Pharmacological, Nutritional, and Rehabilitative Interventions to Improve the Complex Management of Osteoporosis in Patients with Chronic Obstructive Pulmonary Disease: A Narrative Review
Abstract
:1. Introduction
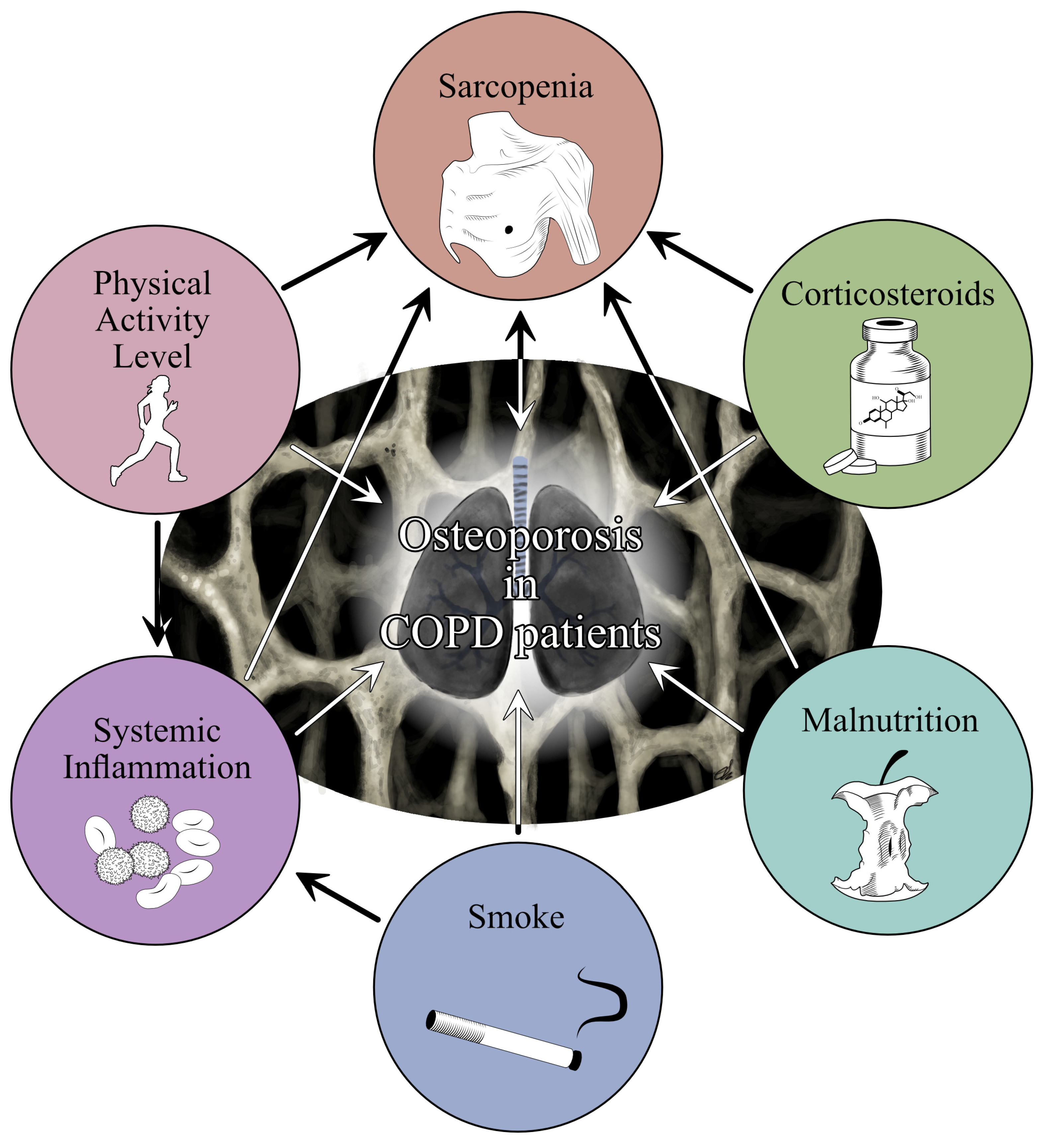
2. COPD and Osteoporosis: A Close Link beyond Corticosteroids and Inflammation
3. Physical Activity in COPD
3.1. Pulmonary Rehabilitation
3.2. Rehabilitation and Therapeutic Exercise
3.3. Barriers and Challenges for the Physical Exercise Programs
3.3.1. Hypoxia and Oxygen Therapy
3.3.2. Anemia
3.3.3. Adherence to Rehabilitation Programs
3.3.4. Environmental and Organizational Issues
3.3.5. Sustainability
4. Nutraceuticals and Dietary Supplements
4.1. Vitamin D and Calcium Supplementation
4.2. Proteins and Amino Acids
4.3. Potential Role of Microbiota: The Gut–Bone Axis
5. Lifestyle Approach and Smoke Cessation
6. Pharmacological Treatments
6.1. Denosumab
6.2. Teriparatide
7. Sustainable Strategies and Digital Innovation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mei, F.; Dalmartello, M.; Bonifazi, M.; Bertuccio, P.; Levi, F.; Boffetta, P.; Negri, E.; La Vecchia, C.; Malvezzi, M. Chronic Obstructive Pulmonary Disease (COPD) mortality trends worldwide: An update to 2019. Respirology 2022. [Google Scholar] [CrossRef] [PubMed]
- Sritharan, S.S.; Østergaard, E.B.; Callesen, J.; Elkjaer, M.; Sand, L.; Hilberg, O.; Skaarup, S.H.; Løkke, A. Barriers toward physical activity in COPD: A quantitative cross-sectional, questionnaire-based study. COPD 2021, 18, 272–280. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, K.U.; Lee, H.; Kim, Y.S.; Lee, M.K.; Park, H.K. Factors associated with low-level physical activity in elderly patients with chronic obstructive pulmonary disease. Korean J. Intern. Med. 2018, 33, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Lippi, L.; D’Abrosca, F.; Folli, A.; Dal Molin, A.; Moalli, S.; Maconi, A.; Ammendolia, A.; de Sire, A.; Invernizzi, M. Closing the gap between inpatient and outpatient settings: Integrating pulmonary rehabilitation and technological advances in the comprehensive management of frail patients. Int. J. Environ. Res. Public Health 2022, 19, 9150. [Google Scholar] [CrossRef]
- Barnsley, J.; Buckland, G.; Chan, P.E.; Ong, A.; Ramos, A.S.; Baxter, M.; Laskou, F.; Dennison, E.M.; Cooper, C.; Patel, H.P. Pathophysiology and treatment of osteoporosis: Challenges for clinical practice in older people. Aging Clin. Exp. Res. 2021, 33, 759–773. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [Green Version]
- Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility fractures in Europe: Burden, management and opportunities. Arch. Osteoporos. 2020, 15, 59. [Google Scholar] [CrossRef] [Green Version]
- Bouxsein, M.L.; Kaufman, J.; Tosi, L.; Cummings, S.; Lane, J.; Johnell, O. Recommendations for optimal care of the fragility fracture patient to reduce the risk of future fracture. J. Am. Acad. Orthop. Surg. 2004, 12, 385–395. [Google Scholar] [CrossRef]
- de Sire, A.; Invernizzi, M.; Baricich, A.; Lippi, L.; Ammendolia, A.; Grassi, F.A.; Leigheb, M. Optimization of transdisciplinary management of elderly with femur proximal extremity fracture: A patient-tailored plan from orthopaedics to rehabilitation. World J. Orthop. 2021, 12, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Bitar, A.N.; Syed Sulaiman, S.A.; Ali, I.A.H.; Khan, I.; Khan, A.H. Osteoporosis among patients with chronic obstructive pulmonary disease: Systematic review and meta-analysis of prevalence, severity, and therapeutic outcomes. J. Pharm. Bioallied Sci. 2019, 11, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Ramsook, A.H.; Coxson, H.O.; Bon, J.; Reid, W.D. Prevalence and risk factors for osteoporosis in individuals with COPD: A systematic review and meta-analysis. Chest 2019, 156, 1092–1110. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.; Aragaki, A.K.; Kooperberg, C.; Watts, N.; Wactawski-Wende, J.; Jackson, R.D.; LeBoff, M.S.; Lewis, C.E.; Chen, Z.; Stefanick, M.L.; et al. Factors associated with 5-year risk of hip fracture in postmenopausal women. JAMA 2007, 298, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- de Filippis, R.; Mercurio, M.; Spina, G.; De Fazio, P.; Segura-Garcia, C.; Familiari, F.; Gasparini, G.; Galasso, O. Antidepressants and vertebral and hip risk fracture: An updated systematic review and meta-analysis. Healthcare 2022, 10, 803. [Google Scholar] [CrossRef]
- Curtis, E.M.; Moon, R.J.; Harvey, N.C.; Cooper, C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone 2017, 104, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gao, H.; Zhao, L.; Wang, J. Osteoporosis in COPD patients: Risk factors and pulmonary rehabilitation. Clin. Respir. J. 2022, 16, 487–496. [Google Scholar] [CrossRef]
- Thorpe, O.; Kumar, S.; Johnston, K. Barriers to and enablers of physical activity in patients with COPD following a hospital admission: A qualitative study. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Liao, K.M.; Chiu, K.L.; Chen, C.Y. Prescription patterns in patients with chronic obstructive pulmonary disease and osteoporosis. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 761–769. [Google Scholar] [CrossRef]
- Caramori, G.; Ruggeri, P.; Arpinelli, F.; Salvi, L.; Girbino, G. Long-term use of inhaled glucocorticoids in patients with stable chronic obstructive pulmonary disease and risk of bone fractures: A narrative review of the literature. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1085–1097. [Google Scholar] [CrossRef] [Green Version]
- Inoue, D.; Watanabe, R.; Okazaki, R. COPD and osteoporosis: Links, risks, and treatment challenges. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 637–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, T.; Schett, G. Pathways for bone loss in inflammatory disease. Curr. Osteoporos. Rep. 2012, 10, 101–108. [Google Scholar] [CrossRef] [PubMed]
- King, P.T. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin. Transl. Med. 2015, 4, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson-Barnes, S.L.; Lanham-New, S.A.; Lambert, H. Modifiable risk factors for bone health & fragility fractures. Best Pract. Res. Clin. Rheumatol. 2022, 101758. [Google Scholar] [CrossRef]
- Al-Bashaireh, A.M.; Haddad, L.G.; Weaver, M.; Chengguo, X.; Kelly, D.L.; Yoon, S. The effect of tobacco smoking on bone mass: An overview of pathophysiologic mechanisms. J. Osteoporos. 2018, 2018, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Roubenoff, R. Physical activity, inflammation, and muscle loss. Nutr. Rev. 2007, 65, S208–S212. [Google Scholar] [CrossRef]
- Hlebichuk, J.L.; Gretebeck, R.J.; Garnier-Villarreal, M.; Piacentine, L.B.; Singh, M.; Gretebeck, K.A. Physical activity, inflammation, and physical function in older adults: Results from the health & retirement study. Biol. Res. Nurs. 2022, 10998004221111217. [Google Scholar] [CrossRef]
- Bernardes, S.; Eckert, I.C.; Burgel, C.F.; Teixeira, P.J.Z.; Silva, F.M. Increased energy and/or protein intake improves anthropometry and muscle strength in COPD patients: A systematic review with meta-analysis on randomized controlled clinical trials. Br. J. Nutr. 2022, 1–55. [Google Scholar] [CrossRef]
- de Sire, A.; Ferrillo, M.; Lippi, L.; Agostini, F.; de Sire, R.; Ferrara, P.E.; Raguso, G.; Riso, S.; Roccuzzo, A.; Ronconi, G.; et al. Sarcopenic dysphagia, malnutrition, and oral frailty in elderly: A comprehensive review. Nutrients 2022, 14, 982. [Google Scholar] [CrossRef]
- Gao, J.; Deng, M.; Li, Y.; Yin, Y.; Zhou, X.; Zhang, Q.; Hou, G. Resistin as a systemic inflammation-related biomarker for sarcopenia in patients with chronic obstructive pulmonary disease. Front. Nutr. 2022, 9, 921399. [Google Scholar] [CrossRef]
- Clynes, M.A.; Gregson, C.L.; Bruyère, O.; Cooper, C.; Dennison, E.M. Osteosarcopenia: Where osteoporosis and sarcopenia collide. Rheumatology 2021, 60, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Peñailillo, L.; Valladares-Ide, D.; Jannas-Velas, S.; Flores-Opazo, M.; Jalón, M.; Mendoza, L.; Nuñez, I.; Diaz-Patiño, O. Effects of eccentric, concentric and eccentric/concentric training on muscle function and mass, functional performance, cardiometabolic health, quality of life and molecular adaptations of skeletal muscle in COPD patients: A multicentre randomised trial. BMC Pulm. Med. 2022, 22, 278. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Baroni, M.; Ranchelli, A.; Lauretani, F.; Maggio, M.; Mecocci, P.; Ruggiero, C. Interaction between bone and muscle in older persons with mobility limitations. Curr. Pharm. Des. 2014, 20, 3178–3197. [Google Scholar] [CrossRef] [Green Version]
- Felsenthal, N.; Zelzer, E. Mechanical regulation of musculoskeletal system development. Development 2017, 144, 4271–4283. [Google Scholar] [CrossRef] [Green Version]
- Agostini, F.; Bernetti, A.; Di Giacomo, G.; Viva, M.G.; Paoloni, M.; Mangone, M.; Santilli, V.; Masiero, S. Rehabilitative good practices in the treatment of sarcopenia: A narrative review. Am. J. Phys. Med. Rehabil. 2021, 100, 280–287. [Google Scholar] [CrossRef]
- Brotto, M.; Invernizzi, M.; Ireland, A.; Klein, G.L. Editorial: Osteoporosis and the role of muscle. Front. Endocrinol. 2022, 13, 951298. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef] [Green Version]
- Rizzoli, R.; Reginster, J.Y.; Arnal, J.F.; Bautmans, I.; Beaudart, C.; Bischoff-Ferrari, H.; Biver, E.; Boonen, S.; Brandi, M.L.; Chines, A.; et al. Quality of life in sarcopenia and frailty. Calcif. Tissue Int. 2013, 93, 101–120. [Google Scholar] [CrossRef] [Green Version]
- Goates, S.; Du, K.; Arensberg, M.B.; Gaillard, T.; Guralnik, J.; Pereira, S.L. Economic impact of hospitalizations in US adults with sarcopenia. J. Frailty Aging 2019, 8, 93–99. [Google Scholar] [CrossRef]
- Burge, A.T.; Cox, N.S.; Abramson, M.J.; Holland, A.E. Interventions for promoting physical activity in people with Chronic Obstructive Pulmonary Disease (COPD). Cochrane Database Syst. Rev. 2020, 4, Cd012626. [Google Scholar] [CrossRef] [PubMed]
- Lewthwaite, H.; Effing, T.W.; Olds, T.; Williams, M.T. Physical activity, sedentary behaviour and sleep in COPD guidelines: A systematic review. Chron. Respir. Dis. 2017, 14, 231–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.; et al. An official American thoracic society/European respiratory society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miravitlles, M.; Ribera, A. Understanding the impact of symptoms on the burden of COPD. Respir. Res. 2017, 18, 67. [Google Scholar] [CrossRef] [Green Version]
- Gloeckl, R.; Schneeberger, T.; Jarosch, I.; Kenn, K. Pulmonary rehabilitation and exercise training in chronic obstructive pulmonary disease. Dtsch. Ärzteblatt Int. 2018, 115, 117–123. [Google Scholar] [CrossRef]
- Holland, A.E.; Cox, N.S.; Houchen-Wolloff, L.; Rochester, C.L.; Garvey, C.; Zuwallack, R.; Nici, L.; Limberg, T.; Lareau, S.C.; Yawn, B.P.; et al. Defining modern pulmonary rehabilitation. An official American thoracic society workshop report. Ann. Am. Thorac. Soc. 2021, 18, e12–e29. [Google Scholar] [CrossRef]
- Hejazi, K.; Askari, R.; Hofmeister, M. Effects of physical exercise on bone mineral density in older postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Arch. Osteoporos. 2022, 17, 102. [Google Scholar] [CrossRef]
- Koshy, F.S.; George, K.; Poudel, P.; Chalasani, R.; Goonathilake, M.R.; Waqar, S.; George, S.; Jean-Baptiste, W.; Yusuf Ali, A.; Inyang, B.; et al. Exercise prescription and the minimum dose for bone remodeling needed to prevent osteoporosis in postmenopausal women: A systematic review. Cureus 2022, 14, e25993. [Google Scholar] [CrossRef]
- Marciniuk, D.D.; Goodridge, D.; Hernandez, P.; Rocker, G.; Balter, M.; Bailey, P.; Ford, G.; Bourbeau, J.; O’Donnell, D.E.; Maltais, F.; et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: A Canadian thoracic society clinical practice guideline. Can. Respir. J. 2011, 18, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Agustí, A.; Calverley, P.M.; Decramer, M.; Stockley, R.A.; Wedzicha, J.A. Prevention of exacerbations in chronic obstructive pulmonary disease: Knowns and unknowns. Chronic Obstr. Pulm. Dis. 2014, 1, 166–184. [Google Scholar] [CrossRef]
- Ambrosino, N.; Fracchia, C. Strategies to relieve dyspnoea in patients with advanced chronic respiratory diseases. A narrative review. Pulmonology 2019, 25, 289–298. [Google Scholar] [CrossRef] [PubMed]
- International Osteoporosis Foundation (IOF). Exercise Recommendations of Bone Health. Available online: https://www.osteoporosis.foundation/health-professionals/prevention/exercise (accessed on 29 September 2022).
- Kortianou, E.A.; Nasis, I.G.; Spetsioti, S.T.; Daskalakis, A.M.; Vogiatzis, I. Effectiveness of interval exercise training in patients with COPD. Cardiopulm. Phys. Ther. J. 2010, 21, 12–19. [Google Scholar] [CrossRef]
- Gloeckl, R.; Marinov, B.; Pitta, F. Practical recommendations for exercise training in patients with COPD. Eur. Respir. Rev. 2013, 22, 178–186. [Google Scholar] [CrossRef]
- Cornelison, S.D.; Pascual, R.M. Pulmonary rehabilitation in the management of chronic lung disease. Med. Clin. N. Am. 2019, 103, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Jaitovich, A.; Barreiro, E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. What we know and can do for our patients. Am. J. Respir. Crit. Care Med. 2018, 198, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Malafarina, V.; Uriz-Otano, F.; Malafarina, C.; Martinez, J.A.; Zulet, M.A. Effectiveness of nutritional supplementation on sarcopenia and recovery in hip fracture patients. A multi-centre randomized trial. Maturitas 2017, 101, 42–50. [Google Scholar] [CrossRef]
- Benedetti, M.G.; Furlini, G.; Zati, A.; Letizia Mauro, G. The effectiveness of physical exercise on bone density in osteoporotic patients. BioMed Res. Int. 2018, 2018, 4840531. [Google Scholar] [CrossRef]
- Hong, A.R.; Kim, S.W. Effects of resistance exercise on bone health. Endocrinol. Metab. 2018, 33, 435–444. [Google Scholar] [CrossRef]
- Loughran, K.J.; Atkinson, G.; Beauchamp, M.K.; Dixon, J.; Martin, D.; Rahim, S.; Harrison, S.L. Balance impairment in individuals with COPD: A systematic review with meta-analysis. Thorax 2020, 75, 539–546. [Google Scholar] [CrossRef]
- Hakamy, A.; Bolton, C.E.; Gibson, J.E.; McKeever, T.M. Risk of fall in patients with COPD. Thorax 2018, 73, 1079–1080. [Google Scholar] [CrossRef]
- Beauchamp, M.K. Balance assessment in people with COPD: An evidence-based guide. Chronic Respir. Dis. 2019, 16, 147997311882031. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Agostini, F.; Lippi, L.; Mangone, M.; Marchese, S.; Cisari, C.; Bernetti, A.; Invernizzi, M. Oxygen-ozone therapy in the rehabilitation field: State of the art on mechanisms of action, safety and effectiveness in patients with musculoskeletal disorders. Biomolecules 2021, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Marotta, N.; Ferrillo, M.; Agostini, F.; Sconza, C.; Lippi, L.; Respizzi, S.; Giudice, A.; Invernizzi, M.; Ammendolia, A. Oxygen-ozone therapy for reducing pro-inflammatory cytokines serum levels in musculoskeletal and temporomandibular disorders: A comprehensive review. Int. J. Mol. Sci. 2022, 23, 2528. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Augustin, I.M.; Vanfleteren, L.E.; Janssen, D.J.; Gaffron, S.; Pennings, H.J.; Smeenk, F.; Pieters, W.; van den Bergh, J.J.; Michels, A.J.; et al. Differential response to pulmonary rehabilitation in COPD: Multidimensional profiling. Eur. Respir. J. 2015, 46, 1625–1635. [Google Scholar] [CrossRef] [Green Version]
- Cesari, M.; Marzetti, E.; Laudisio, A.; Antonica, L.; Pahor, M.; Bernabei, R.; Zuccalà, G. Interaction of HDL cholesterol concentrations on the relationship between physical function and inflammation in community-dwelling older persons. Age Ageing 2010, 39, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Mantoani, L.C.; Dell’Era, S.; MacNee, W.; Rabinovich, R.A. Physical activity in patients with COPD: The impact of comorbidities. Expert Rev. Respir. Med. 2017, 11, 685–698. [Google Scholar] [CrossRef]
- Garvey, C.; Bayles, M.P.; Hamm, L.F.; Hill, K.; Holland, A.; Limberg, T.M.; Spruit, M.A. Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: Review of selected guidelines: An official statement from the American Association of Cardiovascular and Pulmonary Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2016, 36, 75–83. [Google Scholar] [CrossRef]
- Yohannes, A.M.; Ershler, W.B. Anemia in COPD: A systematic review of the prevalence, quality of life, and mortality. Respir. Care 2011, 56, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Wollsching-Strobel, M.; Schwarz, S.B.; Mathes, T.; Majorski, D.S.; Heidari, P.; Kroppen, D.; Magnet, F.S.; Windisch, W. Anemia severely reduces health-related quality of life in COPD patients receiving long-term home non-invasive ventilation. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 2963–2971. [Google Scholar] [CrossRef]
- Rochester, C.L.; Vogiatzis, I.; Holland, A.E.; Lareau, S.C.; Marciniuk, D.D.; Puhan, M.A.; Spruit, M.A.; Masefield, S.; Casaburi, R.; Clini, E.M.; et al. An official American Thoracic Society/European Respiratory Society policy statement: Enhancing implementation, use, and delivery of pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2015, 192, 1373–1386. [Google Scholar] [CrossRef] [Green Version]
- Lucchi, M.; Ambrogi, M.; Aprile, V.; Ribechini, A.; Fontanini, G. Laryngotracheal resection for a post-tracheotomy stenosis in a patient with coronavirus disease 2019 (COVID-19). JTCVS Tech. 2020, 4, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.; Williams, V.; Curtis, F.; Bridle, C.; Jones, A.W. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: A systematic review of qualitative studies. npj Prim. Care Respir. Med. 2018, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A. Pulmonary rehabilitation. Eur. Respir. Rev. 2014, 23, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Jayes, L.R.; Holmes, S.; Sahota, O.; Canavan, M.; Elkin, S.L.; Lim, K.; Murphy, A.C.; Singh, S.; Towlson, E.A.; et al. Management of fracture risk in patients with Chronic Obstructive Pulmonary Disease (COPD): Building a UK consensus through healthcare professional and patient engagement. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.J.; Dale, M.; McKeough, Z.J. Innovative strategies to improve the reach and engagement in pulmonary rehabilitation. J. Thorac. Dis. 2019, 11, S2192–S2199. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.M.M.; Rabinovich, R.; Divgi, K.; Ahmed, S.; Saha, S.K.; Singh, S.; Uddin, A.; Uzzaman, M.N.; Pinnock, H. Systematic review of clinical effectiveness, components, and delivery of pulmonary rehabilitation in low-resource settings. NPJ Prim. Care Respir. Med. 2020, 30, 52. [Google Scholar] [CrossRef]
- Tsutsui, M.; Gerayeli, F.; Sin, D.D. Pulmonary rehabilitation in a post-COVID-19 world: Telerehabilitation as a new standard in patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 379–391. [Google Scholar] [CrossRef]
- Mizuno, S.; Wakabayashi, H.; Wada, F. Rehabilitation nutrition for individuals with frailty, disability, sarcopenic dysphagia, or sarcopenic respiratory disability. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 29–36. [Google Scholar] [CrossRef]
- Damanti, S.; Azzolino, D.; Roncaglione, C.; Arosio, B.; Rossi, P.; Cesari, M. Efficacy of Nutritional Interventions as stand-alone or synergistic treatments with exercise for the management of sarcopenia. Nutrients 2019, 11, 1991. [Google Scholar] [CrossRef] [Green Version]
- Walawska-Hrycek, A.; Galus, W.; Hrycek, E.; Kaczmarczyk, A.; Krzystanek, E. The impact of vitamin D low doses on its serum level and cytokine profile in multiple sclerosis patients. J. Clin. Med. 2021, 10, 2781. [Google Scholar] [CrossRef]
- Behm, C.; Blufstein, A.; Gahn, J.; Kubin, B.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Pleiotropic effects of vitamin D-3 on CD4(+) T lymphocytes mediated by human periodontal ligament cells and inflammatory environment. J. Clin. Periodontol. 2020, 47, 689–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokturk, N.; Baha, A.; Oh, Y.-M.; Young Ju, J.; Jones, P.W. Vitamin D deficiency: What does it mean for chronic obstructive pulmonary disease (COPD)? A compherensive review for pulmonologists. Clin. Respir. J. 2018, 12, 382–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssens, W.; Lehouck, A.; Carremans, C.; Bouillon, R.; Mathieu, C.; Decramer, M. Vitamin D beyond bones in chronic obstructive pulmonary disease: Time to act. Am. J. Respir. Crit. Care Med. 2009, 179, 630–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herr, C.; Greulich, T.; Koczulla, R.A.; Meyer, S.; Zakharkina, T.; Branscheidt, M.; Eschmann, R.; Bals, R. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir. Res. 2011, 12, 31. [Google Scholar] [CrossRef] [Green Version]
- Vieth, R. What is the optimal vitamin D status for health? Prog. Biophys. Mol. Biol. 2006, 92, 26–32. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Mithal, A.; Bonjour, J.P.; Boonen, S.; Burckhardt, P.; Fuleihan, G.E.; Josse, R.G.; Lips, P.; Morales-Torres, J.; Yoshimura, N. IOF position statement: Vitamin D recommendations for older adults. Osteoporos. Int. 2010, 21, 1151–1154. [Google Scholar] [CrossRef] [Green Version]
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J. Am. Geriatr. Soc. 2014, 62, 147–152. [Google Scholar] [CrossRef]
- Jackson, R.D.; LaCroix, A.Z.; Gass, M.; Wallace, R.B.; Robbins, J.; Lewis, C.E.; Bassford, T.; Beresford, S.A.; Black, H.R.; Blanchette, P.; et al. Calcium plus vitamin D supplementation and the risk of fractures. N. Engl. J. Med. 2006, 354, 669–683. [Google Scholar] [CrossRef]
- Daly, R.M.; Brown, M.; Bass, S.; Kukuljan, S.; Nowson, C. Calcium- and vitamin D3-fortified milk reduces bone loss at clinically relevant skeletal sites in older men: A 2-year randomized controlled trial. J. Bone Miner. Res. 2006, 21, 397–405. [Google Scholar] [CrossRef]
- Meier, C.; Woitge, H.W.; Witte, K.; Lemmer, B.; Seibel, M.J. Supplementation with oral vitamin D3 and calcium during winter prevents seasonal bone loss: A randomized controlled open-label prospective trial. J. Bone Miner. Res. 2004, 19, 1221–1230. [Google Scholar] [CrossRef]
- Zhu, K.; Bruce, D.; Austin, N.; Devine, A.; Ebeling, P.R.; Prince, R.L. Randomized controlled trial of the effects of calcium with or without vitamin D on bone structure and bone-related chemistry in elderly women with vitamin D insufficiency. J. Bone Miner. Res. 2008, 23, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- van Bakel, S.I.J.; Gosker, H.R.; Langen, R.C.; Schols, A. Towards personalized management of sarcopenia in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, R.; Prins, H.J.; Boersma, W.G.; Daniels, J.M.; den Heijer, M.; Lips, P.; de Jongh, R.T. Effects of daily vitamin D supplementation on respiratory muscle strength and physical performance in vitamin D-deficient COPD patients: A pilot trial. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2583–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjerk, S.M.; Edgington, B.D.; Rector, T.S.; Kunisaki, K.M. Supplemental vitamin D and physical performance in COPD: A pilot randomized trial. Int. J. Chronic Obstr. Pulm. Dis. 2013, 8, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yumrutepe, T.; Aytemur, Z.A.; Baysal, O.; Taskapan, H.; Taskapan, C.M.; Hacievliyagil, S.S. Relationship between vitamin D and lung function, physical performance and balance on patients with stage I-III chronic obstructive pulmonary disease. Rev. Assoc. Med. Bras. 2015, 61, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Calafiore, D.; Fortunato, L.; Migliario, M. Vitamin D for clinical diseases in women: An indispensable factor in medicine and dentistry. J. Clin. Med. 2022, 11, 3104. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.T.; Watts, N.B.; Genant, H.K.; McKeever, C.D.; Hangartner, T.; Keller, M.; Chesnut, C.H., 3rd; Brown, J.; Eriksen, E.F.; Hoseyni, M.S.; et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: A randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) study group. JAMA 1999, 282, 1344–1352. [Google Scholar] [CrossRef] [Green Version]
- Fujieda, Y.; Horita, T.; Nishimoto, N.; Tanimura, K.; Amasaki, Y.; Kasahara, H.; Furukawa, S.; Takeda, T.; Fukaya, S.; Matsui, K.; et al. Efficacy and safety of sodium RISedronate for glucocorticoid-induced OsTeoporosis with rheumaTOid arthritis (RISOTTO study): A multicentre, double-blind, randomized, placebo-controlled trial. Mod. Rheumatol. 2021, 31, 593–599. [Google Scholar] [CrossRef]
- Mellström, D.D.; Sörensen, O.H.; Goemaere, S.; Roux, C.; Johnson, T.D.; Chines, A.A. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif. Tissue Int. 2004, 75, 462–468. [Google Scholar] [CrossRef]
- Saag, K.G.; Zanchetta, J.R.; Devogelaer, J.P.; Adler, R.A.; Eastell, R.; See, K.; Krege, J.H.; Krohn, K.; Warner, M.R. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: Thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009, 60, 3346–3355. [Google Scholar] [CrossRef]
- Saag, K.G.; Shane, E.; Boonen, S.; Marín, F.; Donley, D.W.; Taylor, K.A.; Dalsky, G.P.; Marcus, R. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N. Engl. J. Med. 2007, 357, 2028–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langdahl, B.L.; Marin, F.; Shane, E.; Dobnig, H.; Zanchetta, J.R.; Maricic, M.; Krohn, K.; See, K.; Warner, M.R. Teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: An analysis by gender and menopausal status. Osteoporos. Int. 2009, 20, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Al Mheid, I.; Quyyumi, A.A. Vitamin D and cardiovascular disease: Controversy unresolved. J. Am. Coll. Cardiol. 2017, 70, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Vitamin D deficiency and diabetes. Biochem. J. 2017, 474, 1321–1332. [Google Scholar] [CrossRef]
- Altieri, B.; Muscogiuri, G.; Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G.; et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Bertoglio, P.; Ricciardi, S.; Alì, G.; Aprile, V.; Korasidis, S.; Palmiero, G.; Fontanini, G.; Mussi, A.; Lucchi, M. N2 lung cancer is not all the same: An analysis of different prognostic groups. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 720–726. [Google Scholar] [CrossRef] [Green Version]
- Lanham-New, S.A.; Webb, A.R.; Cashman, K.D.; Buttriss, J.L.; Fallowfield, J.L.; Masud, T.; Hewison, M.; Mathers, J.C.; Kiely, M.; Welch, A.A.; et al. Vitamin D and SARS-CoV-2 virus/COVID-19 disease. BMJ Nutr. Prev. Health 2020, 3, 106–110. [Google Scholar] [CrossRef]
- Greiller, C.L.; Martineau, A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef] [Green Version]
- Jolliffe, D.A.; Griffiths, C.J.; Martineau, A.R. Vitamin D in the prevention of acute respiratory infection: Systematic review of clinical studies. J. Steroid Biochem. Mol. Biol. 2013, 136, 321–329. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Camargo, C.A.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Amp. Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Dávalos-Yerovi, V.; Marco, E.; Sánchez-Rodríguez, D.; Duran, X.; Meza-Valderrama, D.; Rodríguez, D.A.; Muñoz, E.; Tejero-Sánchez, M.; Muns, M.D.; Guillén-Solà, A.; et al. Malnutrition according to GLIM criteria is associated with mortality and hospitalizations in rehabilitation patients with stable chronic obstructive pulmonary disease. Nutrients 2021, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Rawal, G.; Yadav, S. Nutrition in chronic obstructive pulmonary disease: A review. J. Transl. Intern. Med. 2015, 3, 151–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caffarelli, C.; Alessi, C.; Nuti, R.; Gonnelli, S. Divergent effects of obesity on fragility fractures. Clin. Interv. Aging 2014, 9, 1629–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, P.F.; Elia, M.; Stratton, R.J. Nutritional support and functional capacity in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Respirology 2013, 18, 616–629. [Google Scholar] [CrossRef]
- Kerstetter, J.E.; Kenny, A.M.; Insogna, K.L. Dietary protein and skeletal health: A review of recent human research. Curr. Opin. Lipidol. 2011, 22, 16–20. [Google Scholar] [CrossRef]
- Bikle, D.D.; Tahimic, C.; Chang, W.; Wang, Y.; Philippou, A.; Barton, E.R. Role of IGF-I signaling in muscle bone interactions. Bone 2015, 80, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Sun, S.; Guo, B.; Miao, B.; Luo, Z.; Xia, Z.; Ying, D.; Liu, F.; Guo, B.; Tang, J.; et al. Bioactive peptide isolated from casein phosphopeptides promotes calcium uptake in vitro and in vivo. Food Funct. 2018, 9, 2251–2260. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Harris, S.S.; Rasmussen, H.M.; Dallal, G.E. Comparative effects of oral aromatic and branched-chain amino acids on urine calcium excretion in humans. Osteoporos. Int. 2007, 18, 955–961. [Google Scholar] [CrossRef]
- Lippi, L.; Uberti, F.; Folli, A.; Turco, A.; Curci, C.; d’Abrosca, F.; de Sire, A.; Invernizzi, M. Impact of nutraceuticals and dietary supplements on mitochondria modifications in healthy aging: A systematic review of randomized controlled trials. Aging Clin. Exp. Res. 2022, 3. [Google Scholar] [CrossRef]
- Aldhahir, A.M.; Rajeh, A.M.A.; Aldabayan, Y.S.; Drammeh, S.; Subbu, V.; Alqahtani, J.S.; Hurst, J.R.; Mandal, S. Nutritional supplementation during pulmonary rehabilitation in COPD: A systematic review. Chronic Respir. Dis. 2020, 17, 147997312090495. [Google Scholar] [CrossRef] [Green Version]
- de Sire, A.; de Sire, R.; Curci, C.; Castiglione, F.; Wahli, W. Role of dietary supplements and probiotics in modulating microbiota and bone health: The gut-bone axis. Cells 2022, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut microbiota and obesity: A role for probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Shi, G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Transl. Med. 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, L.; Cheng, Q.; Wang, Y.; Mu, H.; Zhang, Y. COPD and gut-lung axis: How microbiota and host inflammasome influence COPD and related therapeutics. Front. Microbiol. 2022, 13, 868086. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The gut microbiota and inflammation: An overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Yang, R.; Xu, X.; Zhou, X. The microbiota-gut-bone axis and bone health. J. Leukoc. Biol. 2021, 110, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, P. Smoking cessation and COPD. Eur. Respir. Rev. 2013, 22, 37–43. [Google Scholar] [CrossRef]
- Vestergaard, P.; Mosekilde, L. Fracture risk associated with smoking: A meta-analysis. J. Intern. Med. 2003, 254, 572–583. [Google Scholar] [CrossRef]
- Stead, L.F.; Carroll, A.J.; Lancaster, T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst. Rev. 2017, 3, Cd001007. [Google Scholar] [CrossRef]
- Santos, L.; Elliott-Sale, K.J.; Sale, C. Exercise and bone health across the lifespan. Biogerontology 2017, 18, 931–946. [Google Scholar] [CrossRef] [Green Version]
- Lippi, L.; de Sire, A.; Mezian, K.; Curci, C.; Perrero, L.; Turco, A.; Andaloro, S.; Ammendolia, A.; Fusco, N.; Invernizzi, M. Impact of exercise training on muscle mitochondria modifications in older adults: A systematic review of randomized controlled trials. Aging Clin. Exp. Res. 2022, 34, 1495–1510. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; Cisari, C. Sarcopenia and muscular modifications in disabling pathologies of the elderly from the physical and rehabilitation medicine: Point of view. Eur. J. Phys. Rehabil. Med. 2013, 49, 107–109. [Google Scholar] [PubMed]
- Invernizzi, M.; Cisari, C.; Carda, S. The potential impact of new effervescent alendronate formulation on compliance and persistence in osteoporosis treatment. Aging Clin. Exp. Res. 2015, 27, 107–113. [Google Scholar] [CrossRef]
- Geusens, P.; Bevers, M.S.; van Rietbergen, B.; Messina, O.D.; Lespessailles, E.; Oliveri, B.; Chapurlat, R.; Engelke, K.; Chines, A.; Huang, S.; et al. Effect of denosumab compared with risedronate on bone strength in patients initiating or continuing glucocorticoid treatment. J. Bone Miner. Res. 2022, 37, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, Y.; Nozaki, Y.; Inoue, A.; Li, J.; Shiga, T.; Kishimoto, K.; Sugiyama, M.; Kinoshita, K.; Funauchi, M.; Matsumura, I. Effects of denosumab versus teriparatide in glucocorticoid-induced osteoporosis patients with prior bisphosphonate treatment. Bone Rep. 2020, 13, 100293. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Morita, T.; Kumanogoh, A. The therapeutic efficacy of denosumab for the loss of bone mineral density in glucocorticoid-induced osteoporosis: A meta-analysis. Rheumatol. Adv. Pract. 2020, 4, rkaa008. [Google Scholar] [CrossRef]
- Nuti, R.; Brandi, M.L.; Checchia, G.; Di Munno, O.; Dominguez, L.; Falaschi, P.; Fiore, C.E.; Iolascon, G.; Maggi, S.; Michieli, R.; et al. Guidelines for the management of osteoporosis and fragility fractures. Intern. Emerg. Med. 2019, 14, 85–102. [Google Scholar] [CrossRef] [Green Version]
- Scala, R.; Maqoud, F.; Antonacci, M.; Dibenedetto, J.R.; Perrone, M.G.; Scilimati, A.; Castillo, K.; Latorre, R.; Conte, D.; Bendahhou, S.; et al. Bisphosphonates Targeting Ion Channels and Musculoskeletal Effects. Front Pharmacol. 2022, 13, 837534. [Google Scholar] [CrossRef]
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Li, S.S.; He, S.H.; Xie, P.Y.; Li, W.; Zhang, X.X.; Li, T.F.; Li, D.F. Recent Progresses in the Treatment of Osteoporosis. Front. Pharmacol. 2021, 12, 717065. [Google Scholar] [CrossRef]
- Gregson, C.L.; Armstrong, D.J.; Bowden, J.; Cooper, C.; Edwards, J.; Gittoes, N.J.L.; Harvey, N.; Kanis, J.; Leyland, S.; Low, R.; et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2022, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, I.; Falchetti, A.; Merlotti, D.; Eller Vainicher, C.; Gennari, L. Updates in epidemiology, pathophysiology and management strategies of glucocorticoid-induced osteoporosis. Expert Rev. Endocrinol. Metab. 2020, 15, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Cummings, S.R.; Karpf, D.B.; Cauley, J.A.; Thompson, D.E.; Nevitt, M.C.; Bauer, D.C.; Genant, H.K.; Haskell, W.L.; Marcus, R.; et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996, 348, 1535–1541. [Google Scholar] [CrossRef]
- Smith, B.J.; Laslett, L.L.; Pile, K.D.; Phillips, P.J.; Phillipov, G.; Evans, S.M.; Esterman, A.J.; Berry, J.G. Randomized controlled trial of alendronate in airways disease and low bone mineral density. Chron. Respir. Dis. 2004, 1, 131–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesnut, C.H., 3rd; Skag, A.; Christiansen, C.; Recker, R.; Stakkestad, J.A.; Hoiseth, A.; Felsenberg, D.; Huss, H.; Gilbride, J.; Schimmer, R.C.; et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J. Bone Miner. Res. 2004, 19, 1241–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisman, J.A.; Civitelli, R.; Adami, S.; Czerwinski, E.; Recknor, C.; Prince, R.; Reginster, J.Y.; Zaidi, M.; Felsenberg, D.; Hughes, C.; et al. Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J. Rheumatol. 2008, 35, 488–497. [Google Scholar]
- Reginster, J.Y.; Adami, S.; Lakatos, P.; Greenwald, M.; Stepan, J.J.; Silverman, S.L.; Christiansen, C.; Rowell, L.; Mairon, N.; Bonvoisin, B.; et al. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann. Rheum. Dis. 2006, 65, 654–661. [Google Scholar] [CrossRef] [Green Version]
- Martins, L.H.I.; Ferreira, D.C.; Silva, M.T.; Motta, R.H.L.; Franquez, R.T.; Bergamaschi, C.C. Frequency of osteonecrosis in bisphosphonate users submitted to dental procedures: A systematic review. Oral Dis. 2021. [Google Scholar] [CrossRef]
- Beth-Tasdogan, N.H.; Mayer, B.; Hussein, H.; Zolk, O.; Peter, J.U. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst. Rev. 2022, 7, CD012432. [Google Scholar] [CrossRef]
- Ferrillo, M.; Migliario, M.; Marotta, N.; Lippi, L.; Antonelli, A.; Calafiore, D.; Ammendolia, V.; Fortunato, L.; Renò, F.; Giudice, A.; et al. Oral health in breast cancer women with vitamin D deficiency: A machine learning study. J. Clin. Med. 2022, 11, 4662. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Chen, Z.; Xie, X.; Gu, F.; Bi, S.; Yu, T. Effects of Bisphosphonates Treatments in Osteopenic Older Women: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 892091. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.H.; Kim, Y.; Kim, K.; Oh, Y.M.; Yoo, K.H.; Rhee, C.K.; Yoon, H.K.; Kim, Y.S.; Park, Y.B.; et al. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: A national cross-sectional cohort study. BMC Pulm. Med. 2013, 13, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Ma, X.; Wang, T.; Zhai, S. Comparative efficacy of bisphosphonates in short-term fracture prevention for primary osteoporosis: A systematic review with network meta-analyses. Osteoporos. Int. 2016, 27, 3289–3300. [Google Scholar] [CrossRef] [PubMed]
- Compston, J. Practical guidance for the use of bisphosphonates in osteoporosis. Bone 2020, 136, 115330. [Google Scholar] [CrossRef] [PubMed]
- Lekamwasam, S.; Adachi, J.D.; Agnusdei, D.; Bilezikian, J.; Boonen, S.; Borgström, F.; Cooper, C.; Perez, A.D.; Eastell, R.; Hofbauer, L.C.; et al. An appendix to the 2012 IOF-ECTS guidelines for the management of glucocorticoid-induced osteoporosis. Arch. Osteoporos. 2012, 7, 25–30. [Google Scholar] [CrossRef]
- Reid, D.M.; Devogelaer, J.P.; Saag, K.; Roux, C.; Lau, C.S.; Reginster, J.Y.; Papanastasiou, P.; Ferreira, A.; Hartl, F.; Fashola, T.; et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): A multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2009, 373, 1253–1263. [Google Scholar] [CrossRef]
- Black, D.M.; Delmas, P.D.; Eastell, R.; Reid, I.R.; Boonen, S.; Cauley, J.A.; Cosman, F.; Lakatos, P.; Leung, P.C.; Man, Z.; et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N. Engl. J. Med. 2007, 356, 1809–1822. [Google Scholar] [CrossRef] [Green Version]
- Black, D.M.; Reid, I.R.; Boonen, S.; Bucci-Rechtweg, C.; Cauley, J.A.; Cosman, F.; Cummings, S.R.; Hue, T.F.; Lippuner, K.; Lakatos, P.; et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: A randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J. Bone Miner. Res. 2012, 27, 243–254. [Google Scholar] [CrossRef]
- Boonen, S.; Reginster, J.Y.; Kaufman, J.M.; Lippuner, K.; Zanchetta, J.; Langdahl, B.; Rizzoli, R.; Lipschitz, S.; Dimai, H.P.; Witvrouw, R.; et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N. Engl. J. Med. 2012, 367, 1714–1723. [Google Scholar] [CrossRef] [Green Version]
- Saag, K.G.; Wagman, R.B.; Geusens, P.; Adachi, J.D.; Messina, O.D.; Emkey, R.; Chapurlat, R.; Wang, A.; Pannacciulli, N.; Lems, W.F. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: A multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. 2018, 6, 445–454. [Google Scholar] [CrossRef]
- Li, P.; Wu, X.; Li, Y.; Huang, J. Denosumab Versus Bisphosphonates for the Prevention of the Vertebral Fractures in Men with Osteoporosis: An Updated Network Meta-Analysis. Clin Investig. Med. 2022, 45, E14–E22. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, S.; Francomano, D.; Romagnoli, E.; Marocco, C.; Fornari, R.; Resmini, G.; Buffa, A.; Di Pietro, G.; Corvaglia, S.; Gimigliano, F.; et al. Persistence with denosumab therapy in women affected by osteoporosis with fragility fractures: A multicenter observational real practice study in Italy. J. Endocrinol. Invest. 2017, 40, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Bone, H.G.; Bolognese, M.A.; Yuen, C.K.; Kendler, D.L.; Miller, P.D.; Yang, Y.C.; Grazette, L.; San Martin, J.; Gallagher, J.C. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J. Clin. Endocrinol. Metab. 2011, 96, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Zillikens, M.C.; Meier, C.; Body, J.J.; Gonzalez Rodriguez, E.; Anastasilakis, A.D.; Abrahamsen, B.; McCloskey, E.; Hofbauer, L.C.; Guañabens, N.; et al. Fracture risk and management of discontinuation of denosumab therapy: A systematic review and position statement by ECTS. J. Clin. Endocrinol. Metab. 2020, 106, 264–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Potty, A.; Vyas, P.; Lane, J. The role of recombinant PTH in human fracture healing: A systematic review. J. Orthop. Trauma 2014, 28, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.; Krege, J.H.; Marin, F.; Jin, L.; Stepan, J.J. Teriparatide for osteoporosis: Importance of the full course. Osteoporos. Int. 2016, 27, 2395–2410. [Google Scholar] [CrossRef] [Green Version]
- Díez-Pérez, A.; Marin, F.; Eriksen, E.F.; Kendler, D.L.; Krege, J.H.; Delgado-Rodríguez, M. Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: A systematic review and meta-analysis. Bone 2019, 120, 1–8. [Google Scholar] [CrossRef]
- Simpson, E.L.; Martyn-St James, M.; Hamilton, J.; Wong, R.; Gittoes, N.; Selby, P.; Davis, S. Clinical effectiveness of denosumab, raloxifene, romosozumab, and teriparatide for the prevention of osteoporotic fragility fractures: A systematic review and network meta-analysis. Bone 2020, 130, 115081. [Google Scholar] [CrossRef]
- Saag, K.G.; Agnusdei, D.; Hans, D.; Kohlmeier, L.A.; Krohn, K.D.; Leib, E.S.; MacLaughlin, E.J.; Alam, J.; Simonelli, C.; Taylor, K.A.; et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016, 68, 2122–2128. [Google Scholar] [CrossRef] [Green Version]
- Graat-Verboom, L.; Spruit, M.A.; van den Borne, B.E.; Smeenk, F.W.; Martens, E.J.; Lunde, R.; Wouters, E.F. Correlates of osteoporosis in chronic obstructive pulmonary disease: An underestimated systemic component. Respir. Med. 2009, 103, 1143–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinonapoli, G.; Ruggiero, C.; Meccariello, L.; Bisaccia, M.; Ceccarini, P.; Caraffa, A. Osteoporosis in men: A review of an underestimated bone condition. Int. J. Mol. Sci. 2021, 22, 2105. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, D.; Bon, J. CT imaging and comorbidities in COPD: Beyond lung cancer screening. Chest 2021, 159, 147–153. [Google Scholar] [CrossRef]
- Sepúlveda-Loyola, W.; Rodríguez-Sánchez, I.; Pérez-Rodríguez, P.; Ganz, F.; Torralba, R.; Oliveira, D.V.; Rodríguez-Mañas, L. Impact of social isolation due to COVID-19 on health in older people: Mental and physical effects and recommendations. J. Nutr. Health Aging 2020, 24, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, N.; Buta, B.; Xue, Q.-L.; Mohess, D.T.; Bushan, A.; Tran, H.; Batchelor, W.; Defilippi, C.R.; Walston, J.D.; Bandeen-Roche, K.; et al. Interventions for frailty among older adults with cardiovascular disease. J. Am. Coll. Cardiol. 2022, 79, 482–503. [Google Scholar] [CrossRef] [PubMed]
- Bernetti, A.; Farì, G.; Mangone, M.; Fiore, P.; Santilli, V.; Paoloni, M.; Agostini, F. Medical management of osteoarthritis during the COVID-19 pandemic: A challenge for the present and the future. Ann. Ig. 2022, 34, 184–189. [Google Scholar] [CrossRef]
- Md Fadzil, N.H.; Shahar, S.; Rajikan, R.; Singh, D.K.A.; Mat Ludin, A.F.; Subramaniam, P.; Ibrahim, N.; Vanoh, D.; Mohamad Ali, N. A scoping review for usage of telerehabilitation among older adults with mild cognitive impairment or cognitive frailty. Int. J. Environ. Res. Public Health 2022, 19, 4000. [Google Scholar] [CrossRef]
- Marotta, N.; Demeco, A.; Moggio, L.; Ammendolia, A. Why is telerehabilitation necessary? A pre-post COVID-19 comparative study of ICF activity and participation. J. Enabling Technol. 2021, 15, 117–121. [Google Scholar] [CrossRef]
- de Sire, A.; Marotta, N.; Agostini, F.; Drago Ferrante, V.; Demeco, A.; Ferrillo, M.; Inzitari, M.T.; Pellegrino, R.; Russo, I.; Ozyemisci Taskiran, O.; et al. A telerehabilitation approach to chronic facial paralysis in the COVID-19 pandemic scenario: What role for electromyography assessment? J. Pers. Med. 2022, 12, 497. [Google Scholar] [CrossRef]
- Stickland, M.K.; Jourdain, T.; Wong, E.Y.; Rodgers, W.M.; Jendzjowsky, N.G.; Macdonald, G.F. Using telehealth technology to deliver pulmonary rehabilitation to patients with chronic obstructive pulmonary disease. Can. Respir. J. 2011, 18, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Russo, J.E.; McCool, R.R.; Davies, L. VA telemedicine: An analysis of cost and time savings. Telemed. J. E Health 2016, 22, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadi, K.; Jacobs, P.; Sin, D.D. Economic evaluation of a community-based pulmonary rehabilitation program for chronic obstructive pulmonary disease. Lung 2004, 182, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, K.A.; Stefan, M.S.; Priya, A.; Pack, Q.R.; Pekow, P.S.; Lagu, T.; Pinto-Plata, V.M.; ZuWallack, R.L.; Lindenauer, P.K. Participation in pulmonary rehabilitation after hospitalization for chronic obstructive pulmonary disease among medicare beneficiaries. Ann. Am. Thorac. Soc. 2019, 16, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Stafinski, T.; Nagase, F.I.; Avdagovska, M.; Stickland, M.K.; Menon, D. Effectiveness of home-based pulmonary rehabilitation programs for patients with Chronic Obstructive Pulmonary Disease (COPD): Systematic review. BMC Health Serv. Res. 2022, 22, 557. [Google Scholar] [CrossRef]
- Mendes Xavier, D.; Lanza Galvão, E.; Aliane Fonseca, A.; de Souza, G.M.; Pereira Lima, V. Effects of home-based pulmonary rehabilitation on dyspnea, exercise capacity, quality of life and impact of the disease in COPD patients: A systematic review. COPD 2022, 19, 18–46. [Google Scholar] [CrossRef]
- Michaelchuk, W.; Oliveira, A.; Marzolini, S.; Nonoyama, M.; Maybank, A.; Goldstein, R.; Brooks, D. Design and delivery of home-based telehealth pulmonary rehabilitation programs in COPD: A systematic review and meta-analysis. Int. J. Med. Inform. 2022, 162, 104754. [Google Scholar] [CrossRef]
- Rutkowski, S.; Rutkowska, A.; Kiper, P.; Jastrzebski, D.; Racheniuk, H.; Turolla, A.; Szczegielniak, J.; Casaburi, R. Virtual Reality Rehabilitation in patients with chronic obstructive pulmonary disease: A randomized controlled trial. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Almathami, H.K.Y.; Win, K.T.; Vlahu-Gjorgievska, E. Barriers and facilitators that influence telemedicine-based, real-time, online consultation at patients’ homes: Systematic literature review. J. Med. Internet Res. 2020, 22, e16407. [Google Scholar] [CrossRef]
- Gajarawala, S.N.; Pelkowski, J.N. Telehealth benefits and barriers. J. Nurse Pract. 2021, 17, 218–221. [Google Scholar] [CrossRef]
- Elbaz, S.; Cinalioglu, K.; Sekhon, K.; Gruber, J.; Rigas, C.; Bodenstein, K.; Naghi, K.; Lavin, P.; Greenway, K.T.; Vahia, I.; et al. A systematic review of telemedicine for older adults with dementia during COVID-19: An alternative to in-person health services? Front. Neurol. 2021, 12, 761965. [Google Scholar] [CrossRef]
- Doraiswamy, S.; Jithesh, A.; Mamtani, R.; Abraham, A.; Cheema, S. Telehealth use in geriatrics care during the COVID-19 pandemic—A scoping review and evidence synthesis. Int. J. Environ. Res. Public Health 2021, 18, 1755. [Google Scholar] [CrossRef] [PubMed]
- Gani, L.; Tan, F.; King, T. Telecarers improve osteoporosis treatment and compliance rates in secondary osteoporosis prevention for elderly hip fracture patients. Singap. Med. J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ambrens, M.; Alley, S.; Oliveira, J.S.; To, Q.; Delbaere, K.; Vandelanotte, C.; Tiedemann, A. Effect of eHealth-delivered exercise programmes on balance in people aged 65 years and over living in the community: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2022, 12, e051377. [Google Scholar] [CrossRef] [PubMed]

| Area | Interventions | Goals |
|---|---|---|
| Rehabilitation | ||
| Pulmonary rehabilitation | Educational interventions, airway clearance techniques, inspiratory muscle training, action plans for frequent exacerbations, endurance training, and strength training | Relieving dyspnea, reduce risk of exacerbation and hospitalization, and reduce need for medications |
| Exercise program | Weight bearing, progressive resistance exercise, strength training, balance training, tai-chi, endurance training, interval training | Reconditioning skeletal muscle, preventing muscle loss, and improving both muscle mass and strength |
| Lifestyle interventions | Patient and caregiver education, lifestyle counseling and support, smoke cessation | Increasing awareness about unhealthy behavior and improving symptoms through lifestyle changes |
| Sustainable programs | Telemedicine, home-based rehabilitation, telerehabilitation | Increasing adherence, improving PR spreading, and reducing sanitary costs |
| Dietary supplements | ||
| Vitamin D | Oral Supplementation | Increasing bone health, skeletal muscle function and physical performance, positive stimulation of immune response |
| Calcium | Oral Supplementation | Increasing bone health, skeletal muscle function, and physical performance |
| Proteins | Oral Supplementation | Promoting weight gain, improving body composition, and physical performance. Increasing bone health and skeletal muscle mass |
| Aminoacids | Oral Supplementation | Promoting weight gain, improving body composition, and physical performance. Increasing bone health and skeletal muscle mass |
| Probiotics | Oral Supplementation | Promoting anti-inflammatory action and modulating immune systems. Promoting bone health |
| Prebiotics | Oral Supplementation | Promoting anti-inflammatory action and modulating immune systems. Promoting bone health |
| Pharmacological treatment | ||
| Oral bisphosphonate | Alendronate (70 mg/week or 10 mg/day) Risedronate (35 mg/week or 5 mg/day) Ibandronate (150 mg/month) | Inhibiting bone resorption, reducing fracture risk |
| Intravenous bisphosphonate | Zoledronate (5 mg/year) | Inhibiting bone resorption, reducing fracture risk |
| Denosumab | Subcutaneous injection (60 mg/6 months) | Inhibiting bone resorption, reducing fracture risk |
| Teriparatide | Subcutaneous injection (20 mg/day for 24 months) | Promoting skeletal anabolism (with effect on BMD and bone quality), reduce fracture risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sire, A.; Lippi, L.; Aprile, V.; Calafiore, D.; Folli, A.; D’Abrosca, F.; Moalli, S.; Lucchi, M.; Ammendolia, A.; Invernizzi, M. Pharmacological, Nutritional, and Rehabilitative Interventions to Improve the Complex Management of Osteoporosis in Patients with Chronic Obstructive Pulmonary Disease: A Narrative Review. J. Pers. Med. 2022, 12, 1626. https://doi.org/10.3390/jpm12101626
de Sire A, Lippi L, Aprile V, Calafiore D, Folli A, D’Abrosca F, Moalli S, Lucchi M, Ammendolia A, Invernizzi M. Pharmacological, Nutritional, and Rehabilitative Interventions to Improve the Complex Management of Osteoporosis in Patients with Chronic Obstructive Pulmonary Disease: A Narrative Review. Journal of Personalized Medicine. 2022; 12(10):1626. https://doi.org/10.3390/jpm12101626
Chicago/Turabian Stylede Sire, Alessandro, Lorenzo Lippi, Vittorio Aprile, Dario Calafiore, Arianna Folli, Francesco D’Abrosca, Stefano Moalli, Marco Lucchi, Antonio Ammendolia, and Marco Invernizzi. 2022. "Pharmacological, Nutritional, and Rehabilitative Interventions to Improve the Complex Management of Osteoporosis in Patients with Chronic Obstructive Pulmonary Disease: A Narrative Review" Journal of Personalized Medicine 12, no. 10: 1626. https://doi.org/10.3390/jpm12101626
APA Stylede Sire, A., Lippi, L., Aprile, V., Calafiore, D., Folli, A., D’Abrosca, F., Moalli, S., Lucchi, M., Ammendolia, A., & Invernizzi, M. (2022). Pharmacological, Nutritional, and Rehabilitative Interventions to Improve the Complex Management of Osteoporosis in Patients with Chronic Obstructive Pulmonary Disease: A Narrative Review. Journal of Personalized Medicine, 12(10), 1626. https://doi.org/10.3390/jpm12101626










