Calculation of an Improved Stiffness Index Using Decomposed Radial Pulse and Digital Volume Pulse Signals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population, Grouping, and Experimental Procedure
2.1.1. Study Protocol
2.1.2. Grouping
2.1.3. Experimental Procedure
2.2. Measurement Instrument Description and Related Indices
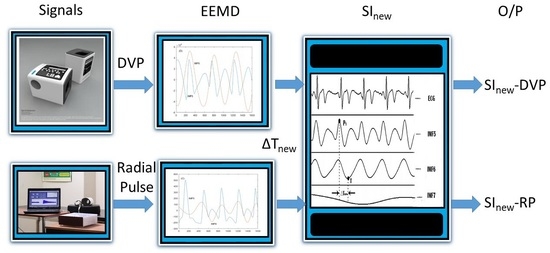
2.2.1. DVP and Radial Pulse Signal Measurements
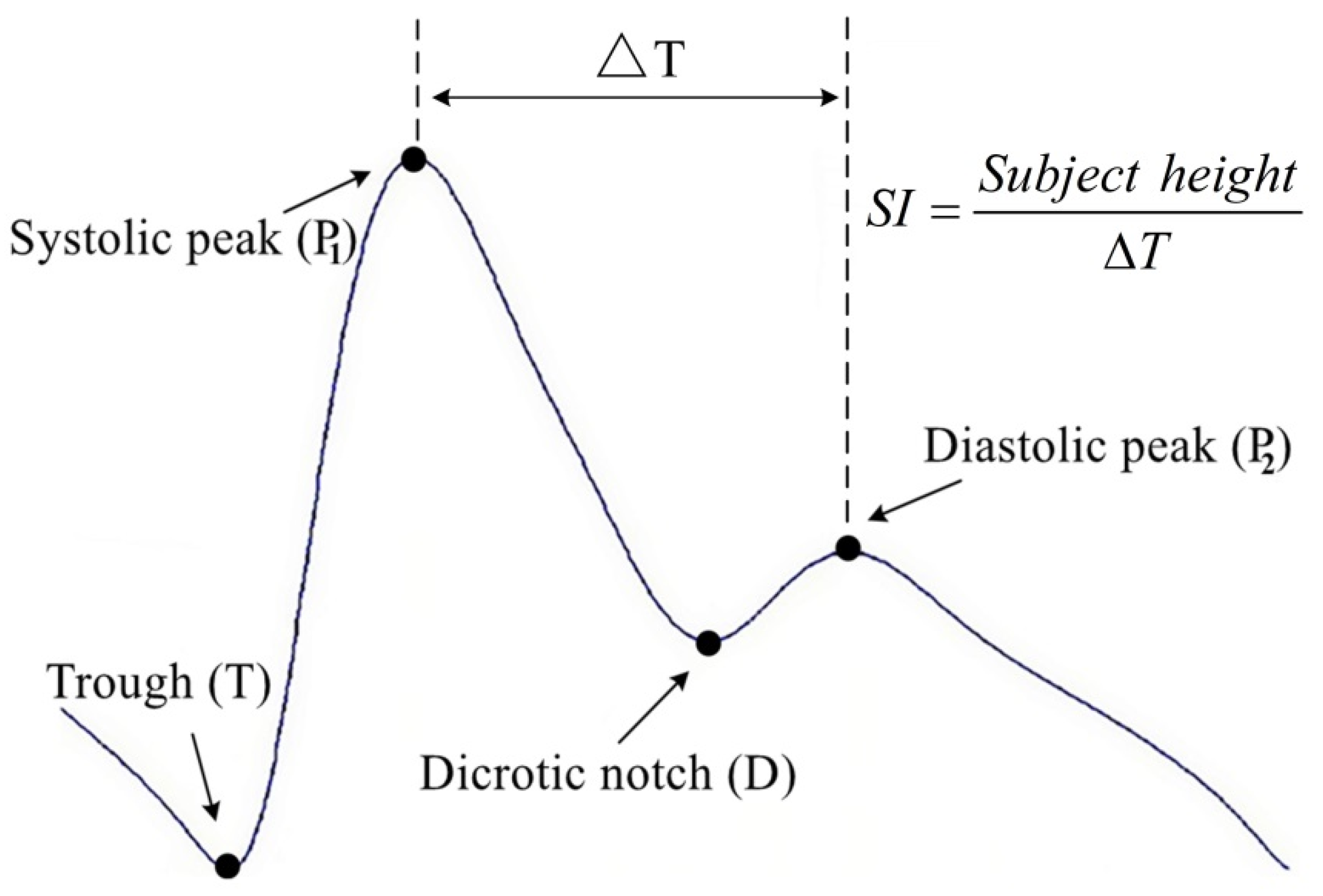
2.2.2. SI Computation
2.3. Statistical Analysis
3. Results
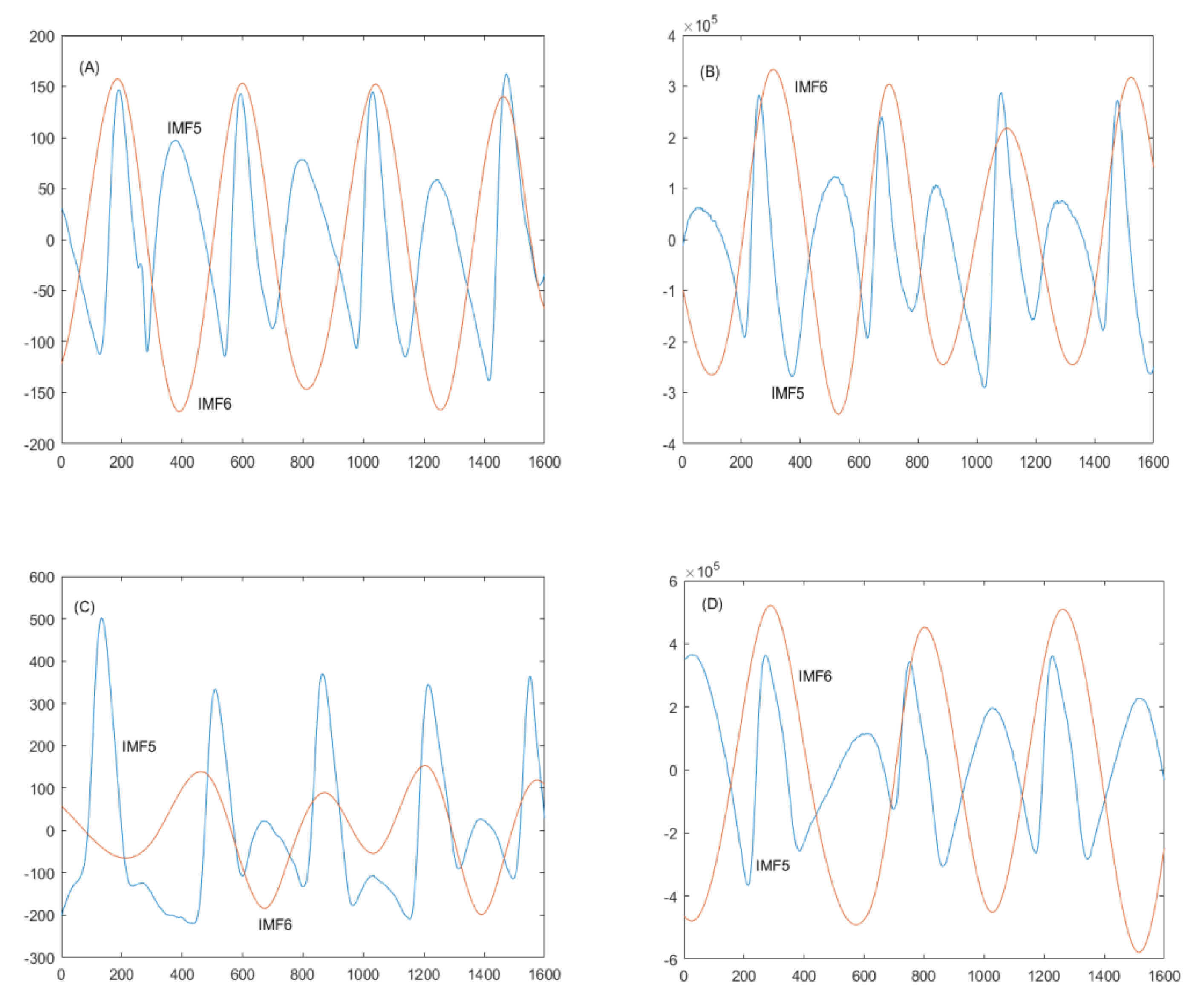
3.1. Use of EEMD for Decomposing IMF5 and IMF6 in Older and Diabetic Subjects
3.2. One-Way ANOVA for Three Arterial Stiffness Indices
3.3. Correlation between SInew-DVP and SInew-RP
3.4. Effects of Risk Factors on SInew-RP and SInew-DVP Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millasseau, S.C.; Ritter, J.M.; Takazawa, K.; Chowienczyk, P.J. Contour Analysis of the Photoplethysmographic Pulse Measured at the Finger. J. Hypertens. 2006, 24, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Said, M.A.; Eppinga, R.N.; Lipsic, E.; Verweij, N.; van der Harst, P. Relationship of Arterial Stiffness Index and Pulse Pressure with Cardiovascular and Disease and Mortality. J. Am. Heart Assoc. 2018, 22, e007621. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.A.; Van Roon, A.M.; Bakker, J.T.; Bijen, H.T.J.; Mulder, D.J.; Brouwers, F.P.; Van Gilst, W.H.; Voors, A.A.; Gansevoort, R.T.; Bakker, S.J.L.; et al. Digital Arterial Pressure Pulse Wave Analysis and Cardiovascular Events in the General Population: The Prevention of Renal and Vascular End-Stage Disease Study. J. Hypertens. 2020, 38, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jin, C.; Li, S.; Zheng, X.; Zhang, X.; Cui, L.; Gao, X. Aging, Arterial Stiffness, and Blood Pressure Association in Chinese Adults. Hypertension 2019, 73, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Boutouyrie, P.; Chowienczyk, P.; Humphrey, J.D.; Mitchell, G.F. Arterial Stiffness and Cardiovascular Risk in Hypertension. Circ. Res. 2021, 128, 864–886. [Google Scholar] [CrossRef]
- Grensemann, J. Cardiac Output Monitoring by Pulse Contour Analysis, the Technical Basics of Less-Invasive Techniques. Front Med. 2018, 5, 64. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Wu, H.-T.; Sun, C.-K. Assessment of Subtle Changes in Diabetes-Associated Arteriosclerosis Using Photoplethysmographic Pulse Wave from Index Finger. J. Med. Syst. 2018, 42, 43. [Google Scholar] [CrossRef]
- Xu, M.; Huang, Y.; Xie, L.; Peng, K.; Ding, L.; Lin, L.; Wang, P.; Hao, M.; Chen, Y.; Sun, Y.; et al. Diabetes and Risk of Arterial Stiffness: A Mendelian Randomization Analysis. Diabetes 2016, 65, 1731–1740. [Google Scholar] [CrossRef]
- London, G.M. Arterial Stiffness in Chronic Kidney Disease and End-Stage Renal Disease. Blood Purif. 2018, 45, 154–158. [Google Scholar] [CrossRef]
- Zekavat, S.M.; Aragam, K.; Emdin, C.; Khera, A.V.; Klarin, D.; Zhao, H.; Natarajan, P. Genetic Association of Finger Photoplethysmography-Derived Arterial Stiffness Index with Blood Pressure and Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Tomson, J.; Hin, H.; Emberson, J.; Kurien, R.; Lay, M.; Cox, J.; Hill, M.; Arnold, L.; Leeson, P.; Armitage, J.; et al. Effects of Vitamin D on Blood Pressure, Arterial Stiffness, and Cardiac Function in Older People after 1 year: BEST-D (Biochemical Efficacy and Safety Trial of Vitamin D). J. Am. Heart Assoc. 2017, 6, e005707. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, N.E. Ensemble Empirical Mode Decomposition: A Noise-Assisted Data Analysis Method. Adv. Adapt. Data Anal. 2009, 1, 1–41. [Google Scholar] [CrossRef]
- Wei, H.-C.; Xiao, M.-X.; Chen, H.-Y.; Li, Y.-Q.; Wu, H.-T.; Sun, C.-K. Instantaneous Frequency from Hilbert-Huang Space as an Indicator of Diabetes and Arterial Stiffness in Middle-Aged and Elderly Subjects. Sci. Rep. 2018, 8, 15771. [Google Scholar] [CrossRef]
- Wei, H.-C.; Li, Y.-Q.; Wu, G.-S.; Xiao, M.-X.; Tang, X.-J.; Chen, J.-J.; Wu, H.-T. New Application of an Instantaneous Frequency Parameter for Assessing Far Infrared Fabric Effect in Aged Subjects. Electronics 2020, 9, 138. [Google Scholar] [CrossRef]
- Wu, H.-T.; Lee, C.-H.; Liu, A.-B.; Chung, W.-S.; Tang, C.-J.; Sun, C.-K.; Yip, H.-K. Arterial Stiffness Using Radial Arterial Waveforms Measured at the Wrist as an Indicator of Diabetic Control in the Elderly. IEEE Trans. Biomed. Eng. 2011, 58, 243–252. [Google Scholar]
- Stallone, A.; Cicone, A.; Materassi, M. New Insights and Best Practices for the Successful Use of Empirical Mode Decomposition, Iterative Filtering and Derived Algorithms. Sci. Rep. 2020, 10, 15161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Yeh, C.H.; Young, H.W.V.; Hu, K.; Lo, M.T. On the Computational Complexity of the Empirical Mode Decomposition Algorithm. Phys. A Stat. Mech. Its Appl. 2014, 400, 159–167. [Google Scholar] [CrossRef]
- Yeh, J.R.; Shieh, J.S.; Huang, N.E. Complementary Ensemble Empirical Mode Decomposition: A Novel Noise Enhanced Data Analysis Method. Adv. Adapt. Data Anal. 2010, 2, 135–156. [Google Scholar] [CrossRef]
- Birukov, A.; Cuadrat, R.; Polemiti, E.; Eichelmann, F.; Schulze, M.B. Advanced Glycation End-Products, Measured as Skin Autofluorescence, Associate with Vascular Stiffness in Diabetic, Pre-diabetic and Normoglycemic Individuals: A Cross-Sectional Study. Cardiovasc. Diabetol. 2021, 20, 110. [Google Scholar] [CrossRef]
- El-Naggar, H.M.; Anwar, H.S.; Helmy, H.A.; Demitry, S.R. Aortic Elasticity Indices as Predictors of Coronary Artery Disease Severity Assessed by SYNTAX Score. J. Cardiovasc. Echogr. 2021, 31, 234–241. [Google Scholar] [CrossRef]
- Guo, C.; Jiang, Z.; He, H.; Liao, Y.; Zhang, D. Wrist Pulse Signal Acquisition and Analysis for Disease Diagnosis: A Review. Comput. Biol. Med. 2022, 143, 105312. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Gupta, R. MoDTRAP: Improved Heart Rate Tracking and Preprocessing of Motion-Corrupted Photoplethysmographic Data for Personalized Healthcare. Biomed. Signal Process. Control. 2020, 56, 101676. [Google Scholar] [CrossRef]
- John, A.; Dingchang, Z.; Panicos, A.K.; Mohamed, E. Photoplethysmography, 1st ed.; Academic Press: London, UK, 2021. [Google Scholar]
- Meng, K.; Xiao, X.; Wei, W.; Chen, G.; Nashalian, A.; Shen, S.; Xiao, X.; Chen, J. Wearable Pressure Sensors for Pulse Wave Monitoring. Adv. Mater. 2022, 34, 2109357. [Google Scholar] [CrossRef] [PubMed]
- Venkata Giri Kumar, P.; Deshpande, S.; Joshi, A.; More, P.; Nagendra, H.R. Significance of Arterial Stiffness in Tridosha Analysis: A Pilot Study. J. Ayurveda Integr. Med. 2017, 8, 252–256. [Google Scholar] [CrossRef]
- Sequí-Domínguez, I.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Pozuelo-Carrascosa, D.P.; Nuñez de Arenas-Arroyo, S.; Martínez-Vizcaíno, V. Accuracy of Pulse Wave Velocity Predicting Cardiovascular and All-Cause Mortality. A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2080. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Cirrincione, A.; Casuccio, A.; Del Cuore, A.; Daidone, M.; Di Chiara, T.; Di Raimondo, D.; Della Corte, V.; Maida, C.; Simonetta, I.; et al. Efficacy of Dulaglutide on Vascular Health Indexes in Subjects with Type 2 Diabetes: A Randomized Trial. Cardiovasc. Diabetol. 2021, 20, 1. [Google Scholar] [CrossRef]
- Bonarjee, V.V.S. Arterial Stiffness: A Prognostic Marker in Coronary Heart Disease. Available Methods and Clinical Application. Front. Cardiovasc. Med. 2018, 5, 64. [Google Scholar] [CrossRef]
- Tajima, T.; Ikeda, A.; Steptoe, A.; Takahashi, K.; Maruyama, K.; Tomooka, K.; Saito, I.; Tanigawa, T. The Independent Association between Salivary Alpha-Amylase Activity and Arterial Stiffness in Japanese Men and Women: The Toon Health Study. Hypertens. Res. 2022, 45, 1249–1262. [Google Scholar] [CrossRef]
- Mej´ıa-Mej´ıa, E.; Allen, J.; Budidha, K.; El-Hajj, C.; Kyriacou, P.A.; Charlton, P.H. Photoplethysmography Signal Processing and Synthesis. In Photoplethysmography; Kyriacou, P.A., Allen, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]


| Parameter | Group 1 | Group 2 | p-Values |
|---|---|---|---|
| Gender (male/female) | 40 (20/20) | 40 (23/17) | N/A |
| Age (years) | 52.45 ± 10.99 | 57.80 ± 7.45 * | 0.048 |
| Body height (cm) | 163.84 ± 7.83 | 163.83 ± 7.39 | 0.994 |
| Body weight (kg) | 64.08 ± 11.72 | 73.96 ± 11.43 ** | <0.001 |
| WC (cm) | 82.58 ± 11.43 | 93.90 ± 10.17 ** | <0.001 |
| BMI (kg/m2) | 23.79 ± 3.69 | 27.54 ± 3.89 ** | <0.001 |
| SBP (mmHg) | 121.20 ± 14.36 | 121.65 ± 27.91 | 0.928 |
| DBP (mmHg) | 75.63 ± 8.57 | 75.30 ± 17.16 | 0.915 |
| PP (mmHg) | 45.58 ± 11.92 | 46.83 ± 15.81 | 0.691 |
| HR (beats/min) | 75.19 ± 17.22 | 87.65 ± 14.81* | 0.001 |
| HDL (mg/dL) | 53.18 ± 17.21 | 40.53 ± 14.97* | 0.001 |
| LDL (mg/dL) | 120.15 ± 41.09 | 128.45 ± 34.29 | 0.330 |
| Cholesterol (mg/dL) | 175.60 ± 50.63 | 190.83 ± 46.55 | 0.165 |
| Triglyceride (mg/dL) | 97.30 ± 47.32 | 171.23 ± 85.22 ** | <0.001 |
| HbA1c (%) | 5.77 ± 0.34 | 8.86 ± 2.11 ** | <0.001 |
| FPG (mg/dL) | 96.85 ± 16.08 | 169.08 ± 48.91 ** | <0.001 |
| Parameter | Group 1 | Group 2 | p-Values |
|---|---|---|---|
| PWVfinger (m/s) | 4.76 ± 0.45 | 5.04 ± 0.34 * | 0.002 |
| SInew-RP (m/s) | 4.51 ± 0.49 | 5.41 ± 1.13 ** | <0.001 |
| SInew-DVP (m/s) | 4.02 ± 0.61 | 4.74 ± 1.09 * | 0.001 |
| (A) | |||||
| Index | N | Mean | SD | ||
| PWVfinger | 80 | 4.90 m/s | 0.42 m/s | ||
| SInew-RP | 80 | 4.96 m/s | 0.98 m/s | ||
| SInew-DVP | 80 | 4.38 m/s | 0.95 m/s | ||
| Total | 240 | 4.75 m/s | 0.86 m/s | ||
| Source of Variance | Sum of Squares | df | Mean Square | F-ratio | p-value |
| Between groups | 16.455 | 2 | 8.227 | 12.112 | <0.001 |
| Within groups | 160.987 | 237 | 0.679 | - | - |
| Total | 177.441 | 239 | - | - | - |
| (B) | |||||
| Multiple Comparisons | N | Mean | Mean Difference | p-Value | |
| PWVfinger vs. SInew-RP | 80 vs. 80 | 4.90 vs. 4.96 m/s | −0.058 | 0.658 | |
| PWVfinger vs. SInew-DVP | 80 vs. 80 | 4.90 vs. 4.38 m/s | 0.524 ** | <0.001 | |
| SInew-RP vs. SInew-DVP | 80 vs. 80 | 4.96 vs. 4.38 m/s | 0.582 ** | <0.001 | |
| Parameter | SInew-RP | SInew-DVP |
|---|---|---|
| Age | r = 0.415 (p < 0.001) | r = 0.439 (p < 0.001) |
| Body weight | r = 0.257 (p = 0.041) | r = 0.233 (p = 0.038) |
| Waist circumference | r = 0.281 (p = 0.012) | r = 0.273 (p = 0.014) |
| Glycosylated hemoglobin | r = 0.400 (p < 0.001) | r = 0.365 (p = 0.001) |
| Cholesterol | r = 0.219 (p = 0.051) | r = 0.229 (p = 0.041) |
| Triglyceride | r = 0.333 (p = 0.003) | r = 0.307 (p = 0.006) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-T.; Chen, J.-J. Calculation of an Improved Stiffness Index Using Decomposed Radial Pulse and Digital Volume Pulse Signals. J. Pers. Med. 2022, 12, 1768. https://doi.org/10.3390/jpm12111768
Wu H-T, Chen J-J. Calculation of an Improved Stiffness Index Using Decomposed Radial Pulse and Digital Volume Pulse Signals. Journal of Personalized Medicine. 2022; 12(11):1768. https://doi.org/10.3390/jpm12111768
Chicago/Turabian StyleWu, Hsien-Tsai, and Jian-Jung Chen. 2022. "Calculation of an Improved Stiffness Index Using Decomposed Radial Pulse and Digital Volume Pulse Signals" Journal of Personalized Medicine 12, no. 11: 1768. https://doi.org/10.3390/jpm12111768
APA StyleWu, H.-T., & Chen, J.-J. (2022). Calculation of an Improved Stiffness Index Using Decomposed Radial Pulse and Digital Volume Pulse Signals. Journal of Personalized Medicine, 12(11), 1768. https://doi.org/10.3390/jpm12111768