Ribosomal DNA Abundance in the Patient’s Genome as a Feasible Marker in Differential Diagnostics of Autism and Childhood-Onset Schizophrenia
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Ethics Expertise
2.3. Patients, Psychometric Scales, Inclusion/Exclusion Criteria
2.4. Clinical Analysis in the Subgroups
2.5. Biochemical Techniques
2.5.1. DNA Isolation and Quantification
2.5.2. Determining rDNA Copy Count in DNA Samples
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Available online: https://icd.who.int/browse10/2019/en (accessed on 27 May 2022).
- Haker, H.; Schneebeli, M.; Stephan, K.E. Can Bayesian Theories of Autism Spectrum Disorder Help Improve Clinical Practice? Front. Psychiatry 2016, 7, 107. [Google Scholar] [CrossRef] [Green Version]
- Cambridge University Press. A Clinician’s Handbook of Child and Adolescent Psychiatry; Gillberg, C., Harrington, R., Steinhausen, H., Eds.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar] [CrossRef]
- Fernandez, A.; Pasquet-Levy, M.; Laure, G.; Thümmler, S.; Askenazy, F. Atteintes Neurodéveloppementales, Comorbidités Psychiatriques Et Pathologies Associées Chez Des Patients Atteints de Schizophrénie Très Précoce Avec Symptômes Autistiques Prémorbides [Neurodevelopmental Disorders, Psychiatric Comorbidities and Associated Pathologies in Patients with Childhood-Onset Schizophrenia and Premorbid Autistic Symptoms]. Can. J. Psychiatry 2021, 66, 1042–1050. [Google Scholar] [CrossRef]
- Unenge Hallerbäck, M.; Lugnegård, T.; Gillberg, C. Is autism spectrum disorder common in schizophrenia? Psychiatry Res. 2012, 198, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Cochran, D.M.; Dvir, Y.; Frazier, J.A. “Autism-plus” spectrum disorders: Intersection with psychosis and the schizophrenia spectrum. Child Adolesc. Psychiatr. Clin. N Am. 2013, 22, 609–627. [Google Scholar] [CrossRef]
- Volkmar, F.R.; Jackson, S.L.J.; Hart, L. Transition Issues and Challenges for Youth with Autism Spectrum Disorders. Pediatr. Ann. 2017, 46, e219–e223. [Google Scholar] [CrossRef]
- Driver, D.I.; Thomas, S.; Gogtay, N.; Rapoport, J.L. Childhood-Onset Schizophrenia and Early-onset Schizophrenia Spectrum Disorders: An Update. Child Adolesc. Psychiatr. Clin. N Am. 2020, 29, 71–90. [Google Scholar] [CrossRef] [Green Version]
- Klyushnik, T.P.; Zozulya, S.A.; Androsova, L.V.; Sarmanova, Z.V.; Otman, I.N.; Panteleeva, G.P. Laboratory diagnostics in monitoring patients with endogenous psychoses “Neuro-immuno-test”. In Medical Technology, 2nd ed.; Medical Information Agency: Moscow, Russia, 2016; p. 32. [Google Scholar]
- Brusov, O.S.; Simashkova, N.V.; Karpova, N.S.; Faktor, M.I.; Nikitina, S.G. Thrombodynamic correlates of catatonia severity in children with autism. Zhurnal Nevrol. I Psikhiatrii Im. SS Korsakova 2019, 119, 57–61. [Google Scholar] [CrossRef]
- Upthegrove, R.; Khandaker, G.M. Cytokines, Oxidative Stress and Cellular Markers of Inflammation in Schizophrenia. Curr. Top Behav. Neurosci. 2020, 44, 49–66. [Google Scholar] [CrossRef]
- Nikitina, S.G.; Ershova, E.S.; Chudakova, J.M.; Shmarina, G.V.; Veiko, N.N.; Martynov, A.V.; Kostuk, S.E.; Modestov Aa Rozhnova, T.M.; Izhevskaya, V.L.; Kostuk, S.V.; et al. Oxidative DNA Damage of Peripheral Blood Cells and Blood Plasma Cell-Free DNA as an Indicator of the Oxidative Stress Level in Children with Autism Spectrum Disorders and Schizophrenia. Psikhiatriya 2021, 19, 15–25. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Dmitrieva, E.M.; Parshukova, D.A.; Kazantseva, D.V.; Vasilieva, A.R.; Smirnova, L.P. Oxidative Stress-Related Mechanisms in Schizophrenia Pathogenesis and New Treatment Perspectives. Oxid. Med. Cell Longev. 2021, 23, 8881770. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, M. Oxidative stress, metabolic and mitochondrial abnormalities associated with autism spectrum disorder. Prog. Mol. Biol. Transl. Sci. 2020, 173, 331–354. [Google Scholar] [CrossRef] [PubMed]
- Emiliani, F.E.; Sedlak, T.W.; Sawa, A. Oxidative stress and schizophrenia: Recent breakthroughs from an old story. Curr. Opin. Psychiatry 2014, 27, 185–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, J.J.; Mortensen, P.B.; Visscher, P.M.; Wray, N.R. Where GWAS and epidemiology meet: Opportunities for the simultaneous study of genetic and environmental risk factors in schizophrenia. Schizophr. Bull. 2013, 39, 955–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul, F.; Sreenivas, N.; Kommu, J.V.S.; Banerjee, M.; Berk, M.; Maes, M.; Leboyer, M.; Debnath, M. Disruption of circadian rhythm and risk of autism spectrum disorder: Role of immune-inflammatory, oxidative stress, metabolic and neurotransmitter pathways. Rev. Neurosci. 2021. [Google Scholar] [CrossRef]
- Chestkov, I.V.; Jestkova, E.M.; Ershova, E.S.; Golimbet, V.E.; Lezheiko, T.V.; Kolesina, N.Y.; Porokhovnik, L.N.; Lyapunova, N.A.; Izhevskaya, V.L.; Kutsev, S.I.; et al. Abundance of ribosomal RNA gene copies in the genomes of schizophrenia patients. Schizophr. Res. 2018, 197, 305–314. [Google Scholar] [CrossRef]
- Ershova, E.S.; Malinovskaya, E.M.; Golimbet, V.E.; Lezheiko, T.V.; Zakharova, N.V.; Shmarina, G.V.; Veiko, R.V.; Umriukhin, P.E.; Kostyuk, G.P.; Kutsev, S.I.; et al. Copy number variations of satellite III (1q12) and ribosomal repeats in health and schizophrenia. Schizophr. Res. 2020, 223, 199–212. [Google Scholar] [CrossRef]
- McStay, B.; Grummt, I. The epigenetics of rRNA genes: From molecular to chromosome biology. Annu. Rev. Cell Dev. Biol. 2008, 24, 131–157. [Google Scholar] [CrossRef] [Green Version]
- Moss, T.; Stefanovsky, V.Y. At the center of eukaryotic life. Cell 2002, 109, 545–548. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, J.G.; Branco, A.T.; Yu, S.; Lemos, B. Ribosomal DNA copy number is coupled with gene expression variation and mitochondrial abundance in humans. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed; American Psychiatric Publishing: Arlington, VA, USA, 2013; p. 992. [Google Scholar]
- Schopler, E.; Reichler, R.; Rochen Renner, B. The Childhood Autism Rating Scale; Western Psychological Services: Los Angeles, CA, USA, 1988. [Google Scholar]
- Opler, L.A.; Kay, S.R.; Lindenmayer, J.P.; Fiszbein, A. Structured Clinical Interview Positive and Negative Syndrome Scale (SCI-PANSS); Multi-Health Systems: Toronto, ON, Canada, 1999. [Google Scholar]
- Nasrallah, H.; Morosini, P.; Gagnon, D.D. Reliability, validity and ability to detect change of the Personal and Social Performance scale in patients with stable schizophrenia. Psychiatry Res. 2008, 161, 213–224. [Google Scholar] [CrossRef]
- Malinovskaya, E.M.; Ershova, E.S.; Golimbet, V.E.; Porokhovnik, L.N.; Lyapunova, N.A.; Kutsev, S.I.; Veiko, N.N.; Kostyuk, S.V. Copy Number of Human Ribosomal Genes With Aging: Unchanged Mean, but Narrowed Range and Decreased Variance in Elderly Group. Front. Genet. 2018, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Kondratyeva, E.I.; Ershova, E.S.; Voronkova AYu Shmarina, G.V.; Krasovsky, S.A.; Zhekaite, E.K.; Petrova, N.V.; Melyanovskaya, Y.L.; Odinaeva, N.D.; Veiko, N.N.; Kostyuk, S.V. Variation in the number of copies of ribosomal genes in the genomes of patients with cystic fibrosis. Med. Genet. 2021, 20, 49–60. (In Russian) [Google Scholar] [CrossRef]
- Sato, A. mTOR, a Potential Target to Treat Autism Spectrum Disorder. CNS Neurol. Disord. Drug Targets 2016, 15, 533–543. [Google Scholar] [CrossRef]
- Santini, E.; Huynh, T.N.; MacAskill, A.F.; Carter, A.G.; Pierre, P.; Ruggero, D.; Kaphzan, H.; Klann, E. Exaggerated translation causes synaptic and behavioural aberrations associated with autism. Nature 2013, 493, 411–415. [Google Scholar] [CrossRef] [Green Version]
- Erickson, C.A.; Davenport, M.H.; Schaefer, T.L.; Wink, L.K.; Pedapati, E.V.; Sweeney, J.A.; Fitzpatrick, S.E.; Brown, W.T.; Budimirovic, D.; Hagerman, R.J.; et al. Fragile X targeted pharmacotherapy: Lessons learned and future directions. J. Neurodev. Disord 2017, 9, 1–14. [Google Scholar] [CrossRef]
- Salim, D.; Gerton, J.L. Ribosomal DNA instability and genome adaptability. Chromosome Res. 2019, 27, 73–87. [Google Scholar] [CrossRef]
| A Group | SZ Group | HC Group | |
|---|---|---|---|
| N | 75 | 43 | 86 |
| Age | 6 ± 3 | 8 ± 3 | 7 ± 2 |
| Gender, M/F (%) | 58/17 (77%/23%) | 34/9 (79%/21%) | 56/30 (65%/35%) |
| Catatonic syndrome | The onset before the age of three. The phenomenon of sensory dissociation combined with catatonic/hyperdynamic disorders in the active period of the disease, replaced by psychopathic and affective symptoms by an age of 3–4. Duration of the active period of the disease is 1.5–2 years. | The onset in the crisis periods of 1.5 and 3 years. Hyper- and hypokinetic catatonia during the attack, motor stereotypies, neurosis-like disorders in remission. The duration of manifest psychosis is 2.5 to 4 years. A developed polymorphic psychotic attack at age points of 6–7 and 12–13 years. | Not observed |
| Delusions, hallucinations, elements of the Kandinsky syndrome | Not observed | Observed from an age of 4–5 during the attacks (not less than 1 month); in the remission, rudimentary deceptions of perception, unsystematic delusions of attitude, damage, poisoning | Not observed |
| Negative impairments | Delay or arrest of speech development. Mild/moderate autism. | Arrest of speech development or speech loss during manifest psychosis and incomplete recovery during remission. Incoherence of speech after the second psychotic attack. Short attention span. Concreteness and torpidity of thinking. Moderate/severe autism | Not observed |
| Cognitive impairments | Perverted type of cognitive dysontogenesis | Deficiency-progredient or defecting type of cognitive dysontogenesis | Not observed |
| Disease course and prognosis | Positive dynamics of the course of the disease with “practical recovery” in 10%, transition to “high functional” autism in 30%, regredient course in 60%. Psychopharmacotherapy exclusively during the active period of the course of disease, correction of emotional or psychopathic disorders in remission. Social rehabilitation. | Paroxysmal-progressive or continuous course. Bad prognosis in case of absence of timely prescribed psychopharmacotherapy. Frequent exacerbations with polymorphic symptoms. Nursing, long-term psychopharmacotherapy, socialization throughout lifetime. | Normal development |
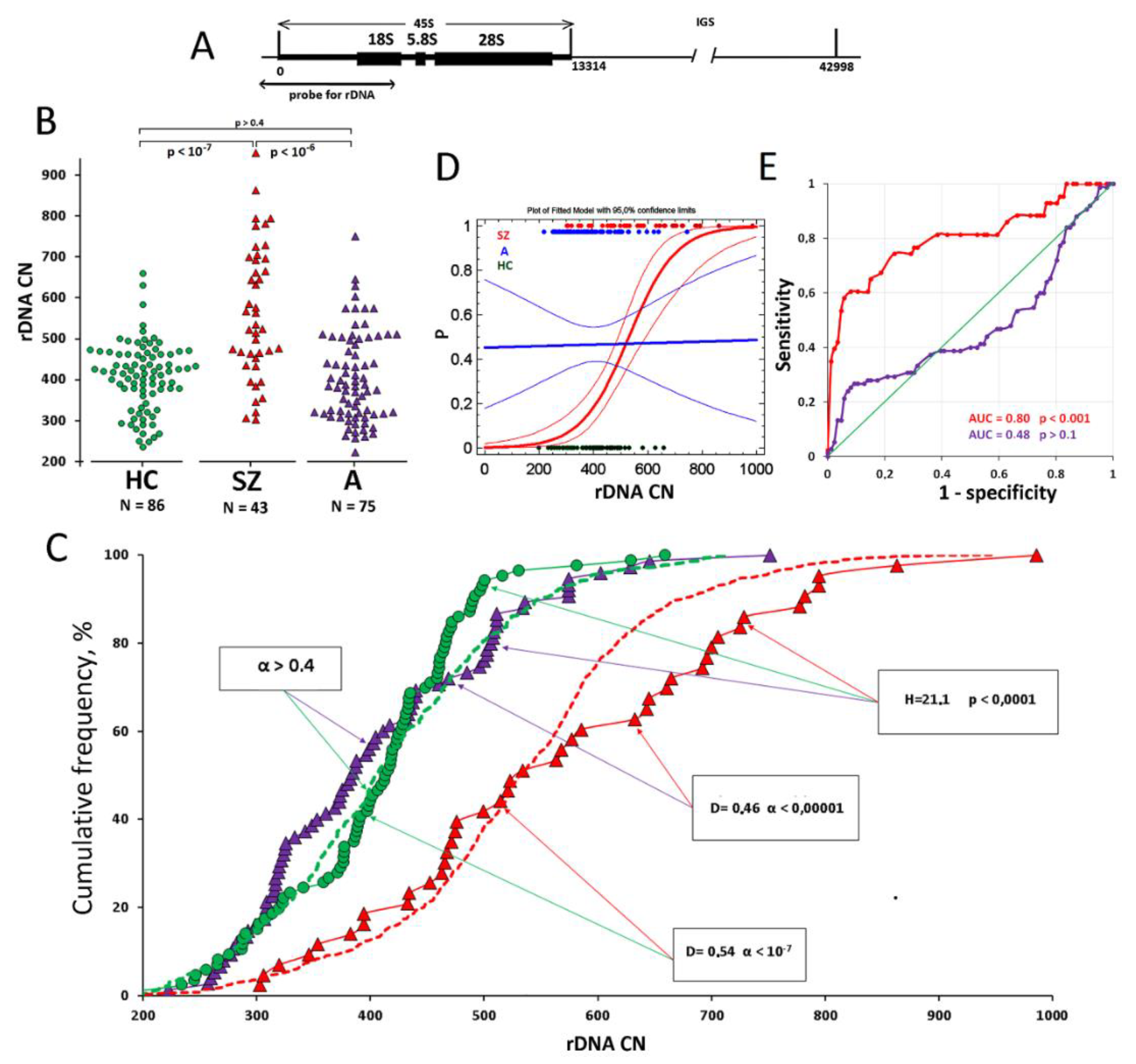
| Schizophrenia Patients (SZ Group) N = 43 | ASD Patients (A Group) N = 75 | Healthy CHILDREN (HC Group) N = 86 | |
|---|---|---|---|
| Mean | 565 | 405 | 403 |
| Standard deviation | 163 | 109 | 86 |
| Standard error | 24 | 12 | 9 |
| Minimum | 303 | 223 | 199 |
| Maximum | 986 | 751 | 659 |
| Range | 683 | 528 | 460 |
| CV (coefficient of variation) | 0.29 | 0.27 | 0.21 |
| Median | 534 | 384 | 414 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ershova, E.S.; Veiko, N.N.; Nikitina, S.G.; Balakireva, E.E.; Martynov, A.V.; Chudakova, J.M.; Shmarina, G.V.; Kostyuk, S.E.; Salimova, N.A.; Veiko, R.V.; et al. Ribosomal DNA Abundance in the Patient’s Genome as a Feasible Marker in Differential Diagnostics of Autism and Childhood-Onset Schizophrenia. J. Pers. Med. 2022, 12, 1796. https://doi.org/10.3390/jpm12111796
Ershova ES, Veiko NN, Nikitina SG, Balakireva EE, Martynov AV, Chudakova JM, Shmarina GV, Kostyuk SE, Salimova NA, Veiko RV, et al. Ribosomal DNA Abundance in the Patient’s Genome as a Feasible Marker in Differential Diagnostics of Autism and Childhood-Onset Schizophrenia. Journal of Personalized Medicine. 2022; 12(11):1796. https://doi.org/10.3390/jpm12111796
Chicago/Turabian StyleErshova, Elizaveta S., Natalia N. Veiko, Svetlana G. Nikitina, Elena E. Balakireva, Andrey V. Martynov, Julia M. Chudakova, Galina V. Shmarina, Svetlana E. Kostyuk, Nataliya A. Salimova, Roman V. Veiko, and et al. 2022. "Ribosomal DNA Abundance in the Patient’s Genome as a Feasible Marker in Differential Diagnostics of Autism and Childhood-Onset Schizophrenia" Journal of Personalized Medicine 12, no. 11: 1796. https://doi.org/10.3390/jpm12111796







