Red Blood Cell Lifespan < 74 Days Can Clinically Reduce Hb1Ac Levels in Type 2 Diabetes
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Data Collection and Laboratory Examination
2.3. Mean Blood Glucose
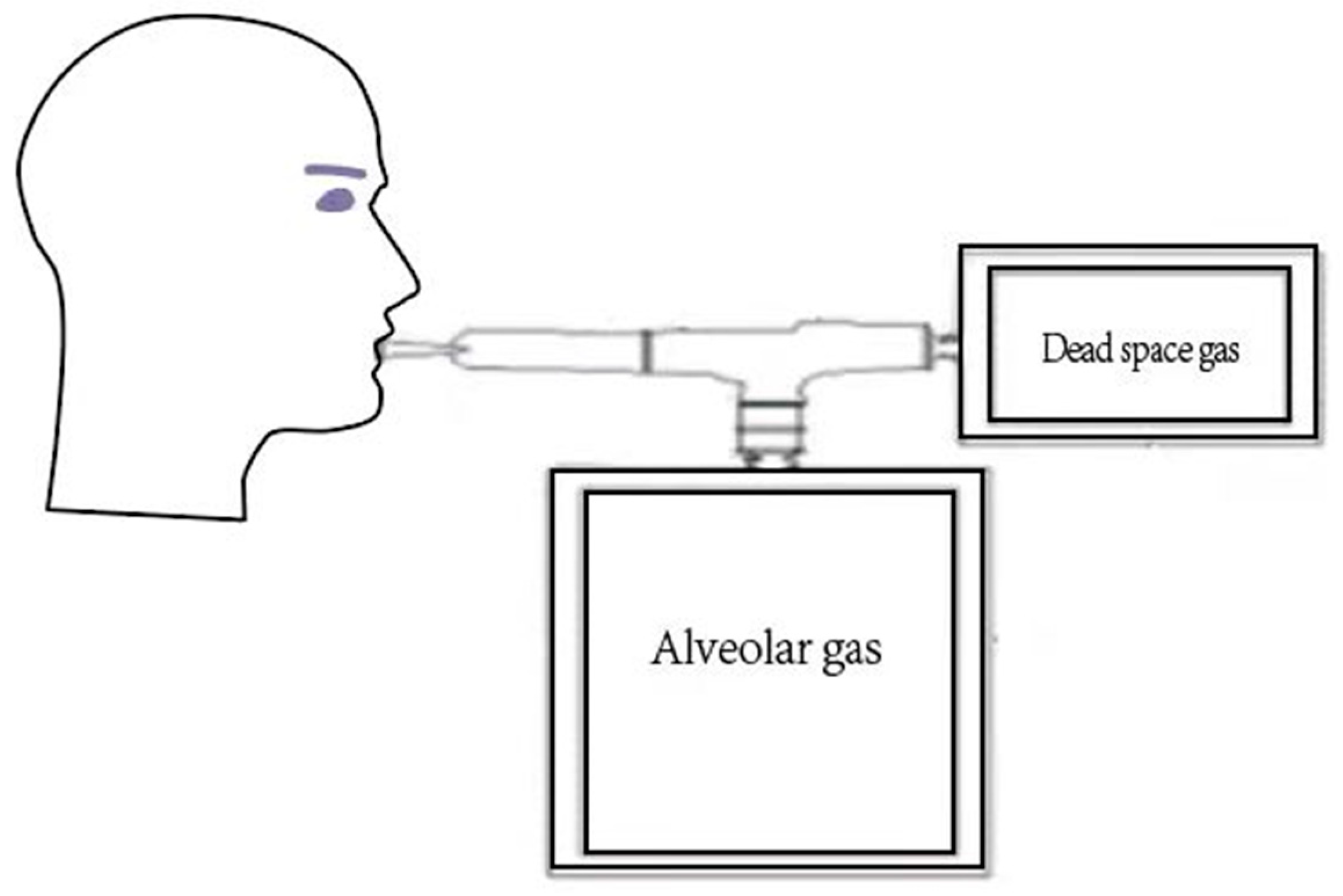
2.4. The CO Breath Test to Measure the RBC Lifespan
2.5. Statistical Analysis
3. Results
3.1. Clinical Data of Patients with T2DM
3.2. The RBC Lifespan Distribution in Patients with T2DM
3.3. Correlation between the RBC Lifespan and Clinical Characteristics of T2DM
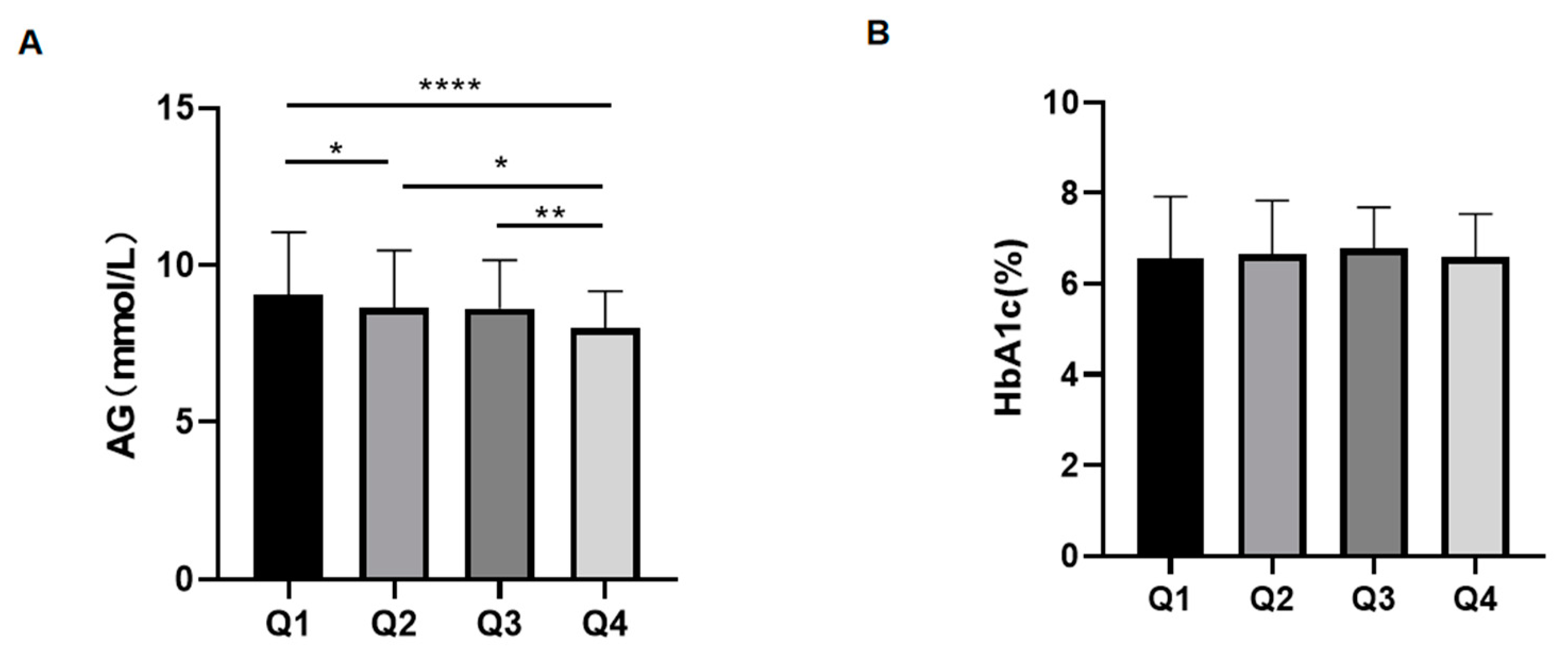
3.4. The Effect of Different RBC Lifespans on HbA1c Test Value
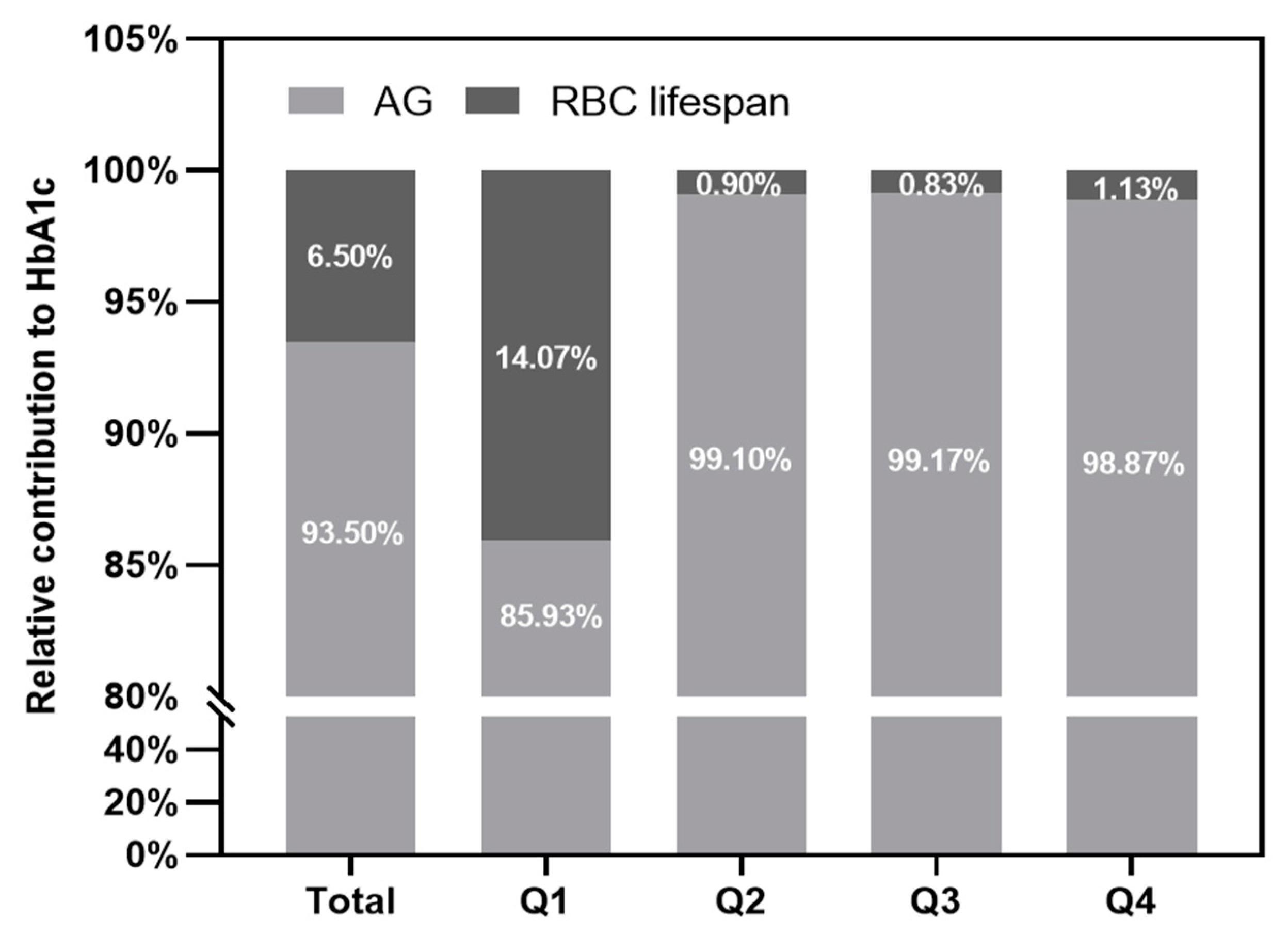
3.5. The Contribution of the RBC Lifespan to HbA1c Test Value
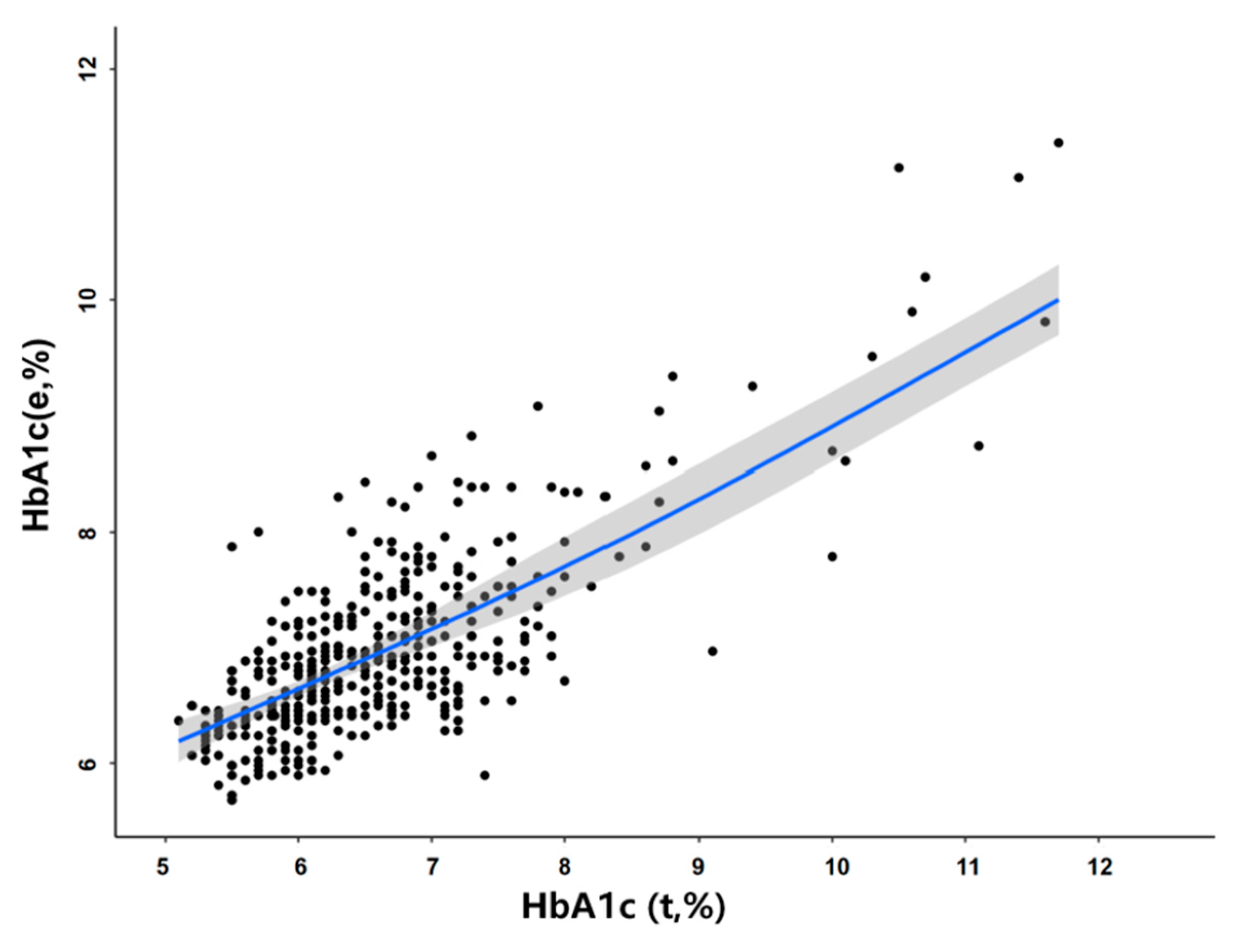
3.6. Adjustment of the Effect of the RBC Lifespan on HbA1c Detection Values
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Glycemic Targets: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. S1), S61–S70. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Heine, R.J.; Holman, R.R.; Sherwin, R.; Zinman, B. Management of hyperglycaemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2006, 49, 1711–1721. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Pofi, R.; Giannetta, E.; Feola, T.; Galea, N.; Barbagallo, F.; Campolo, F.; Badagliacca, R.; Barbano, B.; Ciolina, F.; Defeudis, G.; et al. Sex-specific effects of daily tadalafil on diabetic heart kinetics in RECOGITO, a randomized, double-blind, placebo-controlled trial. Sci. Transl. Med. 2022, 14, eabl8503. [Google Scholar] [CrossRef]
- Pofi, R.; Giannetta, E.; Galea, N.; Francone, M.; Campolo, F.; Barbagallo, F.; Gianfrilli, D.; Venneri, M.A.; Filardi, T.; Cristini, C.; et al. Diabetic Cardiomiopathy Progression is Triggered by miR122-5p and Involves Extracellular Matrix: A 5-Year Prospective Study. JACC Cardiovasc. Imaging 2021, 14, 1130–1142. [Google Scholar] [CrossRef]
- Kourtidou, C.; Rafailidis, V.; Varouktsi, G.; Kanakis, E.; Liakopoulos, V.; Vyzantiadis, T.A.; Stangou, M.; Marinaki, S.; Tziomalos, K. Evaluation of Subclinical Vascular Disease in Diabetic Kidney Disease: A Tool for Personalization of Management of a High-Risk Population. J. Pers. Med. 2022, 12, 1139. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Li, T.; Lou, Q.; Chen, X. Effect of urinary albumin creatinine ratio on type 2 diabetic retinopathy and is cut-off value for early diabetic retinopathy diagnosis. Prim. Care Diabetes 2022, 9, S1751-9918(22)00132-2. [Google Scholar] [CrossRef]
- Morrish, N.J.; Wang, S.L.; Stevens, L.K.; Fuller, J.H.; Keen, H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001, 44 (Suppl. S2), S14–S21. [Google Scholar] [CrossRef]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Malka, R.; Nathan, D.M.; Higgins, J.M. Mechanistic modeling of hemoglobin glycation and red blood cell kinetics enables personalized diabetes monitoring. Sci. Transl. Med. 2016, 8, 359ra130. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Ji, Y.; Zhou, C.; Luo, J.; Zhang, L.; Jing, L.; Zhao, X.; Guo, J.; Gao, Q.; Peng, G.; et al. Comparison of Levitt’s CO breath test and the 15 N-glycine labeling technique for measuring the lifespan of human red blood cells. Am. J. Hematol. 2021, 96, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.J.; Zhang, H.D.; Wu CHZhu, G.L.; Ji, Y.Q.; Huang, J.L.; Du, L.T.; Cao, P.; Zang, D.Y.; Ji, K.M. Rapid CO. Rapid CO breath test screening of drugs for protective effects on ribavirin-induced hemolysis in a rabbit model: A pilot study. Breath Res. 2016, 10, 036010. [Google Scholar]
- Ma, Y.J.; Zhang, H.D.; Ji YQZhu, G.L.; Huang, J.L.; Du, L.T.; Cao, P.; Zang, D.Y.; Du, J.H.; Li, R.; Wang, L. A Modified Carbon Monoxide Breath Test for Measuring Erythrocyte Lifespan in Small Animals. Biomed Res. Int. 2016, 2016, 7173156. [Google Scholar] [CrossRef]
- Chu, H.W.; Ma, Y.J.; Huang, Z.H. A pilot study: Effect of erythrocyte lifespan determined by a modified carbon monoxide breath test on glycosylated hemoglobin interpretation. Breath Res. 2020, 14, 027101. [Google Scholar] [CrossRef]
- Lenters-Westra, E.; Slingerland, R.J. Hemoglobin A1c determination in the A1C-Derived Average Glucose (ADAG) study. Clin. Chem. Lab Med. 2008, 46, 1617–1623. [Google Scholar] [CrossRef]
- Krishnan, S.M.; Dixit, N.M. Estimation of red blood cell lifespan from alveolar carbon monoxide measurements. Transl. Res. 2009, 154, 15–17. [Google Scholar] [CrossRef]
- Stephenson, K.N.; Croxen, R.L.; El-Barbary, A.; Fenstermacher, J.D.; Haspel, H.C. Inhibition of glucose transport and direct interactions with type 1 facilitative glucose transporter (GLUT-1) by etomidate, ketamine, and propofol: A comparison with barbiturates. Biochem. Pharmacol. 2000, 60, 651–659. [Google Scholar] [CrossRef]
- Kameyama, M.; Takeuchi, S.; Ishii, S. Steady-state relationship between average glucose, HbA1c and RBC lifespan. J. Theor. Biol. 2018, 447, 111–117. [Google Scholar] [CrossRef]
- Khera, P.K.; Smith, E.P.; Lindsell, C.J.; Rogge, M.C.; Haggerty, S.; Wagner, D.A.; Palascak, M.B.; Mehta, S.; Hibbert, J.M.; Joiner, C.H.; et al. Use of an oral stable isotope label to confirm variation in red blood cell mean age that influences HbA1c interpretation. Am. J. Hematol. 2015, 90, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Dworatzek, E.; Seeland, U.; Kararigas, G.; Arnal, J.F.; Brunelleschi, S.; Carpenter, T.C.; Erdmann, J.; Franconi, F.; Giannetta, E.; et al. Sex in basic research: Concepts in the cardiovascular field. Cardiovasc. Res. 2017, 113, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Shima, K. Evaluation of error levels in hemoglobin A1c and glycated albumin in type 2 diabetic patients due to inter-individual variability. Diabetes Res. Clin. Pract. 2010, 89, 115–120. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Healthy Control | T2DM Total | T2DM RBC Lifespan Quartile | ap | bp | |||
|---|---|---|---|---|---|---|---|---|
| Q1: <75 d | Q2: 75–89d | Q3: 90–111d | Q4: ≥112d | |||||
| N | 231 | 464 | 113 | 113 | 120 | 118 | 0.0553 | 0.520 |
| Sex (M/F) | (100/115) | (233/231) | (71/42) | (57/56) | (58/62) | (46/72) | 0.369 | 0.006 |
| Duration (year) | 0 | 6 (2.9, 12) | 6.5 (3, 17.4) | 7 (2, 13) | 6 (2.8, 12) | 6.8 (2, 12) | - | 0.240 |
| SBP (mmHg) | 128.1 ± 12.9 | 135.5 ± 16.0 | 139.2 ± 18.1 | 134.2 ± 13.5 | 134.4 ± 14.7 | 134.1 ± 16.7 | <0.0001 | 0.05 |
| DBP (mmHg) | 77.5 ± 10.3 | 80.4 ± 11.2 | 82.9 ± 12.5 | 78.7 ± 10.1 | 79.4 ± 10.6 | 80.5 ± 11.2 | 0.0012 | 0.05 |
| Hb (g/L) | 139.1 ± 17.3 | 142.8 ± 18.8 | 140.7 ± 21.7 | 143.1 ± 18.9 | 144.7 ± 16.0 | 143.1 ± 17.8 | 0.0014 | 0.467 |
| FBG (mmol/L) | 5.06 ± 0.39 | 7.57 ± 1.70 | 8.27 ± 2.47 | 7.55 ± 1.51 | 7.49 ± 1.20 | 7.06 ± 1.29 | <0.0001 | <0.0001 |
| P2BG (mmol/L) | - | 9.75 ± 2.17 | 10.62 ± 2.46 | 9.87 ± 2.38 | 9.70 ± 1.86 | 8.94 ± 1.65 | - | <0.0001 |
| TG (mmol/L) | 1.24 ± 0.50 | 1.82 ± 1.16 | 1.94 ± 1.20 | 1.76 ± 1.06 | 1.90 ± 1.42 | 1.67 ± 0.87 | <0.0001 | 0.2469 |
| TC (mmol/L) | 4.70 ± 0.91 | 4.91 ± 1.11 | 5.19 ± 1.14 | 4.71 ± 1.21 | 4.94 ± 1.05 | 4.77 ± 0.99 | 0.0157 | 0.0596 |
| LDL-c (mmol/L) | 2.93 ± 0.68 | 3.25 ± 0.89 | 3.48 ± 0.92 | 3.06 ± 0.99 | 3.25 ± 0.82 | 3.19 ± 0.79 | <0.0001 | 0.0503 |
| ALT (U/L) | 24.2 ± 12.7 | 24.5 ± 15.5 | 27.9 ± 18.5 | 21.6 ± 11.2 | 23.3 ± 14.5 | 24.8 ± 16.0 | 0.8482 | 0.0508 |
| AST (U/L) | 23.0 ± 8.4 | 22.6 ± 9.7 | 24.6 ± 11.6 | 20.9 ± 7.1 | 21.9 ± 9.0 | 22.9 ± 10.0 | 0.5784 | 0.0605 |
| SCr (umol/L) | 67.1 ± 19.8 | 70.9 ± 20.7 | 68.4 ± 19.8 | 79.5 ± 20.2 | 70.4 ± 24.6 | 65.4 ± 22.7 | 0.2379 | 0.0506 |
| BUN (umol/L) | 5.86 ± 1.79 | 5.87 ± 3.38 | 5.69 ± 1.85 | 6.18 ± 3.15 | 6.32 ± 5.29 | 5.20 ± 2.00 | 0.9489 | 0.5616 |
| UA (umol/L) | 330.0 ± 82.3 | 326.4 ± 86.5 | 333.6 ± 85.6 | 329.9 ± 92.0 | 321.5 ± 81.1 | 309.7 ± 86.4 | 0.6094 | 0.1257 |
| Variables | r-Value | p Value |
|---|---|---|
| Hb (g/L) | 0.08 | 0.089 |
| Duration (years) | −0.077 | 0.108 |
| HbA1c (%) | 0.030 | 0.52 |
| Age (years) | 0.050 | 0.28 |
| AG (mmol/L) | −0.33 | <0.001 |
| GA (%) | −0.14 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Dong, R.; Wang, J.; Zhang, L.; Yu, B.; Shao, X.; Bai, P.; Zhang, R.; Ma, Y.; Yu, P. Red Blood Cell Lifespan < 74 Days Can Clinically Reduce Hb1Ac Levels in Type 2 Diabetes. J. Pers. Med. 2022, 12, 1738. https://doi.org/10.3390/jpm12101738
Zhou S, Dong R, Wang J, Zhang L, Yu B, Shao X, Bai P, Zhang R, Ma Y, Yu P. Red Blood Cell Lifespan < 74 Days Can Clinically Reduce Hb1Ac Levels in Type 2 Diabetes. Journal of Personalized Medicine. 2022; 12(10):1738. https://doi.org/10.3390/jpm12101738
Chicago/Turabian StyleZhou, Saijun, Rongna Dong, Junmei Wang, Li Zhang, Bai Yu, Xian Shao, Pufei Bai, Rui Zhang, Yongjian Ma, and Pei Yu. 2022. "Red Blood Cell Lifespan < 74 Days Can Clinically Reduce Hb1Ac Levels in Type 2 Diabetes" Journal of Personalized Medicine 12, no. 10: 1738. https://doi.org/10.3390/jpm12101738
APA StyleZhou, S., Dong, R., Wang, J., Zhang, L., Yu, B., Shao, X., Bai, P., Zhang, R., Ma, Y., & Yu, P. (2022). Red Blood Cell Lifespan < 74 Days Can Clinically Reduce Hb1Ac Levels in Type 2 Diabetes. Journal of Personalized Medicine, 12(10), 1738. https://doi.org/10.3390/jpm12101738






