromocytoma. Postgrad. Med. J. 1981, 57, 459–460. [Google Scholar] [CrossRef]
- Oberg, K.; Loof, L.; Bostrom, H.; Grimelius, L.; Fahrenkrug, J.; Lundqvist, G. Hypersecretion of calcitonin in patients with the Verner-Morrison syndrome. Scand. J. Gastroenterol. 1981, 16, 135–144. [Google Scholar]
- De Plaen, J.F.; Boemer, F.; van Ypersele De Strihou, C. Hypercalcaemic phaeochromocytoma. Br. Med. J. 1976, 2, 734. [Google Scholar] [CrossRef][Green Version]
- Ghose, R.R.; Winsey, H.S.; Jemmett, J.; Woodhead, J.S. Phaeochromocytoma and hypercalcaemia. Postgrad. Med. J. 1976, 52, 593–595. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gray, R.S.; Gillon, J. Normotensive phaeochromocytoma with hypercalcaemia: Correction after adrenalectomy. Br. Med. J. 1976, 1, 378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cryer, P.E.; Hill, G.J., II. Pancreatic islet cell carcinoma with hypercalcemia and hypergastrinemia: Response to streptozotocin. Cancer 1976, 38, 2217–2221. [Google Scholar] [CrossRef]
- Deftos, L.J.; McMillan, P.J.; Sartiano, G.P.; Abuid, J.; Robinson, A.G. Simultaneous ectopic production of parathyroid hormone and calcitonin. Metabolism 1976, 25, 543–550. [Google Scholar] [CrossRef]
- Hirose, S.; Kobayashi, K.; Kajikawa, K.; Sawabu, N. A case of watery diarrhea, hypokalemia and hypercalcemia associated with nonulcerogenic islet cell tumor of the pancreas. Am. J. Gastroenterol. 1975, 64, 382–391. [Google Scholar]
- Kukreja, S.C.; Hargis, G.K.; Rosenthal, I.M.; Williams, G.A. Pheochromocytoma causing excessive parathyroid hormone production and hypercalcemia. Ann. Intern. Med. 1973, 79, 838–840. [Google Scholar] [CrossRef]
- DeWys, W.D.; Stoll, R.; Au, W.Y.; Salisnjak, M.M. Effects of streptozotocin on an islet cell carcinoma with hypercalcemia. Am. J. Med. 1973, 55, 671–676. [Google Scholar] [CrossRef]
- Swinton, N.W.; Clerkin, E.P., Jr.; Flint, L.D. Hypercalcemia and familial pheochromocytoma. Correction after adrenalectomy. Ann. Intern. Med. 1972, 76, 455–457. [Google Scholar] [CrossRef]
- Lopes, V.M.; Reis, D.D.; Cunha, A.B. Islet-cell adenoma of the pancreas with reversible watery diarrhea and hypokalemia. WDHA syndrome. Am. J. Gastroenterol. 1970, 53, 17–35. [Google Scholar]
- Murray, J.S.; Paton, R.R.; Pope, C.E., 2nd. Pancreatic tumor associated with flushing and diarrhea. Report of a case. New Engl. J. Med. 1961, 26, 436–439. [Google Scholar] [CrossRef]
- Mozar, A.; Lin, H.; Williams, K.; Chin, C.; Li, R.; Kondegowda, N.G.; Stewart, A.F.; Garcia-Ocaña, A.; Vasavada, R.C. Parathyroid Hormone-Related Peptide (1–36) Enhances Beta Cell Regeneration and Increases Beta Cell Mass in a Mouse Model of Partial Pancreatectomy. PLoS ONE 2016, 11, e0158414. [Google Scholar] [CrossRef]
- Bhatia, V.; Cao, Y.; Ko, T.C.; Falzon, M. Parathyroid Hormone-Related Protein Interacts With the Transforming Growth Factor-beta/Bone Morphogenetic Protein-2/Gremlin Signaling Pathway to Regulate Proinflammatory and Profibrotic Mediators in Pancreatic Acinar and Stellate Cells. Pancreas 2016, 45, 659–670. [Google Scholar] [CrossRef]
- Wysolmerski, J.J. Parathyroid hormone-related protein: An update. J. Clin. Endocrinol. Metab. 2012, 97, 2947–2956. [Google Scholar] [CrossRef]
- McCauley, L.K.; Martin, T.J. Twenty-five years of PTHrP progress: From cancer hormone to multifunctional cytokine. J. Bone Miner. Res. 2012, 27, 1231–1239. [Google Scholar] [CrossRef]
- Mundy, G.R.; Edwards, J.R. PTH-related peptide (PTHrP) in hypercalcemia. J. Am. Soc. Nephrol. 2008, 19, 672–675. [Google Scholar] [CrossRef]
- Walker, R.E.; Lawson, M.A.; Buckle, C.H.; Snowden, J.A.; Chantry, A.D. Myeloma bone disease: Pathogenesis, current treatments and future targets. Br. Med. Bull. 2014, 111, 117–138. [Google Scholar] [CrossRef][Green Version]
- Uchimura, K.; Mokuno, T.; Nagasaka, A.; Hayakawa, N.; Kato, T.; Yamazaki, N.; Kobayashi, T.; Nagata, M.; Kotake, M.; Itoh, M.; et al. Lung cancer associated with hypercalcemia induced by concurrently elevated parathyroid hormone and parathyroid hormone-related protein levels. Metabolism 2002, 51, 871–875. [Google Scholar] [CrossRef]
- Weiss, E.S.; Doty, J.; Brock, M.V.; Halvorson, L.; Yang, S.C. A case of ectopic parathyroid hormone production by a pulmonary neoplasm. J. Thorac. Cardiovasc. Surg. 2006, 131, 923–924. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Dinh, T.A.; Haque, A. Small cell carcinoma of the ovary with hypercalcemia and ectopic parathyroid hormone production. Arch. Pathol. Lab. Med. 2005, 129, 531–533. [Google Scholar] [CrossRef]
- Donovan, P.J.; Sundac, L.; Pretorius, C.J.; d’Emden, M.C.; McLeod, D.S. Calcitriol-mediated hypercalcemia: Causes and course in 101 patients. J. Clin. Endocrinol. Metab. 2013, 98, 4023–4029. [Google Scholar] [CrossRef]
- Seymour, J.F.; Gagel, R.F. Calcitriol: The major humoral mediator of hypercalcemia in Hodgkin’s disease and non-Hodgkin’s lymphomas. Blood 1993, 82, 1383–1394. [Google Scholar] [CrossRef]
- Mudde, A.H.; Berg, H.V.D.; Boshuis, P.G.; Breedveld, F.C.; Markusse, H.M.; Kluin, P.M.; Bijvoet, O.L.M.; Papapoulos, S.E. Ectopic production of 1,25-dihydroxyvitamin D by B-cell lymphoma as a cause of hypercalcemia. Cancer 1987, 59, 1543–1546. [Google Scholar] [CrossRef]
- Hewison, M.; Kantorovich, V.; Liker, H.R.; Van Herle, A.J.; Cohan, P.; Zehnder, D.; Adams, J. Vitamin D-mediated hypercalcemia in lymphoma: Evidence for hormone production by tumor-adjacent macrophages. J. Bone Miner. Res. 2003, 18, 579–582. [Google Scholar] [CrossRef]
- Zandee, W.T.; Brabander, T.; Blažević, A.; Minczeles, N.S.; Feelders, R.A.; de Herder, W.W.; Hofland, J. Peptide Receptor Radionuclide Therapy With 177Lu-DOTATATE for Symptomatic Control of Refractory Carcinoid Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, e3665–e3672. [Google Scholar] [CrossRef]
- Parghane, R.V.; Mitra, A.; Bannore, T.U.; Rakshit, S.; Banerjee, S.; Basu, S. Initial clinical evaluation of indigenous (90)Y-DOTATATE in sequential duo-PRRT approach ((177)Lu-DOTATATE and (90)Y-DOTATATE) in neuroendocrine tumors with large bulky disease: Observation on tolerability, (90)Y-DOTATATE post- PRRT imaging characteristics (bremsstrahlung and PETCT) and early adverse effects. World J. Nucl. Med. 2021, 20, 73–81. [Google Scholar]
- Raymond, L.M.; Korzun, T.; Kardosh, A.; Kolbeck, K.J.; Pommier, R.; Mittra, E.S. The State of Peptide Receptor Radionuclide Therapy and Its Sequencing among Current Therapeutic Options for Gastroenteropancreatic Neuroendocrine Tumors. Neuroendocrinology 2021, 111, 1086–1098. [Google Scholar] [CrossRef]
- Pusceddu, S.; Prinzi, N.; Tafuto, S.; Ibrahim, T.; Filice, A.; Brizzi, M.P.; Panzuto, F.; Baldari, S.; Grana, C.M.; Campana, D.; et al. Association of Upfront Peptide Receptor Radionuclide Therapy with Progression-Free Survival Among Patients With Enteropancreatic Neuroendocrine Tumors. JAMA Netw. Open. 2022, 5, e220290. [Google Scholar] [CrossRef]
- Thakker, R.V.; Newey, P.J.; Walls, G.V.; Bilezikian, J.; Dralle, H.; Ebeling, P.R.; Melmed, S.; Sakurai, A.; Tonelli, F.; Brandi, M.L. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J. Clin. Endocrinol. Metab. 2012, 97, 2990–3011. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Asa, S.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.Y.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
| Author, Year | N° of Cases | Sex, Age (y) | Primary Site of NEN | Grade, Ki67 | Initial Staging (ENETS) | Metastasis at Diagnosis of Paraneoplastic Hypercalcemia | Time to Onset of Paraneoplastic Hypercalcemia (Months) | Paraneoplastic Hypercalcemia-Inducing Molecules | Other Peptide Secretion | Paraneoplastic Hypercalcemia Therapy | NEN Therapy | Paraneoplastic Hypercalcemia Responsive to | Survival from Onset of Paraneoplastic Hypercalcemia (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Giannetta E et al., 2021 [14] | 4 | M, 40 | Pancreatic | G2, Ki67 = 5% | IV | Liver | At diagnosis | PTHrP | Calcitonin | Denosumab | SSA PRRT | Denosumab | 36 |
| M, 45 | Pancreatic | G2, Ki67 = 5% | II | - | 96 | NA | - | IV hydration Loop diuretics Cinacalcet Biphosphonate Denosumab Corticosteroids | Surgery SSA Everolimus PRRT | Corticosteroids | 60 | ||
| F, 49 | Pancreatic | G1, Ki67 < 1% | III | - | At diagnosis | 1,25(OH) vitamin D | - | Hemodyalisis Biphosphonate Calcitonin | TAE Surgery | Surgery | 156 | ||
| M, 69 | Pulmonary | Atypical carcinoid, Ki67 = 9% | IV | Liver | At diagnosis | PTHrP | Calcitonin | Biphosphonate | Surgery | Surgery | NA | ||
| Copur MS et al., 2020 [15] | 1 | F, 62 | Pancreatic | G3, Ki67 = 30% | IV | Liver | At diagnosis | PTHrP | - | Biphosphonate IV hydration | SSA 5-FU Oxaliplatin Surgery Pembrolizumab | Biphosphonate IV hydration | 6 |
| Ataallah B et al., 2020 [16] | 1 | F, 22 | Pancreatic | NA | IV | Liver | At diagnosis | NA | VIP | Biphosphonate IV hydration | - | Biphosphonate | NA |
| Van Lierop AH et al., 2019 [17] | 1 | M, 50 | Pancreatic | G2, Ki67 = 10% | IV | Spleen Liver | 94 | 1,25(OH) vitamin D | - | Biphosphonate IV hydration | Surgery CapTem Nivolumab | Surgery | 24 |
| Gild ML et al., 2018 [18] | 1 | M, 47 | Pancreatic | G1, Ki67 < 1% | NA | Lymphonodes | At diagnosis | NA | Glucagon PP | Biphosphonate Denosumab | SSA Surgery | Surgery | 27 |
| Daskalakis K et al., 2018 [19] | 1 | NA | Pancreatic | NA | IV | Liver | NA | PTHrP | - | Bisphosphonate IV hydration Cinacalcet | Surgery SSA Streptzotocine 5-FU IFNα PRRT Bevacizumab CapTem TAE Everolimus Sunitinib | TAE CapTem | NA |
| Symington M et al., 2017 [20] | 1 | F, 54 | Pancreatic | G1, Ki67 < 1% | IV | Liver | At diagnosis | PTHrP | Gastrin | Bisphosphonate IV hydration | SSA | SSA | 3 |
| Lu C et al., 2017 [21] | 1 | M, 65 | Mediastinal | Typical carcinoid | NA | - | At diagnosis | PTH | - | - | Surgery | Surgery | NA |
| Ranade R et al., 2017 [22] | 1 | M, 49 | Pancreatic | G2, Ki67 = 12% | IV | Liver Bone | At diagnosis | PTHrP | - | NA | SSA PRRT CapTem | PRRT CapTem | 21 |
| Valdes-Socin H et al., 2016 [23] | 1 | M, 52 | Pancreatic | G1, Ki67 = 2% | IV | Liver Spleen | At diagnosis | - | Calcitonin | Bisphosphonate Calcitonin IV hydration Cinacalcet | Streptozotocine Adriamycin FOLFOX SSA Sunitinib | Cinacalcet | 48 |
| Iliuta IA et al., 2015 [24] | 1 | M, 48 | Pancreatic | NA | IV | Liver | At diagnosis | PTHrP | - | Bisphosphonate IV hydration Calcitonin Corticosteroids | SSA Everolimus PRRT | PRRT | NA |
| Teng J et al., 2014 [25] | 1 | M, 38 | Pancreatic | G1, Ki67 < 2% | IV | Liver | At diagnosis | PTHrP | - | Hemodialysis Bisphosphonate Calcitonin IV hydration Denosumab Corticosteroids | Carboplatin Etoposide PRRT | Bisphosphonate Denosumab PRRT | 22 |
| Zhu V et al., 2014 [26] | 1 | F, 43 | Pancreatic | G1-2, Ki67 = 2–5% | IV | Liver | 96 | 1,25(OH) vitamin D | - | Bisphosphonate IV hydration Denosumab Calcitonin | SSA HACE Sunitinib CapTem | CapTem | 24 |
| Kamp K et al., 2014 [27] | 9 | M, 41 | Pancreatic | NA | IV | Liver | At diagnosis | PTHrP | - | NA | NA | NA | NA |
| M, 58 | Pancreatic | G1 | IV | Liver Lymphnodes | NA | PTHrP | - | NA | NA | NA | NA | ||
| F, 40 | Pancreatic | NA | IV | Liver Lymphnodes | At diagnosis | PTHrP | VIP | NA | NA | NA | NA | ||
| M, 61 | Pancreatic | G1 | IV | Liver | At diagnosis | PTHrP | - | Biphosphonates IV hydration Denosumab | SSA PRRT Sunitinib | SSA PRRT | NA | ||
| M, 60 | Unknown | G2 | IV | Liver | At diagnosis | PTHrP | - | NA | NA | NA | NA | ||
| M, 38 | Pancreatic | G1 | IV | Liver | At diagnosis | PTHrP | - | NA | NA | NA | NA | ||
| F, 42 | Pancreatic | NA | IV | Liver Lymphnodes | At diagnosis | PTHrP | - | NA | NA | NA | NA | ||
| F, 51 | Pancreatic | NA | IV | Liver | At diagnosis | PTHrP | - | NA | NA | NA | NA | ||
| F, 49 | Pancreatic | G1 | IV | Liver Lymphnodes | NA | PTHrP | - | Biphosphonates Corticosteroids IV hydration Loop diuretics | Surgery SSA PRRT TAE | - | 18 | ||
| Rossi RE et al., 2014 [28] | 1 | F, 25 | Pancreatic | G2, Ki67 = 5% | IIA | - | At diagnosis | PTHrP | - | Biphosphonates IV hydration | Surgery TAE OLT Streptozotocin 5-FU Cisplatin | Surgery | 192 |
| Milanesi A et al., 2013 [29] | 5 | F, 49 | Pancreatic | G1, Ki67 < 2% | IV | Liver | 18 | PTHrP | Somatostatin PP | Biphosphonates | SSA PRRT | Biphosphonates | 36 |
| M, 53 | Pancreatic | G2, Ki67 = 5–10% | IV | Liver | 108 | PTHrP | - | Biphosphonates IV hydration | Surgery SSA HACE CapTem | HACE Biphosphonates CapTem | 96 | ||
| M, 52 | Pancreatic | G2, Ki67 < 5% | IIB | Liver | 48 | PTHrP | Glucagon | Biphosphonates | Surgery HACE | Biphosphonates HACE | 60 | ||
| F, 40 | Unknown | G2, Ki67 = 10% | IV | Liver Lung | 2 | PTHrP | Gastrin | Biphosphonates IV hydration | Sunitinib Everolimus Carboplatin Etoposide | - | 17, DOD | ||
| M, 54 | Pancreatic | G1, Ki67 = 2% | IV | Liver Bone | At diagnosis | PTHrP | - | Biphosphonates Calcitonin | PRRT SSA CapTem | CapTem | 15 | ||
| Shah RH et al., 2013 [30] | 1 | F, 53 | Pancreatic | G1, Ki67 < 1% | IV | Liver Adrenal gland | At diagnosis | PTHrP | PP | Biphosphonates | SSA | Biphosphonates SSA | NA |
| Kanakis G et al., [31] | 1 | M, 58 | Pancreatic | G2, Ki67 = 4% | IV | Liver | 48 | PTHrP | PP | Biphosphonates IV hydration Cinacalcet Glucocorticoids | Streptozotocine 5-FU IFNα SSA PRRT CapTem HACE Bevacizumab Everolimus | HACE Combined chemotherapy | 72 |
| Kandil E et al., 2011 [32] | 1 | F, 73 | Neck | NA | NA | - | At diagnosis | PTH | - | - | Surgery | Surgery | 6 |
| Ghazi AA et al., 2011 [33] | 1 | F, 35 | Pancreatic | G1-2, Ki67 = 1–3% | IIIA | - | At diagnosis | PTHrP | - | IV hydration Biphosphonates Calcitonin | Surgery Etoposide Platinum | Biphosphonates Surgery | 6 |
| Shirai K et al., 2011 [34] | 1 | F, 53 | Pancreatic | NA | IIIA | - | At diagnosis | PTHrP | Glucagon | IV hydration | Surgery HACE RFA | Surgery | 84 |
| Takeda K et al., 2010 [35] | 1 | M, 12 | Pheochromocytoma | NA | NA | - | At diagnosis | PTHrP | - | Biphosphonates Loop diuretics | Surgery | Surgery | 12 |
| Morita Y et al., 2010 [36] | 1 | F, 58 | Pancreatic | NA | IV | Liver | At diagnosis | PTHrP | Gastrin | - | Surgery | Surgery | 19 |
| Demura M et al., 2010 [37] | 1 | F, 20 | MTC | NA | NA | Lymphnodes | At diagnosis | PTH | Calcitonin | - | Surgery | Surgery | NA |
| Srirajaskanthan R et al., 2009 [38] | 5 | F, 25 | Pancreatic | NA | NA | - | At diagnosis | PTHrP | - | NA | SSA TAE Surgery OLT | Surgery | NA |
| F, 44 | Pancreatic | NA | IV | Liver | At diagnosis | PTHrP | - | NA | SSA Streptozotocine 5-FU | SSA | NA | ||
| M, 26 | Pancreatic | NA | IV | Liver | At diagnosis | PTHrP | - | NA | SSA Streptozotocine 5-FU Cisplatin Etoposide | NA | |||
| F, 64 | Pancreatic | NA | NA | - | At diagnosis | PTHrP | - | NA | SSA Streptozotocine 5-FU Cisplatin Etoposide | NA | |||
| F, 34 | Pancreatic | NA | IV | Liver | At diagnosis | PTHrP | - | NA | SSA Streptozotocine 5-FU Cisplatin Carboplatin Etoposide Surgery TAE | Surgery | NA | ||
| Brzozowska MM et al., 2009 [39] | 1 | F, 77 | Unknown | NA | IV | Liver Spleen | At diagnosis | PTHrP | - | Biphosphonates Corticosteroids | Etoposide Carboplatin SSA | - | 14 |
| Van den Eynden GG et al., 2007 [40] | 1 | M, 59 | Pancreatic | G1 | IIIA | - | At diagnosis | PTHrP | Calcitonin | Biphosphonates IV hydration | Surgery SSA IFNα | IFNα | 57 |
| Barakat MT et al., 2004 [41] | 1 | F, 47 | Pancreatic | NA | IV | Liver | 24 | PTHrP | - | Biphosphonates IV hydration | TAE SSA | TAE SSA | 40 |
| Mullerpatan PM et al., 2004 [42] | 1 | F, 56 | Pancreatic | NA | IIB | - | At diagnosis | NA | Calcitonin VIP | IV hydration | Surgery | IV hydration | 18 |
| Abraham P et al., 2002 [43] | 1 | F, 25 | Pancreatic | NA | NA | - | At diagnosis | PTHrP | - | Biphosphonates | Surgery | Biphosphonates Surgery | 24 |
| Clemens P et al., 2001 [44] | 1 | M, 34 | Pancreatic | NA | IIIA | - | At diagnosis | PTHrP | - | IV hydration Biphosphonates Calcitonin Corticosteroids | Streptozotocin 5-FU Doxorubicin SSA Carboplatin Etoposide | Chemotherapy | 32, DOD |
| Papazachariou IM et al., 2001 [45] | 2 | F, 33 | Pancreatic | NA | IV | Liver | At diagnosis | PTHrP | Somatostatin | Biphosphonates IV hydration | Surgery TAE SSA | Surgery TAE | 60 |
| M, 41 | Pancreatic | NA | IV | Liver | 48 | PTHrP | Somatostatin | - | TAE Surgery | TAE Surgery | 4, DOD | ||
| Loh K et al., 1998 [46] | 1 | M, 15 | Retroperitoneal paraganglioma | - | IV | Liver Bone Mediastinum | At diagnosis | PTHrP | - | Biphosphonates Calcitonin IV hydration | Surgery | Biphosphonates Surgery | 4 |
| van de Loosdrecht AA et al., 1998 [47] | 1 | F, 45 | Pancreatic | NA | IV | Liver | 128 | PTHrP | - | IV hydration Corticosteroids | SSA | - | 16, DOD |
| Mantzoros CS et al., 1997 [48] | 1 | F, 59 | Unknown | NA | IV | Liver | At diagnosis | PTHrP | - | IV hydration Biphosphonates Calcitonin Plicamycin Gallium nitrate | 5-FU Carboplatin | - | 3, DOD |
| Wu TJ et al., 1997 [49] | 9 | M, 66 | Pancreatic | NA | IV | Liver Spleen | NA | NA | - | NA | NA | NA | NA |
| F, 42 | Pancreatic | NA | IV | Liver Spleen | NA | PTHrP | - | NA | NA | NA | NA | ||
| F, 45 | Pancreatic | NA | IV | Liver | NA | PTHrP | - | NA | NA | NA | NA | ||
| M, 64 | Pancreatic | NA | IV | Liver | NA | PTHrP | Glucagon | NA | NA | NA | NA | ||
| M, 61 | Pancreatic | NA | NA | - | NA | PTHrP | - | NA | NA | NA | NA | ||
| F, 38 | Pancreatic | NA | IV | Liver | NA | PTHrP | - | NA | NA | NA | NA | ||
| M, 20 | Pancreatic | NA | NA | - | NA | PTHrP | - | NA | NA | NA | NA | ||
| F, 47 | Pancreatic | NA | IV | Liver | NA | PTHrP | - | NA | NA | NA | NA | ||
| F, 51 | Pancreatic | NA | IV | Liver | NA | PTHrP | Somatostatin | NA | NA | NA | NA | ||
| Mao C et al., 1995 [50] | 3 | M, 41 | Pancreatic | NA | IV | Liver Lymphnodes | 0.5 | NA | - | IV hydration Calcitonin Plicamycin | Surgery | - | 32, DOD |
| M, 43 | Pancreatic | NA | IV | Liver | 120 | PTHrP | - | - | Surgery Chemotherapy | - | 6, DOD | ||
| M, 64 | Pancreatic | NA | IV | Liver Lymphnodes Kidney Pleurae | At diagnosis | PTHrP | - | Corticosteroids | - | - | 1.5, DOD | ||
| Anthony LB et al., 1995 [51] | 1 | F, 75 | Pancreatic | NA | NA | - | 60 | PTHrP | PP | IV hydration Plicamycin | Streptzotocin 5-FU SSA | SSA | 3 |
| Ratcliffe WA et al. [52] | 1 | F, 39 | Pancreatic | NA | NA | - | At diagnosis | PTHrP | - | IV hydration Calcitonin Biphosphonates | Surgery | Surgery | 9 |
| Yoshikawa T et al., 1994 [53] | 1 | M, 43 | Thymic | NA | NA | - | At diagnosis | PTH (serum) PTHrP (immunohistochemistry) | - | - | RT | - | 15 |
| Mune T et al., 1993 [54] | 1 | M, 58 | Pheochromocytoma | NA | NA | - | At diagnosis | PTHrP | - | Alpha-blockers | Surgery | Alpha-blockers | NA |
| Williams EJ et al., 1992 [55] | 1 | M, 30 | Pancreatic | NA | IIIA | - | At diagnosis | PTHrP | Somatostatin PP | Biphosphonates IV hydration Calcitonin Plicamycin | Streptozotocin | Plicamycin Streptozotocin | 23, DOD |
| Bridgewater JA et al., 1993 [56] | 1 | M, 68 | Pheochromocytoma | NA | III | Lymphonodes | 94 | PTHrP | - | Biphosphonates | Surgery | Biphosphonates | 1, DOD |
| Miraliakbari BA et al., 1992 [57] | 1 | F, 47 | Pancreatic | NA | IIIA | - | At diagnosis | PTHrP | - | IV hydration Calcitonin Corticosteroids | Surgery | Surgery | 36 |
| Tarver DS et al., 1992 [58] | 1 | M, 36 | Pancreatic | NA | IV | Liver | 48 | PTHrP | - | IV hydration Calcitonin Biphosphonates Corticosteroids | TAE | TAE | 18 |
| Mitlak BH et al., 1991 [59] | 1 | F, 77 | Pancreatic | NA | NA | - | At diagnosis | PTHrP | - | Biphosphonates | Surgery Streptozotocin 5-FU | Surgery Biphosphonates Streptozotocin 5-FU | 58 |
| Bresler L et al., 1991 [60] | 1 | M, 45 | Pancreatic | NA | NA | - | At diagnosis | NA a | - | NA | Surgery Streptozotocin 5-FU | Surgery | 60 |
| Harrison M et al., 1990 [61] | 1 | M, 51 | Pheochromocytoma | NA | NA | - | At diagnosis | PTHrP | - | Biphosphonates IV hydration | Surgery Cisplatin Doxorubicin Metotrexate 5-FU Lomustine SSA | Surgery SSA | 49 |
| Kimura S et al., 1990 [62] | 1 | M, 54 | Pheochromocytoma | NA | NA | - | At diagnosis | PTHrP | - | - | Surgery | Surgery | NA |
| Rizzoli R et al., 1990 [63] | 2 | M, 30 | Pancreatic | NA | IV | Liver | At diagnosis | PTHrP | - | Bisphosphonates | Surgery | Surgery | NA |
| F, 60 | Pancreatic | NA | IV | Liver Bone | At diagnosis | PTHrP | - | Bisphosphonates | IFNα | Bisphosphonates | 12, DOD | ||
| Dodwell D et al., 1990 [64] | 1 | F, 42 | Pancreatic | NA | IV | Liver | At diagnosis | PTHrP | - | Corticosteroids Bisphosphonates | IFNα SSA Surgery | SSA Surgery | 30 |
| Wynick D et al., 1990 [65] | 1 | F, 37 | Pancreatic | NA | IV | Liver | At diagnosis | PTHrP | - | Corticosteroids | SSA | SSA | 48 |
| Heitz PU et al., 1989 [66] | 1 | F, 52 | Pancreatic | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Venkatesh S et al., 1989 [67] | 1 | M, 54 | Pancreatic | NA | IV | Liver | 72 | NA | VIP | IV hydration | TAE Surgery SSA | IV hydration SSA | 48 |
| Friesen SR, 1987 [68] | 1 | M, 8 | Pancreatic | NA | IV | Liver | At diagnosis | NA a | Phosphate enemas | Surgery | Phosphate enemas Surgery | 2, DOD | |
| Sarfati E et al., 1987 [69] | 1 | M, 64 | Pulmonary | NA | NA | - | At diagnosis | NA | - | - | Surgery | Surgery | 18 |
| Shetty MR, 1987 [70] | 1 | M, 44 | Pancreatic | NA | IV | Liver | NA | NA | NA | NA | NA | NA | NA |
| Vair DB et al., 1987 [71] | 1 | F, 47 | Pancreatic | NA | NA | - | At diagnosis | NA a | - | NA | NA | NA | NA |
| Arps H et al., 1986 [72] | 1 | M, 48 | Pancreatic | NA | IV | Liver | 76 | PTH | - | NA | Surgery TAE | - | 7 |
| Grossman E et al., 1985 [73] | 4 | M, 16 | Pheochromocytoma | NA | NA | NA | At diagnosis | NA | - | NA | Surgery | Surgery | NA |
| F, 75 | Pheochromocytoma | NA | NA | NA | At diagnosis | NA | - | NA | Surgery | NA | NA | ||
| M, 26 | Pheochromocytoma | NA | NA | NA | At diagnosis | NA | - | NA | Surgery | Surgery | NA | ||
| M, 58 | Pheochromocytoma | NA | NA | NA | At diagnosis | NA | - | NA | Surgery | NA | NA | ||
| Baba T et al., 1985 [74] | 1 | M, 15 | Pheochromocytoma | NA | IV | Bone | 204 | NA | - | IV hydration Calcitonin Plicamycin | Surgery | Plicamycin | 3, DOD |
| Loveridge N et al., 1985 [75] | 1 | F, 68 | Pulmonary | NA | IV | Liver | At diagnosis | NA a | - | Bisphosphonates IV hydration Corticosteroids | - | IV hydration | 12, DOD |
| Shanberg AM et al., 1985 [76] | 1 | M, 53 | Pheochromocytoma | NA | NA | - | At diagnosis | NA | - | IV hydration Corticosteroids | Surgery | IV hydration Surgery | NA |
| Stewart AF et al., 1985 [77] | 1 | F, 11 | Pheochromocytoma | NA | NA | - | At diagnosis | NA a | - | - | Surgery | Surgery | NA |
| Rasbach DA et al., 1985 [78] | 1 | F, 68 | Pancreatic | NA | IV | Liver | At diagnosis | NA a | - | IV hydration Prednisone | Streptozotocine 5-FU | Streptozotocine 5-FU | 3 |
| Fairhurst JB et al., 1981 [79] | 1 | M, 47 | Pheochromocytoma | NA | NA | - | At diagnosis | NA a | - | - | Surgery | Surgery | 9 |
| Öberg K et al., 1981 [80] | 3 | M, 52 | Pancreatic | NA | NA | - | At diagnosis | NA | Calcitonin VIP Gastrin PP | IV hydration | Surgery Streptozotocin | Surgery | 20 |
| M, 38 | Pancreatic | NA | IV | Liver Omentum | 94 | NA | Calcitonin VIP | IV hydration | Surgery Streptozotocin | Surgery | 7 | ||
| F, 54 | Pancreatic | NA | IV | Omentum | At diagnosis | NA | Calcitonin PP | IV hydration | Surgery Streptozotocin | Streptozotocin | 6 | ||
| De Plaen JF et al., 1976 [81] | 1 | M, 45 | Pheochromocytoma | NA | NA | - | At diagnosis | NA | - | - | Surgery | Surgery | 1, DOD |
| Ghose RR et al., 1976 [82] | 1 | M, 14 | Pheochromocyotma | NA | NA | - | At diagnosis | PTH | - | - | Surgery | Surgery | NA |
| Gray RS et al., 1976 [83] | 1 | M, 66 | Pheochromocytoma | NA | NA | - | At diagnosis | NA | - | - | Surgery | Surgery | 18 |
| Cryer PE et al., 1976 [84] | 1 | F, 61 | Pancreatic | NA | IV | Liver | 189 | NA | Gastrin | Calcitonin Plicamycin | Streptozotocin | Streptozotocin | 13 |
| Deftos LJ et al., 1976 [85] | 1 | F, 27 | Gastric | NA | IV | Liver | At diagnosis | PTH | Calcitonin | - | Melphalan | - | 18, DOD |
| Hirose S et al., 1975 [86] | 1 | M, 62 | Pancreatic | NA | IV | Liver | At diagnosis | PTH | - b | IV hydration | - | - | 4, DOD |
| Kukreja SC et al., 1973 [87] | 1 | M, 16 | Pheochromocytoma | NA | NA | - | At diagnosis | PTH | - | Diuretics | Surgery | Surgery | 36 |
| DeWys WD et al., 1973 [88] | 1 | M, 57 | Pancreatic | NA | IV | Liver | At diagnosis | NA a | ACTH | IV hydration Calcitonin | Streptozotocin | Streptozotocin | 14 |
| Swinton NW et al., 1972 [89] | 1 | M, 12 | Pheochromocytoma | NA | NA | - | At diagnosis | NA | - | - | Surgery | Surgery | 25 |
| Lopes VM et al., 1970 [90] | 1 | F, 42 | Pancreatic | NA | NA | - | At diagnosis | NA | - b | IV hydration | Surgery | Surgery | 24 |
| Murray JS et al., 1961 [91] | 1 | M, 49 | Pancreatic | NA | NA | - | At diagnosis | NA | - b | IV hydration | Surgery | - | 14 |
| Total Number of Cases | 114 (100%) |
|---|---|
| Sex | n = 113 |
| Male | 62 (54.9%) |
| Female | 51 (45.1%) |
| Mean age ± standard deviation | 46.3 ± 15.8 |
| Primary NEN histology | n = 114 |
| Pancreatic NEN | 83 (72.8%) |
| Pheochromocytoma | 18 (15.8%) |
| Unknown NEN | 4 (3.5%) |
| Lung NEN | 3 (2.6%) |
| Gastric NEN | 1 (0.9%) |
| Neck NEN | 1 (0.9%) |
| Mediastinal NEN | 1 (0.9%) |
| Medullary thyroid cancer | 1 (0.9%) |
| Paraganglioma | 1 (0.9%) |
| Thymic NEN | 1 (0.9%) |
| Tumor grade | n = 23 |
| G1 | 10 (43.5%) |
| G2 | 12 (52.2%) |
| G2 | 1 (4.3%) |
| Metastatic disease at paraneoplastic hypercalcemia onset | 66 (57.9%) |
| Metastatic sites | n = 110 |
| Liver | 65 (59.1%) |
| Lymphnode | 9 (8.2%) |
| Bone | 6 (5.5%) |
| Lung | 1 (0.9%) |
| Other sites | 12 (10.1%) |
| Presence of paraneoplastic hypercalcemia at NEN diagnosis | 79 (69.3%) |
| Mean time to onset of paraneoplastic hypercalcemia, months ± standard deviation | 83.4 ± 56.3 |
| Causes of metachronous paraneoplastic hypercalcemia | n = 16 |
| Local recurrence/development of distant metastases | 7 (43.8%) |
| Tumor progression | 8 (50%) |
| No disease progression | 1 (6.3%) |
| Calcemic levels at onset of paraneoplastic hypercalcemia, mean ± SD (mg/dl) | 14 ± 2.7 |
| Calcemic levels at onset of paraneoplastic hypercalcemia in pancreatic NEN patients | 14.3 ± 2.9 |
| Calcemic levels at onset of paraneoplastic hypercalcemia in Pheochromocytoma patients | 12.4 ± 1.4 |
| Paraneoplastic hypercalcemia-producing molecules | n = 80 |
| PTHrP | 68 (85%) |
| PTH | 9 (11.3%) |
| 1,25(OH) vitamin D | 3 (3.8%) |
| Cosecretion of other peptides | 32 (28.1%) |
| Calcitonin | 10 (31.3%) |
| VIP | 8 (25%) |
| Pancreatic polypeptide | 6 (18.8%) |
| Gastrin | 5 (15.6%) |
| Somatostatin | 5 (15.6%) |
| Glucagon | 4 (12.5%) |
| ACTH | 1 (3.1%) |
| Cosecretion of more than one peptide | 7 (21.9%) |
| Median survival, months (IQR range) | 18 months (range, 7–37) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannetta, E.; Sesti, F.; Modica, R.; Grossrubatscher, E.M.; Ragni, A.; Zanata, I.; Colao, A.; Faggiano, A. What Lies behind Paraneoplastic Hypercalcemia Secondary to Well-Differentiated Neuroendocrine Neoplasms? A Systematic Review of the Literature. J. Pers. Med. 2022, 12, 1553. https://doi.org/10.3390/jpm12101553
Giannetta E, Sesti F, Modica R, Grossrubatscher EM, Ragni A, Zanata I, Colao A, Faggiano A. What Lies behind Paraneoplastic Hypercalcemia Secondary to Well-Differentiated Neuroendocrine Neoplasms? A Systematic Review of the Literature. Journal of Personalized Medicine. 2022; 12(10):1553. https://doi.org/10.3390/jpm12101553
Chicago/Turabian StyleGiannetta, Elisa, Franz Sesti, Roberta Modica, Erika Maria Grossrubatscher, Alberto Ragni, Isabella Zanata, Annamaria Colao, and Antongiulio Faggiano. 2022. "What Lies behind Paraneoplastic Hypercalcemia Secondary to Well-Differentiated Neuroendocrine Neoplasms? A Systematic Review of the Literature" Journal of Personalized Medicine 12, no. 10: 1553. https://doi.org/10.3390/jpm12101553
APA StyleGiannetta, E., Sesti, F., Modica, R., Grossrubatscher, E. M., Ragni, A., Zanata, I., Colao, A., & Faggiano, A. (2022). What Lies behind Paraneoplastic Hypercalcemia Secondary to Well-Differentiated Neuroendocrine Neoplasms? A Systematic Review of the Literature. Journal of Personalized Medicine, 12(10), 1553. https://doi.org/10.3390/jpm12101553








