Sonographic Assessment of Uterine Biometry for the Diagnosis of Diffuse Adenomyosis in a Tertiary Outpatient Clinic
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol and Selection Criteria
2.2. Case and Control Goups
2.3. Study Outcomes
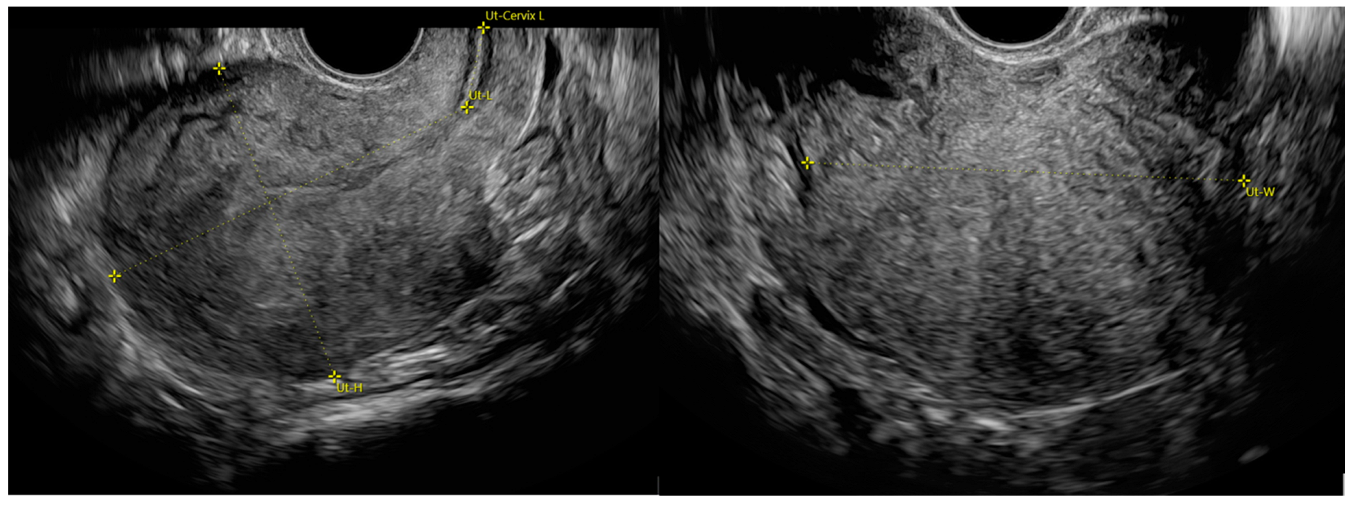
2.4. Patient Assessment and Ultrasound Details
2.5. Statistical Analysis
2.6. Ethical Statement and Informed Consent
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cunningham, R.K.; Horrow, M.M.; Smith, R.J.; Springer, J. Adenomyosis: A Sonographic Diagnosis. RadioGraphics 2018, 38, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, T.; Dueholm, M.; Leone, F.P.G.; Valentin, L.; Rasmussen, C.K.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installé, A.J.; Guerriero, S.; et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: A consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, T.; de Bruijn, A.M.; de Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Bourne, T.; Timmerman, D.; Huirne, J.A.F. Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet. Gynecol. 2019, 53, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Exacoustos, C.; Morosetti, G.; Conway, F.; Camilli, S.; Martire, F.G.; Lazzeri, L.; Piccione, E.; Zupi, E. New Sonographic Clas-sification of Adenomyosis: Do Type and Degree of Adenomyosis Correlate to Severity of Symptoms? J. Minim. Invasive Gynecol. 2020, 27, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Khine, Y.M.; Kaponis, A.; Nikellis, T.; Decavalas, G.; Taniguchi, F. The Impact of Adenomyosis on Women’s Fertility. Obstet. Gynecol. Survey. 2016, 71, 557–568. [Google Scholar] [CrossRef] [PubMed]
- di Donato, N.; Montanari, G.; Benfenati, A.; Leonardi, D.; Bertoldo, V.; Monti, G.; Raimondo, D.; Seracchioli, R. Prevalence of adenomyosis in women undergoing surgery for endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Seracchioli, R.; Raimondo, D.; Maletta, M.; Travaglino, A.; Raimondo, I.; Giaquinto, I.; Orsini, B.; Insabato, L.; Pellicano, M.; et al. Prevalence of adenomyosis in endometrial cancer patients: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2021, 303, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Raimondo, D.; Mastronardi, M.; Raimondo, I.; Del Forno, S.; Arena, A.; Sutherland, N.; Borgia, A.; Mattioli, G.; Terzano, P.; et al. Endometriosis of the Appendix: When to Predict and How to Manage—A Multivariate Analysis of 1935 Endometriosis Cases. J. Minim. Invasive Gynecol. 2020, 27, 100–106. [Google Scholar] [CrossRef]
- Naftalin, J.; Hoo, W.; Pateman, K.; Mavrelos, D.; Holland, T.; Jurkovic, D. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum. Reprod. 2012, 27, 3432–3439. [Google Scholar] [CrossRef] [PubMed]
- Andres, M.P.; Borrelli, G.M.; Ribeiro, J.; Baracat, E.C.; Abrão, M.S.; Kho, R.M. Transvaginal Ultrasound for the Diagnosis of Adenomyosis: Systematic Review and Meta-Analysis. J. Minim. Invasive Gynecol. 2018, 25, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Tellum, T.; Nygaard, S.; Lieng, M. Noninvasive Diagnosis of Adenomyosis: A Structured Review and Meta-analysis of Diagnostic Accuracy in Imaging. J. Minim. Invasive Gynecol. 2020, 27, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Leonardi, M.; Condous, G.; Da Silva Costa, F.; Mol, B.W.; Wong, L. Diagnostic Accuracy of Transvaginal Ul-trasound and Magnetic Resonance Imaging for Adenomyosis. J. Ultrasound Med. 2021, 40, 2289–2306. [Google Scholar] [CrossRef] [PubMed]
- Bromley, B.; Shipp, T.D.; Benacerraf, B. Adenomyosis: Sonographic findings and diagnostic accuracy. J. Ultrasound Med. 2000, 19, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, L.; Morosetti, G.; Centini, G.; Monti, G.; Zupi, E.; Piccione, E.; Exacoustos, C. A sonographic classification of adeno-myosis: Interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil. Steril. 2018, 110, 1154–1161. [Google Scholar] [CrossRef]
- da Silva, J.R.; Andres, M.P.; Leite, A.P.K.; Gomes, M.T.N.A.; Neto, J.S.; Baracat, E.C.; Carmona, F.; Abrão, M.S. Comparison of Sensitivity and Specificity of Structured and Narrative Reports of Transvaginal Ultrasonogaphy for Adenomyosis. J. Min-im. Invasive Gynecol. 2021, 28, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Kepkep, K.; Tuncay, Y.A.; Göynümer, G.; Tutal, E. Transvaginal sonography in the diagnosis of adenomyosis: Which findings are most accurate? Ultrasound Obstet. Gynecol. 2007, 30, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Wang, C.B.; Lee, C.Y.; Wun, T.H.; Lin, P.; Lin, Y.H.; Tseng, C.C.; Chen, C.H.; Tseng, C.J. Transvaginal So-nographic Criteria for the Diagnosis of Adenomyosis Based on Histopathologic Correlation. Taiwan J. Obstet. Gynecol. 2010, 49, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Zannoni, L.; Ambrosio, M.; Raimondo, D.; Arena, A.; Del Forno, S.; Borghese, G.; Paradisi, R.; Seracchioli, R. Question Mark Sign and Transvaginal Ultrasound Uterine Tenderness for the Diagnosis of Adenomyosis. J. Ultrasound Med. 2020, 39, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Mooney, S.; Roberts, R.; McGinnes, D.; Ellett, L.; Maher, P.; Ireland-Jenkin, K.; Stone, K. The myometrial-cervical ratio (MCR): Assessing the diagnostic accuracy of a novel ultrasound measurement in the diagnosis of adenomyosis. Aust. N. Z. J. Obstet. Gynaecol. 2022, 62, 110–117. [Google Scholar] [CrossRef]
- McCaughey, T.; Mooney, S.; Harlow, K.; Healey, M.; Stone, K. The use of the myometrial-cervical ratio in the ultrasound diagnosis of adenomyosis—A validation study. Aust. N. Z. J. Obstet. Gynaecol. 2022, 62, 560–565. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577, Erratum in Ann. Intern. Med. 2008, 148, 168. [Google Scholar] [CrossRef]
- Exacoustos, C.; Brienza, L.; Di Giovanni, A.; Szabolcs, B.; Romanini, M.E.; Zupi, E.; Arduini, D. Adenomyosis: Three-dimensional sonographic findings of the junctional zone and correlation with histology. Ultrasound Obstet. Gynecol. 2011, 37, 471–479. [Google Scholar] [CrossRef] [PubMed]
- di Donato, N.; Bertoldo, V.; Montanari, G.; Zannoni, L.; Caprara, G.; Seracchioli, R. Question mark form of uterus: A simple sonographic sign associated with the presence of adenomyosis. Ultrasound Obstet. Gynecol. 2015, 46, 126–127. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, M.; Dueholm, M. Imaging for Adenomyosis: Making the Diagnosis by Sonography. J. Minim. Invasive Gynecol. 2020, 27, 267. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, D.; Raffone, A.; Aru, A.C.; Salucci, P.; Travaglino, A.; Maletta, M.; Ambrosio, M.; Borghese, G.; Iodice, R.; Casa-dio, P.; et al. C-reactive Protein for Predicting Early Postoperative Complications in Patients Undergoing Laparoscopic Shav-ing for Deep Infiltrating Endometriosis. J. Minim. Invasive Gynecol. 2022, 29, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, M.J.; Van den Bosch, T.; de Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Hehenkamp, W.J.K.; Groen-man, F.; De Bruyn, C.; Rasmussen, C.; et al. Consensus on revised definitions of Morphological Uterus Sonographic Assess-ment (MUSA) features of adenomyosis: Results of modified Delphi procedure. Ultrasound Obstet. Gynecol. 2022, 60, 118–131. [Google Scholar] [CrossRef]
- Ferenczy, A. Pathophysiology of adenomyosis. Hum. Reprod. Update 1998, 4, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.K.; Van den Bosch, T.; Exacoustos, C.; Manegold-Brauer, G.; Benacerraf, B.R.; Froyman, W.; Landolfo, C.; Condorelli, M.; Egekvist, A.G.; Josefsson, H.; et al. Intra- and Inter-Rater Agreement Describing Myometrial Lesions Using Morphologic Uterus Sonographic Assessment: A Pilot Study. J. Ultrasound Med. 2019, 38, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Struble, J.; Reid, S.; Bedaiwy, M.A. Adenomyosis: A Clinical Review of a Challenging Gynecologic Condition. J. Minim. Invasive Gynecol. 2016, 23, 164–185. [Google Scholar] [CrossRef] [PubMed]
- Levgur, M.; Abadi, M.A.; Tucker, A. Adenomyosis: Symptoms, histology, and pregnancy terminations. Obstet. Gynecol. 2000, 95, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Bergholt, T.; Eriksen, L.; Berendt, N.; Jacobsen, M.; Hertz, J.B. Prevalence and risk factors of adenomyosis at hysterectomy. Hum. Reprod. 2001, 16, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Zannoni, L.; Del Forno, S.; Raimondo, D.; Arena, A.; Giaquinto, I.; Paradisi, R.; Casadio, P.; Meriggiola, M.C.; Seracchioli, R. Adenomyosis and endometriosis in adolescents and young women with pelvic pain: Prevalence and risk factors. Minerva Pediatr. 2020. online ahead of print. [Google Scholar] [CrossRef]
- Exacoustos, C.; Zupi, E. A new era in diagnosing adenomyosis is coming. Fertil. Steril. 2018, 110, 858. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Y.W.; Wang, S.; Fan, Q.B.; Shi, H.H.; Leng, J.H.; Sun, D.W.; Lang, J.H.; Zhu, L. Clinical Manifestations of Ade-nomyosis Patients with or Without Pain Symptoms. J. Pain Res. 2019, 12, 3127–3133. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | All (n = 112) | Adenomyosis | ||
|---|---|---|---|---|
| Yes | No | p-Value | ||
| (n = 56) | (n = 56) | |||
| Age, y | 37.6 ± 7.7 | 37.5 ± 7.7 | 37.7 ± 7.7 | 0.913 |
| BMI | 24.0 ± 3.3 | 23.6 ± 3.3 | 24.2 ± 3.4 | 0.098 |
| Age group, y | 0.996 | |||
| ≤25 | 9 (8%) | 5 (9%) | 4 (7%) | |
| 26–30 | 15 (13%) | 7 (13%) | 8 (14%) | |
| 31–35 | 12 (11%) | 6 (11%) | 6 (11%) | |
| 36–40 | 34 (30%) | 16 (29%) | 18 (32%) | |
| 41–45 | 24 (21%) | 13 (23%) | 11 (20%) | |
| >45 | 18 (16%) | 9 (16%) | 9 (16%) | |
| Parity | 0.555 | |||
| 0 | 76 (68%) | 38 (68%) | 38 (68%) | |
| 1 | 25 (22%) | 11 (20%) | 14 (25%) | |
| 2 | 11 (10%) | 7 (12%) | 4 (7%) | |
| Hormonal therapy for ≥3 months before study | 0.157 | |||
| None | 46 (41%) | 23 (41%) | 23 (41%) | |
| Progestin | 34 (30%) | 21 (38%) | 13 (23%) | |
| Intrauterine device | 17 (15%) | 8 (14%) | 9 (16%) | |
| Estrogen/progestin | 15 (13%) | 4 (7%) | 11 (20%) | |
| Previous surgery for endometriosis | 42 (38%) | 30 (54%) | 12 (21%) | <0.001 a |
| Pain symptoms (NRS ≥ 5) | ||||
| Dysmenorrhea | 14 (13%) | 9 (16%) | 5 (9%) | 0.253 |
| Dyspareunia | 15 (13%) | 11 (20%) | 4 (7%) | 0.052 |
| Ovulation pain | 4 (4%) | 3 (5%) | 1 (2%) | 0.618 |
| Chronic pelvic pain | 7 (6%) | 6 (11%) | 1 (2%) | 0.113 |
| Dysuria | 1 (1%) | 0 (0%) | 1 (2%) | 1.000 |
| Dyschezia | 3 (3%) | 3 (5%) | 0 (0%) | 0.243 |
| Heavy menstrual bleeding (PBAC ≥ 100) | 9 (8%) | 8 (14%) | 1 (2%) | 0.016 a |
| Characteristic | All (n = 112) | Adenomyosis | ||
|---|---|---|---|---|
| Yes | No | p-Value | ||
| (n = 56) | (n = 56) | |||
| Globular uterus | 46 (41%) | 41 (73%) | 5 (9%) | <0.001 a |
| Fan-shaped shadows | 38 (34%) | 37 (66%) | 1 (2%) | <0.001 a |
| Hyperechogenic islands | 36 (32%) | 36 (64%) | 0 (0%) | <0.001 a |
| JZ interruption/irregularities | 36 (32%) | 30 (54%) | 6 (11%) | <0.001 a |
| Anechoic myometrial cysts | 35 (31%) | 34 (61%) | 1 (2%) | <0.001 a |
| Trans-lesional vascularity | 33 (29%) | 33 (59%) | 0 (0%) | <0.001 a |
| Echogenic sub-endometrial lines and buds | 32 (29%) | 32 (57%) | 0 (0%) | <0.001 a |
| Asymmetry of the uterine walls | 52 (46%) | 45 (80%) | 7 (13%) | <0.001 a |
| QM sign | 18 (16%) | 18 (32%) | 0 (0%) | <0.001 a |
| Measurement | Optimal Cutoff | Sensitivity at Cutoff | Specificity at Cutoff | AUC |
|---|---|---|---|---|
| Est. (95% CI) | Est. (95% CI) | Est. (95% CI) | Est. (95% CI) | |
| LD, cm | 78.5 (73.6, 83.4) | 0.64 (0.51, 0.76) | 0.66 (0.53, 0.77) | 0.67 (0.57, 0.77) |
| APD, cm | 39.5 (36.2, 42.8) | 0.70 (0.57, 0.80) | 0.71 (0.59, 0.82) | 0.75 (0.66, 0.84) |
| TD, cm | 52.5 (46.6, 58.4) | 0.50 (0.37, 0.63) | 0.82 (0.70, 0.90) | 0.70 (0.60, 0.80) |
| Volume, ×103 cm3 | 71.1 (45.6, 96.6) | 0.79 (0.66, 0.87) | 0.64 (0.51, 0.76) | 0.73 (0.64, 0.83) |
| LD/APD, cm | 2.05 (1.96, 2.13) | 0.70 (0.57, 0.80) | 0.70 (0.57, 0.80) | 0.72 (0.62, 0.81) |
| LD/TD, cm | 1.67 (1.56, 1.78) | 0.73 (0.60, 0.83) | 0.52 (0.39, 0.64) | 0.62 (0.52, 0.73) |
| APD/TD, cm | 0.76 (0.70, 0.81) | 0.77 (0.64, 0.86) | 0.48 (0.36, 0.61) | 0.63 (0.53, 0.74) |
| APD+TD, cm | 90.5 (84.4, 96.6) | 0.66 (0.53, 0.77) | 0.77 (0.64, 0.86) | 0.74 (0.65, 0.83) |
| LD/(APD+TD), cm | 0.90 (0.85, 0.96) | 0.64 (0.51, 0.76) | 0.64 (0.51, 0.76) | 0.68 (0.58, 0.78) |
| LD+APD, cm | 117.5 (107.8, 127.2) | 0.70 (0.57, 0.80) | 0.68 (0.55, 0.79) | 0.72 (0.62, 0.82) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raimondo, D.; Lazzeri, L.; Raffone, A.; Giorgi, M.; Orsini, B.; Verrelli, L.; Lenzi, J.; Travaglino, A.; De Meis, L.; Mollo, A.; et al. Sonographic Assessment of Uterine Biometry for the Diagnosis of Diffuse Adenomyosis in a Tertiary Outpatient Clinic. J. Pers. Med. 2022, 12, 1572. https://doi.org/10.3390/jpm12101572
Raimondo D, Lazzeri L, Raffone A, Giorgi M, Orsini B, Verrelli L, Lenzi J, Travaglino A, De Meis L, Mollo A, et al. Sonographic Assessment of Uterine Biometry for the Diagnosis of Diffuse Adenomyosis in a Tertiary Outpatient Clinic. Journal of Personalized Medicine. 2022; 12(10):1572. https://doi.org/10.3390/jpm12101572
Chicago/Turabian StyleRaimondo, Diego, Lucia Lazzeri, Antonio Raffone, Matteo Giorgi, Benedetta Orsini, Ludovica Verrelli, Jacopo Lenzi, Antonio Travaglino, Lucia De Meis, Antonio Mollo, and et al. 2022. "Sonographic Assessment of Uterine Biometry for the Diagnosis of Diffuse Adenomyosis in a Tertiary Outpatient Clinic" Journal of Personalized Medicine 12, no. 10: 1572. https://doi.org/10.3390/jpm12101572
APA StyleRaimondo, D., Lazzeri, L., Raffone, A., Giorgi, M., Orsini, B., Verrelli, L., Lenzi, J., Travaglino, A., De Meis, L., Mollo, A., Zupi, E., Seracchioli, R., & Casadio, P. (2022). Sonographic Assessment of Uterine Biometry for the Diagnosis of Diffuse Adenomyosis in a Tertiary Outpatient Clinic. Journal of Personalized Medicine, 12(10), 1572. https://doi.org/10.3390/jpm12101572








