Weight Recidivism and Dumping Syndrome after Roux-En-Y Gastric Bypass: Exploring the Therapeutic Role of Transoral Outlet Reduction
Abstract
:1. Introduction
2. TORe for Weight Regain
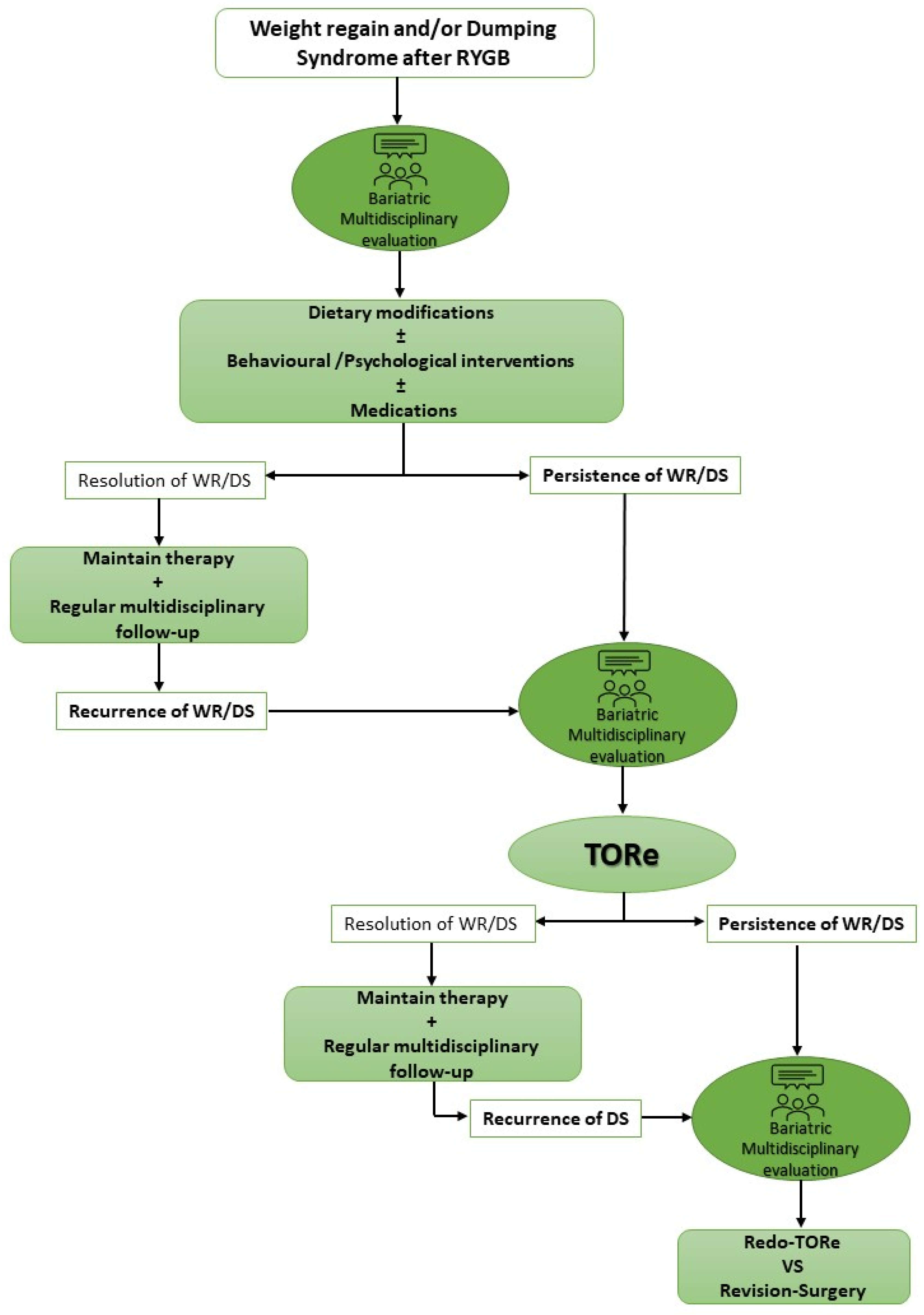
3. TORe for Dumping Syndrome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight Factsheet, 2018. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 December 2018).
- Cambi, M.P.C.; Baretta, G.A.P.; De Oliveira Magro, D.; Boguszewski, C.L.; Ribeiro, I.B.; Jirapinyo, P.; de Moura, D.T.H. Multidisciplinary Approach for Weight Regain—how to Manage this Challenging Condition: An Expert Review. Obes. Surg. 2021, 31, 1290–1303. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Stoll, C.R.T.; Song, J.; Varela, J.E.; Eagon, C.J.; Colditz, G.A. The Effectiveness and Risks of Bariatric Surgery: An Updated Systematic Review and Meta-analysis, 2003-2012. JAMA 2014, 149, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjöström, L.; Lindroos, A.-K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjöström, C.D.; et al. Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004, 23, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.D.; Davidson, L.E.; Litwin, S.E. Weight and metabolic outcomes 12 years after gastric bypass. N. Engl. J. Med. 2018, 378, 93–96. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, E.J.; Pate, V.; Warthen, M.; Winegar, D.A. Baseline data from American Society for Metabolic and Bariatric Surgery-designated Bariatric Surgery Centers of Excellence using the Bariatric Outcomes Longitudinal Database. Surg. Obes. Relat. Dis. 2010, 6, 347–355. [Google Scholar] [CrossRef]
- Han, Y.; Jia, Y.; Wang, H.; Cao, L.; Zhao, Y. Comparative analysis of weight loss and resolution of comorbidities between laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass: A systematic review and meta-analysis based on 18 studies. Int. J. Surg. 2020, 76, 101–110. [Google Scholar] [CrossRef]
- Christou, N.V.; Look, D.; Maclean, L.D. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann. Surg. 2006, 244, 734–740. [Google Scholar] [CrossRef]
- Magro, D.O.; Geloneze, B.; Delfini, R.; Pareja, B.C.; Callejas, F.; Pareja, J.C. Long-term weight regain after gastric bypass: A 5-year prospective study. Obes. Surg. 2008, 18, 648–651. [Google Scholar] [CrossRef]
- Scarpellini, E.; Arts, J.; Karamanolis, G.; Laurenius, A.; Siquini, W.; Suzuki, H.; Ukleja, A.; Van Beek, A.; Vanuytsel, T.; Bor, S.; et al. International consensus on the diagnosis and management of dumping syndrome. Nat. Rev. Endocrinol. 2020, 16, 448–466. [Google Scholar] [CrossRef]
- Velapati, S.R.; Shah, M.; Kuchkuntla, A.R.; Abu-Dayyeh, B.; Grothe, K.; Hurt, R.T.; Mundi, M.S. Weight Regain After Bariatric Surgery: Prevalence, Etiology, and Treatment. Curr. Nutr. Rep. 2018, 7, 329–334. [Google Scholar] [CrossRef]
- Westerveld, D.; Yang, D. Through Thick and Thin: Identifying Barriers to Bariatric Surgery, Weight Loss Maintenance, and Tailoring Obesity Treatment for the Future. Surg. Res. Prac. 2016, 2016, 8616581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emous, M.; Wolffenbuttel, B.H.R.; Totté, E.; van Beek, A.P. The short- to mid-term symptom prevalence of dumping syndrome after primary gastric-bypass surgery and its impact on health-related quality of life. Surg. Obes. Relat. Dis. 2017, 13, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Ukleja, A. Dumping syndrome: Pathophysiology and treatment. Nutr. Clin. Pract. 2005, 20, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Arts, J.; Caenepeel, P.; De Wulf, D.; Bisschops, R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Arts, J.; Caenepeel, P.; Bisschops, R.; Dewulf, D.; Holvoet, L.; Piessevaux, H.; Bourgeois, S.; Sifrim, D.; Janssens, J.; Tack, J. Efficacy of the long-acting repeatable formulation of the somatostatin analogue octreotide in postoperative dumping. Clin. Gastroenterol. Hepatol. 2009, 7, 432–437. [Google Scholar] [CrossRef]
- Sigstad, H. A clinical diagnostic index in the diagnosis of the dumping syndrome. Changes in plasma volume and blood sugar after a test meal. Acta Med. Scand. 1970, 188, 479–486. [Google Scholar] [CrossRef]
- Andersen, J.R.; Holtug, K.; Uhrenholt, A. Trial of pectin-enriched muffins in patients with severe dumping syndrome after gastric resection. Observations on symptoms and gastric emptying pattern. Acta Chir. Scand. 1989, 155, 39–41. [Google Scholar]
- Harju, E.; Larmi, T.K. Efficacy of guar gum in preventing the dumping syndrome. J. Parenter. Enteral. Nutr. 1983, 7, 470–472. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Gassull, M.A.; Leeds, A.R.; Metz, G.; Dilawari, J.B.; Slavin, B.; Blendis, L.M. Effect of dietary fiber on complications of gastric surgery: Prevention of postprandial hypoglycemia by pectin. Gastroenterology 1977, 73, 215–217. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Bloom, S.R.; Albuquerque, R.H.; Leeds, A.R.; Sarson, D.L.; Metz, G.L.; Alberti, K.G. Pectin and complications after gastric surgery: Normalisation of postprandial glucose and endocrine responses. Gut 1980, 21, 574–579. [Google Scholar] [CrossRef] [Green Version]
- De Cunto, A.; Barbi, E.; Minen, F.; Ventura, A. Safety and efficacy of high-dose acarbose treatment for dumping syndrome. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Didden, P.; Penning, C.; Masclee, A.A. Octreotide therapy in dumping syndrome: Analysis of long-term results. Aliment. Pharmacol. Ther. 2006, 24, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Thompson, C.C. Transoral outlet reduction for weight regain after gastric bypass: Long-term follow-up. Gastrointest. Endosc. 2016, 83, 776–779. [Google Scholar] [CrossRef]
- Coakley, B.A.; Deveney, C.W.; Spight, D.H.; Thompson, S.K.; Le, D.; Jobe, B.A.; Wolfe, B.M.; McConnell, D.B.; O’Rourke, R.W. Revisional bariatric surgery for failed restrictive procedures. Surg. Obes. Relat. Dis. 2008, 4, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.M.; Ziemelis, M.; Paparodis, R.; Ahmed, M.; Davis, D.B. Laparoscopic reversal of Roux-en-Y gastric bypass: Technique and utility for treatment of endocrine complications. Surg. Obes. Relat. Dis. 2014, 10, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Lakdawala, M.; Limas, P.; Dhar, S.; Remedios, C.; Dhulla, N.; Sood, A.; Bhasker, A.G. Laparoscopic revision of Roux-en-Y gastric bypass to sleeve gastrectomy: A ray of hope for failed Roux-en-Y gastric bypass. Asian J. Endosc. Surg. 2016, 9, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, D.D.; Nwokeabia, I.D.; Purnell, S.; Zafar, S.N.; Ortega, G.; Hughes, K.; Fullum, T.M. Revision of Roux-En-Y Gastric Bypass for Weight Regain: A Systematic Review of Techniques and Outcomes. Obes. Surg. 2016, 26, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, B.S.; Saghir, S.M.; Naga, Y.; Dhaliwal, A.; Ramai, D.; Cross, C.; Singh, S.; Bhat, I.; Adler, D.G. Efficacy of transoral outlet reduction in Roux-en-Y gastric bypass patients to promote weight loss: A systematic review and meta-analysis. Endosc. Int. Open. 2020, 8, E1332–E1340. [Google Scholar] [CrossRef]
- Brunaldi, V.O.; Jirapinyo, P.; de Moura, D.T.H.; Okazaki, O.; Bernardo, W.M.; Galvão Neto, M.; Campos, J.M.; Santo, M.A.; de Moura, E.G.H. Endoscopic Treatment of Weight Regain Following Roux-en-Y Gastric Bypass: A Systematic Review and Meta-analysis. Obes. Surg. 2018, 28, 266–276. [Google Scholar] [CrossRef]
- Jirapinyo, P.; Slattery, J.; Ryan, M.B.; Abu Dayyeh, B.K.; Lautz, D.B.; Thompson, C.C. Evaluation of an endoscopic suturing device for transoral outlet reduction in patients with weight regain following Roux-en-Y gastric bypass. Endoscopy 2013, 45, 532–536. [Google Scholar] [CrossRef]
- Relly, R.; Mati, S.; Aviv, C.N.; Fishman, S. Endoscopic trans-oral outlet reduction after bariatric surgery is safe and effective for dumping syndrome. Surg. Endosc. 2021, 35, 6846–6852. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.J.; Bazerbachi, F.; Rizk, M.; Rustagi, T.; Acosta, A.; Wilson, E.B.; Wilson, T.; Neto, M.G.; Zundel, N.; Mundi, M.S.; et al. Transoral outlet reduction with full thickness endoscopic suturing for weight regain after gastric bypass: A large multicenter international experience and meta-analysis. Surg. Endosc. 2018, 32, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L.; et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jirapinyo, P.; Kumar, N.; AlSamman, M.A.; Thompson, C.C. Five-year outcomes of transoral outlet reduction for the treatment of weight regain after Roux-en-Y gastric bypass. Gastrointest. Endosc. 2020, 91, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Callahan, Z.M.; Su, B.; Kuchta, K.; Linn, J.; Carbray, J.; Ujiki, M. Five-year results of endoscopic gastrojejunostomy revision (transoral outlet reduction) for weight gain after gastric bypass. Surg. Endosc. 2020, 34, 2164–2171. [Google Scholar] [CrossRef]
- Abu Dayyeh, B.K.; Lautz, D.B.; Thompson, C.C. Gastrojejunal stoma diameter predicts weight regain after Roux-en-Y gastric bypass. Clin. Gastroenterol. Hepatol. 2011, 9, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Patel, L.Y.; Lapin, B.; Brown, C.S.; Stringer, T.; Gitelis, M.E.; Linn, J.G.; Denham, W.E.; Farwell, E.; Haggerty, S.; Ujiki, M.B. Outcomes following 50 consecutive endoscopic gastrojejunal revisions for weight gain following Roux-en-Y gastric bypass: A comparison of endoscopic suturing techniques for stoma reduction. Surg. Endosc. 2017, 31, 2667–2677. [Google Scholar] [CrossRef]
- Schulman, A.R.; Kumar, N.; Thompson, C.C. Transoral outlet reduction: A comparison of purse-string with interrupted stitch technique. Gastrointest. Endosc. 2018, 87, 1222–1228. [Google Scholar] [CrossRef]
- Hollenbach, M.; Selig, L.; Lellwitz, S.; Beer, S.; Feisthammel, J.; Rosendahl, J.; Schaumburg, T.; Mössner, J.; Hoffmeister, A. Endoscopic full-thickness transoral outlet reduction with semicircumferential endoscopic submucosal dissection. Endoscopy 2019, 51, 684–688. [Google Scholar] [CrossRef]
- Jirapinyo, P.; de Moura, D.T.H.; Thompson, C.C. Endoscopic submucosal dissection with suturing for the treatment of weight regain after gastric bypass: Outcomes and comparison with traditional transoral outlet reduction (with video). Gastrointest. Endosc. 2020, 91, 1282–1288. [Google Scholar] [CrossRef]
- Wong, S.K.H. Endoscopic full-thickness transoral outlet reduction with endoscopic submucosal dissection or argon plasma coagulation: Does it make a difference? Endoscopy 2019, 51, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Steffen, R.; Kessler, U.; Merki, H.; Zehetner, J. Endoscopic Gastrojejunal Revisions Following Gastric Bypass: Lessons Learned in More Than 100 Consecutive Patients. J. Gastrointest. Surg. 2019, 23, 58–66. [Google Scholar] [CrossRef]
- Stier, C.; Chiappetta, S. Endoluminal Revision (OverStitch (TM), Apollo Endosurgery) of the Dilated Gastroenterostomy in Patients with Late Dumping Syndrome After Proximal Roux-en-Y Gastric Bypass. Obes. Surg. 2016, 26, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Steffen, R.; Kessler, U.; Merki, H.; Zehetner, J. Short-term outcomes of endoscopic gastro-jejunal revisions for treatment of dumping syndrome after Roux-En-Y gastric bypass. Surg. Endosc. 2020, 34, 3626–3632. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.J.; Abu Dayyeh, B.K.; Storm, A.C.; Bazerbachi, F.; Matar, R.; Vella, A.; Kellogg, T.; Stier, C. Endoscopic management of dumping syndrome after Roux-en-Y gastric bypass: A large international series and proposed management strategy. Gastrointest. Endosc. 2020, 92, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Zaman, J.; Arellano, J.; Binetti, B.; Singh, T.P. Sa2011 Endoscopic gastrojejunostomy revision following Roux-en-Y gastric bypass: Outcomes at 2-year follow-up. Gastrointest. Endosc. 2017, 85, AB275. [Google Scholar] [CrossRef]
- Petchers, A.; Walker, A.; Bertram, C.; Feustel, P.; Singh, T.P.; Zaman, J. Evaluation of Endoscopic Gastrojejunostomy Revision Following Roux-en-Y Gastric Bypass for Treatment of Dumping Syndrome. Gastrointest. Endosc. 2022, 96, 639–644. [Google Scholar] [CrossRef]
- Cooper, T.C.; Simmons, E.B.; Webb, K.; Burns, J.L.; Kushner, R.F. Trends in Weight Regain Following Roux-en-Y Gastric Bypass (RYGB) Bariatric Surgery. Obes. Surg. 2015, 25, 1474–1481. [Google Scholar] [CrossRef]
- Athanasiadis, D.I.; Martin, A.; Kapsampelis, P.; Monfared, S.; Stefanidis, D. Factors associated with weight regain post-bariatric surgery: A systematic review. Surg. Endosc. 2021, 35, 4069–4084. [Google Scholar] [CrossRef]
- Ryou, M.; McQuaid, K.R.; Thompson, C.C.; Edmundowicz, S.; Mergener, K.; ASGE EndoVators Task Force. ASGE EndoVators Summit: Defining the role and value of endoscopic therapies in obesity management. Surg. Endosc. 2018, 32, 1–13. [Google Scholar] [CrossRef]
- Hui, C.; Dhakal, A.; Bauza, G.J. Dumping Syndrome. 2022 Jun 27. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bessler, M.; Daud, A.; DiGiorgi, M.F.; Inabnet, W.B.; Schrope, B.; Olivero-Rivera, L.; Davis, D. Adjustable gastric banding as revisional bariatric procedure after failed gastric bypass--intermediate results. Surg. Obes. Relat. 2010, 6, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Irani, K.; Youn, H.A.; Ren-Fielding, C.J.; Fielding, G.A.; Kurian, M. Midterm results for gastric banding as salvage procedure for patients with weight loss failure after Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2011, 7, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Chin, P.; Ali, M.; Francis, K.; Leport, P. Adjustable gastric band placed around gastric bypass pouch as revision operation for failed gastric bypass. Surg. Obes. Relat. Dis. 2009, 5, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, A.; Schneck, A.; Hébuterne, X.; Gugenheim, J. Gastric pouch resizing for Roux-en-Y gastric bypass failure in patients with a dilated pouch. Surg. Obes. Relat. Dis. 2013, 9, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, A.; Julien, C.; Brown, P.; Woods, I.; Hamdi, A.; Ortega, G.; Fullum, T.; Tran, D. Midterm outcomes of revisional surgery for gastric pouch and gastrojejunal anastomotic enlargement in patients with weight regain after gastric bypass for morbid obesity. Obes. Surg. 2014, 24, 1386–1390. [Google Scholar] [CrossRef]
- Rawlins, M.; Teel, D.; Hedgcorth, K.; Maguire, J. Revision of Roux-en Y gastric bypass to distal bypass for failed weight loss. Surg. Obes. Relat. Dis. 2011, 7, 45–49. [Google Scholar] [CrossRef]
- Srikanth, M.; Oh, K.; Fox, S. Revision to malabsorption Roux-en-Y gastric bypass (MRNYGBP) provides long-term (10 years) durable weight loss in patients with failed anatomically intact gastric restric tive operations. Obes. Surg. 2011, 21, 825–831. [Google Scholar] [CrossRef]
- Sugerman, H.; Kellum, J.; Demaria, E. Conversion of proximal to distal gastric bypass for failed gastric bypass for superobesity. J. Gastrointest. Surg. 1997, 1, 517–525. [Google Scholar] [CrossRef]
- Fobi, M.A.; Lee, H.; Igwe, D., Jr.; Felahy, B.; James, E.; Stanczyk, M.; Tambi, J.; Eyong, P. Revision of failed gastric bypass to distal Roux-en-Y gastric bypass: A review of 65 cases. Obes. Surg. 2001, 11, 190–195. [Google Scholar] [CrossRef]
- Parikh, M.; Pomp, A.; Gagner, M. Laparoscopic conversion of failed gastric bypass to duodenal switch: Technical considerations and preliminary outcomes. Surg. Obes. Relat. Dis. 2007, 3, 611–618. [Google Scholar] [CrossRef]
- Keshishian, A.; Zahriya, K.; Hartoonian, T.; Ayagian, C. Duodenal switch is a safe operation for patients who have failed other bariatric operations. Obes. Surg. 2004, 14, 1187–1192. [Google Scholar] [CrossRef] [PubMed]

| Study | N of Patients | Time RYGB–TORe (Years) | Pre-TORe Weight Parameters | Weight Regain after RYGB | Pre-TORe GJA Diameter (mm) | Post-TORe GJA Diameter (mm) | Weight Loss 6m | Weight Loss 12m | Weight Loss 2y | Weight Loss 3y | Weight Loss 5y | Redo-TORe | Surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jirapinyo et al. [31] | 25 | 6 (2–10) | BMI: 43 kg/m2 | 24 kg (1.4–59) (37.9%) ** | 26.4 (18–40) | 6 (3–10) | 11.7 kg (2.3–27.2) | 10.8 kg (0.7–27.2) | NA | NA | NA | NA | NA |
| Kumar et al. [24] | 150 | 8.6 ± 0.3 | Weight: 110.7 ± 2.2 kg BMI: 40.1 ± 0.7 kg/m2 | 4.1 ± 0.3 kg (49.7 ± 4.3%) | 24.1 ± 0.6 | 9.0 ± 0.2 | AWL: 10.6 ± 0.7 kg TBWL: 9.6 ± 0.6% EWL: 28.8 ± 2.7% | AWL: 10.5 ± 1.2 kg TBWL: 9.5 ± 0.9% EWL: 24.9 ± 2.6% | AWL: 9.0 ± 1.7 kg TBWL: 8.1 ± 1.4% EWL: 20.0 ± 6.4% | AWL: 9.5 ± 2.1 kg TBWL: 8.6 ± 1.5% EWL: 19.2 ± 4.6% | NA | NA | NA |
| Vargas et al. [33] | 130 | 8.4 ± 4.78 | BMI: 36.8 ± 6.84 kg/m2 | 24.6 ± 16.6 kg (38.8%) | 28 ± 4.74 | 8.3 ± 1.42 | AWL: 9.31 ± 6.7 kg | AWL: 7.75 ± 8.4 kg TBW: 6 ± 7.0% EWL: 20.2 ± 10% | AWL: 8 ± 8.8 kg* | NA | NA | NA | NA |
| Tsai et al. [43] | 81 | 6.7 (0.8–18.5) | Weight: 94.9 kg BMI: 33.6 kg/m2 | 18.2 kg (36.1%) ** | 22 (13–40) | 6 (4–14) | AWL: 6.0 (0.2–24.8) kg | AWL: 8.0 (0.2–8) | NA | NA | NA | 16 (19.9%) | 11 (13.65%) lap pouch revision |
| Jirapinyo et al. [35] | 331 | 9.3 ± 4.7 | Weight: 110.0 ± 26.3 kg BMI: 40.1 ± 9.1 kg/m2 | 55.2 kg (51.0%) ** | 23.4 ± 6.0 | 8.4 ± 1.6 | NA | AWL: 9.4 ± 12.3 kg TBWL: 8.5 ± 8.5% | NA | AWL: 8.7 ± 13.8 kg TBWL: 6.9 ± 10.1% | AWL: 10.3 ± 14.6 kg TBWL 8.8 ± 12.5% | 95 (28.7%) | 4 (1.2%) GJA reconstruction or limb distalization |
| Callahan et al. [36] | 70 | 7.7 ± 4.0 | Weight: 116.1 ± 25.2 kg BMI: 42.3 ± 8.5 kg/m2 | 27.5 ± 32.1 kg (42.8 ± 18.7%) | 30.6 ± 6.2 | 5.8 ± 2.0 | AWL: 10.7 ± 11.6 kg EWL: 18.5 ± 18.2% | AWL: 8.5 ± 11.5 kg EWL: 14.9 ± 20.6% | NA | AWL: 5.3 ± 9.1 kg EWL: 8.7 ± 14.9% | AWL: 3.9 ± 13.1 kg EWL: 7.0 ± 23.8% | NA | NA |
| Study | N. of Patients | Time RYGB–TORe (Years) | Pre-TORe GJA Diameter (mm) | Post-TORe GJA Diameter (mm) | SDS Baseline | Dumping Syndrome Outcomes | Redo-TORe | Surgery |
|---|---|---|---|---|---|---|---|---|
| Stier et al. [44] | 14 | 4.6 ± 2.6 | NA | 8 | 12.7 ± 4.2 | 1 month: SDS 3.1 ± 2.1 | 0/14 (0%) | 1 (reconstruction of the upper gastrointestinal tract + sleeve gastrectomy) |
| Brown et al. [47] | 27 | NA | NA | 12 | NA | 3 months: 92% DS resolution 2 years: 80% DS resolution | NA | NA |
| Tsai et al. [45] | 40 | 6.7 (0.8–19.0) | 22.6 (18–35) | < 10 | 13.9 (0–28) | 14.8 (3–32) months: SDS 8.6 (0–28) | 9/40 (22.5%) | 2 (laparoscopic pouch revision) |
| Vargas et al. [46] | 115 | 8.9 ± 1.1 | 39.8 ± 6.7 | 6.2 (4–13) | 17.2 ± 5.9 | 3 months: SDS 2.6 ± 1.9 | 3/115 (2.6%) | 3 (surgical enteral feeding tube placement) |
| Relly et al. [32] | 13 | 5.5 (1–9) | 25.2 (15–30) | 5.6 (5–10) | 19.4 ± 3.6 | 6 months: SDS 5.2 ± 5.5 | 2/13 (15.2%) | NA |
| Petchers et al. [48] | 98 | 9 ± 4.6 | NA | 15 | NA | 1 month: 88% DS resolution 3.5 years: 84% DS resolution | 7/98 (7.1%) | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matteo, M.V.; Gallo, C.; Pontecorvi, V.; Bove, V.; De Siena, M.; Carlino, G.; Costamagna, G.; Boškoski, I. Weight Recidivism and Dumping Syndrome after Roux-En-Y Gastric Bypass: Exploring the Therapeutic Role of Transoral Outlet Reduction. J. Pers. Med. 2022, 12, 1664. https://doi.org/10.3390/jpm12101664
Matteo MV, Gallo C, Pontecorvi V, Bove V, De Siena M, Carlino G, Costamagna G, Boškoski I. Weight Recidivism and Dumping Syndrome after Roux-En-Y Gastric Bypass: Exploring the Therapeutic Role of Transoral Outlet Reduction. Journal of Personalized Medicine. 2022; 12(10):1664. https://doi.org/10.3390/jpm12101664
Chicago/Turabian StyleMatteo, Maria Valeria, Camilla Gallo, Valerio Pontecorvi, Vincenzo Bove, Martina De Siena, Giorgio Carlino, Guido Costamagna, and Ivo Boškoski. 2022. "Weight Recidivism and Dumping Syndrome after Roux-En-Y Gastric Bypass: Exploring the Therapeutic Role of Transoral Outlet Reduction" Journal of Personalized Medicine 12, no. 10: 1664. https://doi.org/10.3390/jpm12101664
APA StyleMatteo, M. V., Gallo, C., Pontecorvi, V., Bove, V., De Siena, M., Carlino, G., Costamagna, G., & Boškoski, I. (2022). Weight Recidivism and Dumping Syndrome after Roux-En-Y Gastric Bypass: Exploring the Therapeutic Role of Transoral Outlet Reduction. Journal of Personalized Medicine, 12(10), 1664. https://doi.org/10.3390/jpm12101664









