Cardiovascular Complications in Hematopoietic Stem Cell Transplanted Patients
Abstract
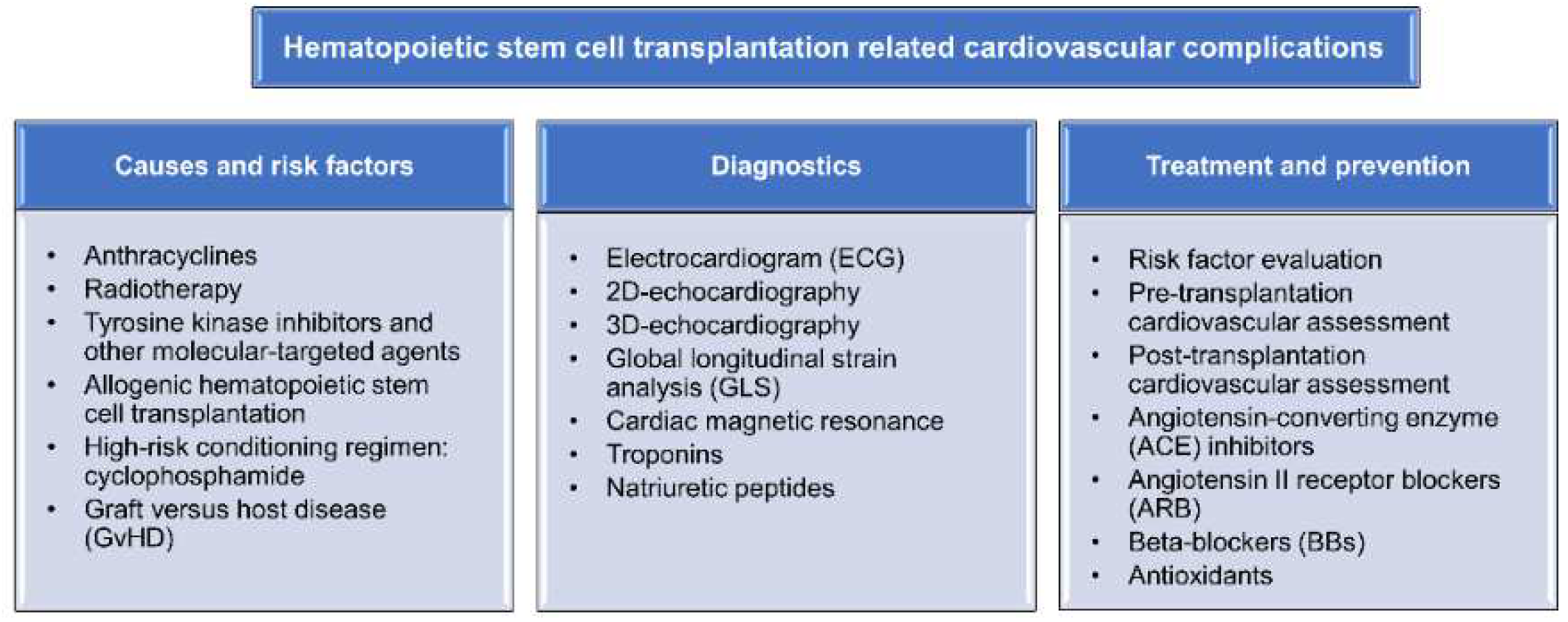
:1. Introduction
2. Cardiovascular Complications Associated with HSCT
2.1. Short-Term Cardiotoxicity during HSCT
2.2. Long-Term HSCT-Related Cardiovascular Complications
3. Factors and Causes of Cardiovascular Complications in HSCT
3.1. Anthracyclines
3.2. Radiotherapy
3.3. Tyrosine Kinase Inhibitors and Other Molecular-Targeted Agents
3.4. Conditioning Regimens and Cyclophosphamide
3.5. Graft-Versus-Host Disease (GvHD)
4. Diagnostics of Cardiovascular Complications
5. Treatment and Prevention Procedures
5.1. Risk Prediction
5.2. Bone Marrow Transplant Societies’ Guideline and Recommendation
5.3. European Society of Cardiology Guidelines and Recommendations
5.4. Cardioprotective Agents and Treatments
5.5. Antioxidants as Cardio-Protective Treatment
6. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Appelbaum, F.R. Hematopoietic-cell transplantation at 50. N. Engl. J. Med. 2007, 357, 1472–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copelan, E.A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 2006, 354, 1813–1826. [Google Scholar] [CrossRef]
- Niederwieser, D.; Baldomero, H.; Atsuta, Y.; Aljurf, M.; Seber, A.; Greinix, H.T.; Koh, M.; Worel, N.; Galeano, S.; Jaimovich, G.; et al. One and Half Million Hematopoietic Stem Cell Transplants (HSCT). Dissemination, Trends and Potential to Improve Activity By Telemedicine from the Worldwide Network for Blood and Marrow Transplantation (WBMT). Blood 2019, 134, 2035. [Google Scholar] [CrossRef]
- Morken, C.M.; Tevaarwerk, A.J.; Swiecichowski, A.K.; Haine, J.E.; Williams, Z.T.; Norslien, K.; Arroyo, N.; Zhang, X.; Campbell, B.; Mendonca, E.A.; et al. Survivor and Clinician Assessment of Survivorship Care Plans for Hematopoietic Stem Cell Transplantation Patients: An Engineering, Primary Care, and Oncology Collaborative for Survivorship Health. Biol. Blood Marrow Transplant. 2019, 25, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Passweg, J.R.; Baldomero, H.; Gratwohl, A.; Bregni, M.; Cesaro, S.; Dreger, P.; de Witte, T.; Farge-Bancel, D.; Gaspar, B.; Marsh, J.; et al. The EBMT activity survey: 1990–2010. Bone Marrow Transplant. 2012, 47, 906–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, S. Long-term health impacts of hematopoietic stem cell transplantation inform recommendations for follow-up. Expert Rev. Hematol. 2011, 4, 437–452. [Google Scholar] [CrossRef] [Green Version]
- Martin, P.J.; Counts, G.W.; Appelbaum, F.R.; Lee, S.J.; Sanders, J.E.; Deeg, H.J.; Flowers, M.E.D.; Syrjala, K.L.; Hansen, J.A.; Storb, R.F.; et al. Life Expectancy in Patients Surviving More than 5 Years After Hematopoietic Cell Transplantation. J. Clin. Oncol. 2010, 28, 1011–1016. [Google Scholar] [CrossRef] [Green Version]
- Bresters, D.; van Gils, I.C.; Kollen, W.J.; Ball, L.M.; Oostdijk, W.; van der Bom, J.G.; Egeler, R.M. High burden of late effects after haematopoietic stem cell transplantation in childhood: A single-centre study. Bone Marrow Transplant. 2010, 45, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.L.; Francisco, L.; Kawashima, T.; Leisenring, W.; Robison, L.L.; Baker, K.S.; Weisdorf, D.J.; Forman, S.J.; Bhatia, S. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: A report from the Bone Marrow Transplant Survivor Study. Blood 2010, 116, 3129–3139. [Google Scholar] [CrossRef] [Green Version]
- Ohmoto, A.; Fuji, S. Cardiac complications associated with hematopoietic stem-cell transplantation. Bone Marrow Transplant. 2021, 56, 2637–2643. [Google Scholar] [CrossRef]
- Tichelli, A.; Bucher, C.; Rovo, A.; Stussi, G.; Stern, M.; Paulussen, M.; Halter, J.; Meyer-Monard, S.; Heim, D.; Tsakiris, D.A.; et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood 2007, 110, 3463–3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotz, S.J.; Ryan, T.D.; Hayek, S.S. Cardiovascular disease and its management in children and adults undergoing hematopoietic stem cell transplantation. J. Thromb. Thrombolysis 2021, 51, 854–869. [Google Scholar] [CrossRef] [PubMed]
- Buja, L.M.; Ferrans, V.J.; Graw, R.G., Jr. Cardiac pathologic findings in patients treated with bone marrow transplantation. Hum. Pathol. 1976, 7, 17–45. [Google Scholar] [CrossRef]
- Roziakova, L.; Mistrik, M.; Batorova, A.; Kruzliak, P.; Bojtarova, E.; Dubrava, J.; Gergel, J.; Mladosievicova, B. Can we predict clinical cardiotoxicity with cardiac biomarkers in patients after haematopoietic stem cell transplantation? Cardiovasc Toxicol. 2015, 15, 210–216. [Google Scholar] [CrossRef]
- Tuzovic, M.; Mead, M.; Young, P.A.; Schiller, G.; Yang, E.H. Cardiac Complications in the Adult Bone Marrow Transplant Patient. Curr. Oncol. Rep. 2019, 21, 28. [Google Scholar] [CrossRef] [Green Version]
- Tonorezos, E.S.; Stillwell, E.E.; Calloway, J.J.; Glew, T.; Wessler, J.D.; Rebolledo, B.J.; Pham, A.; Steingart, R.M.; Lazarus, H.; Gale, R.P.; et al. Arrhythmias in the setting of hematopoietic cell transplants. Bone Marrow Transplant. 2015, 50, 1212–1216. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, S.L.; Klumpp, T.R.; Magdalinski, A.J.; Mangan, K.F. Value of the pretransplant evaluation in predicting toxic day-100 mortality among blood stem-cell and bone marrow transplant recipients. J. Clin. Oncol. 1998, 16, 3796–3802. [Google Scholar] [CrossRef]
- Versluys, A.B.; Grotenhuis, H.B.; Boelens, M.J.J.; Mavinkurve-Groothuis, A.M.C.; Breur, J. Predictors and Outcome of Pericardial Effusion after Hematopoietic Stem Cell Transplantation in Children. Pediatr. Cardiol. 2018, 39, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, D.C.; de Oliveira, J.S.; Parisio, K.; Ramalho, F.M. Pericardial effusion and cardiac tamponade: Clinical manifestation of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Rev. Bras. Hematol. Hemoter. 2014, 36, 159–161. [Google Scholar] [CrossRef] [Green Version]
- Peres, E.; Levine, J.E.; Khaled, Y.A.; Ibrahim, R.B.; Braun, T.M.; Krijanovski, O.I.; Mineishi, S.; Abidi, M.H. Cardiac complications in patients undergoing a reduced-intensity conditioning hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010, 45, 149–152. [Google Scholar] [CrossRef]
- Gul, Z.; Bashir, Q.; Cremer, M.; Yusuf, S.W.; Gunaydin, H.; Arora, S.; Slone, S.; Nieto, Y.; Sherwani, N.; Parmar, S.; et al. Short-term cardiac toxicity of autologous hematopoietic stem cell transplant for multiple myeloma. Leuk. Lymphoma 2015, 56, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.D.; Xu, L.P.; Liu, D.H.; Zhang, X.H.; Chen, H.; Chen, Y.H.; Han, W.; Wang, Y.; Wang, F.R.; Wang, J.Z.; et al. Heart failure after allogeneic hematopoietic stem cell transplantation. Int. J. Cardiol. 2013, 167, 2502–2506. [Google Scholar] [CrossRef] [PubMed]
- Murdych, T.; Weisdorf, D.J. Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977-1997. Bone Marrow Transplant. 2001, 28, 283–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alblooshi, R.; Kanfar, S.; Lord, B.; Atenafu, E.G.; Michelis, F.V.; Pasic, I.; Gerbitz, A.; Al-Shaibani, Z.; Viswabandya, A.; Kim, D.D.H.; et al. Clinical prevalence and outcome of cardiovascular events in the first 100 days postallogeneic hematopoietic stem cell transplant. Eur. J. Haematol. 2021, 106, 32–39. [Google Scholar] [CrossRef]
- Hertenstein, B.; Stefanic, M.; Schmeiser, T.; Scholz, M.; Goller, V.; Clausen, M.; Bunjes, D.; Wiesneth, M.; Novotny, J.; Kochs, M. Cardiac toxicity of bone marrow transplantation: Predictive value of cardiologic evaluation before transplant. J. Clin. Oncol. 1994, 12, 998–1004. [Google Scholar] [CrossRef]
- Kupari, M.; Volin, L.; Suokas, A.; Hekali, P.; Ruutu, T. Cardiac involvement in bone marrow transplantation: Serial changes in left ventricular size, mass and performance. J. Intern. Med. 1990, 227, 259–266. [Google Scholar] [CrossRef]
- Fujimaki, K.; Maruta, A.; Yoshida, M.; Sakai, R.; Tanabe, J.; Koharazawa, H.; Kodama, F.; Asahina, S.; Minamizawa, M.; Matsuzaki, M.; et al. Severe cardiac toxicity in hematological stem cell transplantation: Predictive value of reduced left ventricular ejection fraction. Bone Marrow Transplant. 2001, 27, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Sakata-Yanagimoto, M.; Kanda, Y.; Nakagawa, M.; Asano-Mori, Y.; Kandabashi, K.; Izutsu, K.; Imai, Y.; Hangaishi, A.; Kurokawa, M.; Tsujino, S.; et al. Predictors for severe cardiac complications after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004, 33, 1043–1047. [Google Scholar] [CrossRef] [Green Version]
- Armenian, S.H.; Sun, C.L.; Shannon, T.; Mills, G.; Francisco, L.; Venkataraman, K.; Wong, F.L.; Forman, S.J.; Bhatia, S. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood 2011, 118, 6023–6029. [Google Scholar] [CrossRef] [Green Version]
- Armenian, S.H.; Sun, C.L.; Francisco, L.; Steinberger, J.; Kurian, S.; Wong, F.L.; Sharp, J.; Sposto, R.; Forman, S.J.; Bhatia, S. Late congestive heart failure after hematopoietic cell transplantation. J. Clin. Oncol. 2008, 26, 5537–5543. [Google Scholar] [CrossRef]
- Leger, K.J.; Baker, K.S.; Cushing-Haugen, K.L.; Flowers, M.E.D.; Leisenring, W.M.; Martin, P.J.; Mendoza, J.A.; Reding, K.W.; Syrjala, K.L.; Lee, S.J.; et al. Lifestyle factors and subsequent ischemic heart disease risk after hematopoietic cell transplantation. Cancer 2018, 124, 1507–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leger, K.J.; Cushing-Haugen, K.; Hansen, J.A.; Fan, W.; Leisenring, W.M.; Martin, P.J.; Zhao, L.P.; Chow, E.J. Clinical and Genetic Determinants of Cardiomyopathy Risk among Hematopoietic Cell Transplantation Survivors. Biol. Blood Marrow Transplant. 2016, 22, 1094–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, E.J.; Mueller, B.A.; Baker, K.S.; Cushing-Haugen, K.L.; Flowers, M.E.; Martin, P.J.; Friedman, D.L.; Lee, S.J. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann. Intern. Med. 2011, 155, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Sun, C.L.; Vase, T.; Ness, K.K.; Blum, E.; Francisco, L.; Venkataraman, K.; Samoa, R.; Wong, F.L.; Forman, S.J.; et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: Role in development of subsequent cardiovascular disease. Blood 2012, 120, 4505–4512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Lacchetti, C.; Lenihan, D. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. J. Oncol. Pract. 2017, 13, 270–275. [Google Scholar] [CrossRef]
- Curigliano, G.; Cardinale, D.; Dent, S.; Criscitiello, C.; Aseyev, O.; Lenihan, D.; Cipolla, C.M. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J. Clin. 2016, 66, 309–325. [Google Scholar] [CrossRef] [Green Version]
- Heidenreich, P.A.; Kapoor, J.R. Radiation induced heart disease: Systemic disorders in heart disease. Heart 2009, 95, 252–258. [Google Scholar] [CrossRef]
- Armenian, S.H.; Sun, C.L.; Mills, G.; Teh, J.B.; Francisco, L.; Durand, J.B.; Wong, F.L.; Forman, S.J.; Bhatia, S. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2010, 16, 1138–1144. [Google Scholar] [CrossRef] [Green Version]
- Mulrooney, D.A.; Nunnery, S.E.; Armstrong, G.T.; Ness, K.K.; Srivastava, D.; Donovan, F.D.; Kurt, B.A.; Metzger, M.L.; Krasin, M.J.; Joshi, V.; et al. Coronary artery disease detected by coronary computed tomography angiography in adult survivors of childhood Hodgkin lymphoma. Cancer 2014, 120, 3536–3544. [Google Scholar] [CrossRef]
- Sivgin, S.; Eser, B. The management of iron overload in allogeneic hematopoietic stem cell transplant (alloHSCT) recipients: Where do we stand? Ann. Hematol. 2013, 92, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.; Loscocco, F.; Visani, G.; Chiarucci, M.; Musto, P.; Kubasch, A.S.; Platzbecker, U.; Vinchi, F. Iron Toxicity and Chelation Therapy in Hematopoietic Stem Cell Transplant. Transplant. Cell 2021, 27, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Gagelmann, N.; Wolschke, C.; Klyuchnikov, E.; Christopeit, M.; Ayuk, F.; Kroger, N. TKI Maintenance After Stem-Cell Transplantation for FLT3-ITD Positive Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 630429. [Google Scholar] [CrossRef] [PubMed]
- Burchert, A.; Bug, G.; Fritz, L.V.; Finke, J.; Stelljes, M.; Rollig, C.; Wollmer, E.; Wasch, R.; Bornhauser, M.; Berg, T.; et al. Sorafenib Maintenance after Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia with FLT3-Internal Tandem Duplication Mutation (SORMAIN). J. Clin. Oncol. 2020, 38, 2993–3002. [Google Scholar] [CrossRef]
- Moslehi, J.J. Cardiovascular Toxic Effects of Targeted Cancer Therapies. N. Engl. J. Med. 2016, 375, 1457–1467. [Google Scholar] [CrossRef]
- Schmidinger, M.; Zielinski, C.C.; Vogl, U.M.; Bojic, A.; Bojic, M.; Schukro, C.; Ruhsam, M.; Hejna, M.; Schmidinger, H. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2008, 26, 5204–5212. [Google Scholar] [CrossRef] [PubMed]
- Ratain, M.J.; Moslehi, J.J.; Lichter, A.S. Ibrutinib’s Cardiotoxicity-An Opportunity for Postmarketing Regulation. JAMA Oncol. 2021, 7, 177–178. [Google Scholar] [CrossRef]
- Caldeira, D.; Alves, D.; Costa, J.; Ferreira, J.J.; Pinto, F.J. Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta-analysis. PLoS ONE 2019, 14, e0211228. [Google Scholar] [CrossRef] [Green Version]
- Ganatra, S.; Sharma, A.; Shah, S.; Chaudhry, G.M.; Martin, D.T.; Neilan, T.G.; Mahmood, S.S.; Barac, A.; Groarke, J.D.; Hayek, S.S.; et al. Ibrutinib-Associated Atrial Fibrillation. JACC Clin. Electrophysiol. 2018, 4, 1491–1500. [Google Scholar] [CrossRef]
- Heeringa, J.; van der Kuip, D.A.; Hofman, A.; Kors, J.A.; van Herpen, G.; Stricker, B.H.; Stijnen, T.; Lip, G.Y.; Witteman, J.C. Prevalence, incidence and lifetime risk of atrial fibrillation: The Rotterdam study. Eur. Heart J. 2006, 27, 949–953. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Ballen, K.; Rizzo, D.; Giralt, S.; Lazarus, H.; Ho, V.; Apperley, J.; Slavin, S.; Pasquini, M.; Sandmaier, B.M.; et al. Defining the intensity of conditioning regimens: Working definitions. Biol. Blood Marrow Transplant. 2009, 15, 1628–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanholtz, C. Acute life-threatening toxicity of cancer treatment. Crit. Care Clin. 2001, 17, 483–502. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Antin, J.H.; Guinan, E.C.; Rappeport, J.M. Cyclophosphamide cardiotoxicity: An analysis of dosing as a risk factor. Blood 1986, 68, 1114–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braverman, A.C.; Antin, J.H.; Plappert, M.T.; Cook, E.F.; Lee, R.T. Cyclophosphamide cardiotoxicity in bone marrow transplantation: A prospective evaluation of new dosing regimens. J. Clin. Oncol. 1991, 9, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Doki, N.; Shingai, N.; Yoshioka, K.; Kakihana, K.; Sakamaki, H.; Ohashi, K. The clinical features of fatal cyclophosphamide-induced cardiotoxicity in a conditioning regimen for allogeneic hematopoietic stem cell transplantation (allo-HSCT). Ann. Hematol. 2016, 95, 1145–1150. [Google Scholar] [CrossRef]
- Marumo, A.; Omori, I.; Tara, S.; Otsuka, Y.; Konuma, R.; Adachi, H.; Wada, A.; Kishida, Y.; Konishi, T.; Nagata, A.; et al. Cyclophosphamide-induced cardiotoxicity at conditioning for allogeneic hematopoietic stem cell transplantation would occur among the patients treated with 120 mg/kg or less. Asia Pac. J. Clin. Oncol. 2022, 18, e507–e514. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, Y.; Han, T.; Cheng, Y.; Mo, X.; Wang, J.; Chen, Y.; Wang, F.; Tang, F.; Han, W.; et al. A modified conditioning regimen based on low-dose cyclophosphamide and fludarabine for haploidentical hematopoietic stem cell transplant in severe aplastic anemia patients at risk of severe cardiotoxicity. Clin. Transplant. 2022, 36, e14514. [Google Scholar] [CrossRef]
- Al-Hashmi, S.; Boels, P.J.M.; Zadjali, F.; Sadeghi, B.; Sällström, J.; Hultenby, K.; Hassan, Z.; Arner, A.; Hassan, M. Busulphan-cyclophosphamide cause endothelial injury, remodeling of resistance arteries and enhanced expression of endothelial nitric oxide synthase. PLoS ONE 2012, 7, e30897. [Google Scholar] [CrossRef] [Green Version]
- Bolanos-Meade, J.; Reshef, R.; Fraser, R.; Fei, M.; Abhyankar, S.; Al-Kadhimi, Z.; Alousi, A.M.; Antin, J.H.; Arai, S.; Bickett, K.; et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: A randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019, 6, e132–e143. [Google Scholar]
- Luznik, L.; O’Donnell, P.V.; Symons, H.J.; Chen, A.R.; Leffell, M.S.; Zahurak, M.; Gooley, T.A.; Piantadosi, S.; Kaup, M.; Ambinder, R.F.; et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol. Blood Marrow Transplant. 2008, 14, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Luznik, L.; Bolanos-Meade, J.; Zahurak, M.; Chen, A.R.; Smith, B.D.; Brodsky, R.; Huff, C.A.; Borrello, I.; Matsui, W.; Powell, J.D.; et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood 2010, 115, 3224–3230. [Google Scholar] [CrossRef] [PubMed]
- Kanakry, C.G.; O’Donnell, P.V.; Furlong, T.; de Lima, M.J.; Wei, W.; Medeot, M.; Mielcarek, M.; Champlin, R.E.; Jones, R.J.; Thall, P.F.; et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J. Clin. Oncol. 2014, 32, 3497–3505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, J.; Whited, L.; Saliba, R.M.; Rondon, G.; Banchs, J.; Shpall, E.; Champlin, R.; Popat, U. Cardiac toxicity after matched allogeneic hematopoietic cell transplant in the posttransplant cyclophosphamide era. Blood Adv. 2021, 5, 5599–5607. [Google Scholar] [CrossRef] [PubMed]
- Dulery, R.; Mohty, R.; Labopin, M.; Sestili, S.; Malard, F.; Brissot, E.; Battipaglia, G.; Mediavilla, C.; Banet, A.; Van de Wyngaert, Z.; et al. Early Cardiac Toxicity Associated With Post-Transplant Cyclophosphamide in Allogeneic Stem Cell Transplantation. JACC CardioOncol. 2021, 3, 250–259. [Google Scholar] [CrossRef]
- Ritchie, D.S.; Seymour, J.F.; Roberts, A.W.; Szer, J.; Grigg, A.P. Acute left ventricular failure following melphalan and fludarabine conditioning. Bone Marrow Transplant. 2001, 28, 101–103. [Google Scholar] [CrossRef] [Green Version]
- Phillips, G.L.; Meisenberg, B.; Reece, D.E.; Adams, V.R.; Badros, A.; Brunner, J.; Fenton, R.; Filicko, J.; Grosso, D.; Hale, G.A.; et al. Amifostine and autologous hematopoietic stem cell support of escalating-dose melphalan: A phase I study. Biol. Blood Marrow Transplant. 2004, 10, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Flowers, M.E.; Parker, P.M.; Johnston, L.J.; Matos, A.V.; Storer, B.; Bensinger, W.I.; Storb, R.; Appelbaum, F.R.; Forman, S.J.; Blume, K.G.; et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: Long-term follow-up of a randomized trial. Blood 2002, 100, 415–419. [Google Scholar] [CrossRef]
- Armenian, S.H.; Chow, E.J. Cardiovascular disease in survivors of hematopoietic cell transplantation. Cancer 2014, 120, 469–479. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.L.; Rule, S.; Martin, P.; Goy, A.; Auer, R.; Kahl, B.S.; Jurczak, W.; Advani, R.H.; Romaguera, J.E.; Williams, M.E.; et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2013, 369, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Mesa, R.A.; Verstovsek, S.; Gupta, V.; Mascarenhas, J.O.; Atallah, E.; Burn, T.; Sun, W.; Sandor, V.; Gotlib, J. Effects of ruxolitinib treatment on metabolic and nutritional parameters in patients with myelofibrosis from COMFORT-I. Clin. Lymphoma Myeloma Leuk. 2015, 15, 214–221.e1. [Google Scholar] [CrossRef] [Green Version]
- Tichelli, A.; Gratwohl, A. Vascular endothelium as ‘novel’ target of graft-versus-host disease. Best Pract. Res. Clin. Haematol. 2008, 21, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wright, L.; Negishi, T.; Negishi, K.; Liu, J.; Marwick, T.H. Research to Practice: Assessment of Left Ventricular Global Longitudinal Strain for Surveillance of Cancer Chemotherapeutic-Related Cardiac Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1196–1201. [Google Scholar] [CrossRef]
- Liu, J.; Banchs, J.; Mousavi, N.; Plana, J.C.; Scherrer-Crosbie, M.; Thavendiranathan, P.; Barac, A. Contemporary Role of Echocardiography for Clinical Decision Making in Patients During and After Cancer Therapy. JACC Cardiovasc. Imaging 2018, 11, 1122–1131. [Google Scholar] [CrossRef]
- Ryan, T.D.; Moore, R.A.; Lang, S.M.; Khoury, P.; Dandoy, C.E.; Davies, S.M.; Taylor, M.D. Rapid cardiac MRI protocol for cardiac assessment in paediatric and young adult patients undergoing haematopoietic stem cell transplant: A feasibility study. Cardiol. Young 2021, 31, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.A.; Hill, G.R.; Hunt, P.; Carnoutsos, S.; Spearing, R.L.; Espiner, E.; Hart, D.N. Assessment of cardiotoxicity during haemopoietic stem cell transplantation with plasma brain natriuretic peptide. Bone Marrow Transplant. 2000, 26, 309–313. [Google Scholar] [CrossRef] [Green Version]
- Roziakova, L.; Bojtarova, E.; Mistrik, M.; Dubrava, J.; Gergel, J.; Lenkova, N.; Mladosievicova, B. Serial measurements of cardiac biomarkers in patients after allogeneic hematopoietic stem cell transplantation. J. Exp. Clin. Cancer Res. 2012, 31, 13. [Google Scholar] [CrossRef] [Green Version]
- Roziakova, L.; Bojtarova, E.; Mistrik, M.; Krajcovicova, I.; Mladosievicova, B. Abnormal cardiomarkers in leukemia patients treated with allogeneic hematopoietic stem cell transplantation. Bratisl. Lek. Listy 2012, 113, 159–162. [Google Scholar] [CrossRef] [Green Version]
- Armenian, S.H.; Yang, D.; Teh, J.B.; Atencio, L.C.; Gonzales, A.; Wong, F.L.; Leisenring, W.M.; Forman, S.J.; Nakamura, R.; Chow, E.J. Prediction of cardiovascular disease among hematopoietic cell transplantation survivors. Blood Adv. 2018, 2, 1756–1764. [Google Scholar] [CrossRef]
- Oliveira, G.H.; Al-Kindi, S.G.; Guha, A.; Dey, A.K.; Rhea, I.B.; deLima, M.J. Cardiovascular risk assessment and management of patients undergoing hematopoietic cell transplantation. Bone Marrow Transplant. 2021, 56, 544–551. [Google Scholar] [CrossRef]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 23, e333–e465. [Google Scholar]
- Miller, L.W. Cardiovascular toxicities of immunosuppressive agents. Am. J. Transplant. 2002, 2, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Gharib, M.I.; Burnett, A.K. Chemotherapy-induced cardiotoxicity: Current practice and prospects of prophylaxis. Eur J. Heart Fail. 2002, 4, 235–242. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch, X.; Rovira, M.; Sitges, M.; Domenech, A.; Ortiz-Perez, J.T.; de Caralt, T.M.; Morales-Ruiz, M.; Perea, R.J.; Monzo, M.; Esteve, J. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: The OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J. Am. Coll. Cardiol. 2013, 61, 2355–2362. [Google Scholar]
- Cardinale, D.; Colombo, A.; Sandri, M.T.; Lamantia, G.; Colombo, N.; Civelli, M.; Martinelli, G.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006, 114, 2474–2481. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.; Kumar Singh, S.; Agrawal, V.; Bali Singh, T. Role of ACE inhibitors in anthracycline-induced cardiotoxicity: A randomized, double-blind, placebo-controlled trial. Pediatr. Blood Cancer 2018, 65, e27308. [Google Scholar] [CrossRef]
- Wang, W.; Kang, P.M. Oxidative Stress and Antioxidant Treatments in Cardiovascular Diseases. Antioxidants 2020, 9, 1292. [Google Scholar] [CrossRef]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Sabri, A.; Hughie, H.H.; Lucchesi, P.A. Regulation of hypertrophic and apoptotic signaling pathways by reactive oxygen species in cardiac myocytes. Antioxid. Redox Signal. 2003, 5, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181-90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kameda, K.; Matsunaga, T.; Abe, N.; Hanada, H.; Ishizaka, H.; Ono, H.; Saitoh, M.; Fukui, K.; Fukuda, I.; Osanai, T.; et al. Correlation of oxidative stress with activity of matrix metalloproteinase in patients with coronary artery disease. Possible role for left ventricular remodelling. Eur. Heart J. 2003, 24, 2180–2185. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.A.; Yuan Yuan, D.; Nawaz, W.; Ze, H.; Zhuo, C.X.; Talal, B.; Taleb, A.; Mais, E.; Qilong, D. Antioxidant therapy for management of oxidative stress induced hypertension. Free Radic. Res. 2017, 51, 428–438. [Google Scholar] [CrossRef]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef] [Green Version]
- Vincent, D.T.; Ibrahim, Y.F.; Espey, M.G.; Suzuki, Y.J. The role of antioxidants in the era of cardiooncology. Cancer Chemother. Pharm. 2013, 72, 1157–1168. [Google Scholar] [CrossRef] [Green Version]
- Monsuez, J.J. Detection and prevention of cardiac complications of cancer chemotherapy. Arch. Cardiovasc. Dis. 2012, 105, 593–604. [Google Scholar] [CrossRef] [Green Version]
- de Baat, E.C.; Mulder, R.L.; Armenian, S.; Feijen, E.A.; Grotenhuis, H.; Hudson, M.M.; Mavinkurve-Groothuis, A.M.; Kremer, L.C.; van Dalen, E.C. Dexrazoxane for preventing or reducing cardiotoxicity in adults and children with cancer receiving anthracyclines. Cochrane Database Syst. Rev. 2022, 9, CD014638. [Google Scholar] [CrossRef]
- Cvetkovic, R.S.; Scott, L.J. Dexrazoxane: A review of its use for cardioprotection during anthracycline chemotherapy. Drugs 2005, 65, 1005–1024. [Google Scholar] [CrossRef]
- Testore, F.; Milanese, S.; Ceste, M.; de Conciliis, E.; Parello, G.; Lanfranco, C.; Manfredi, R.; Ferrero, G.; Simoni, C.; Miglietta, L.; et al. Cardioprotective effect of dexrazoxane in patients with breast cancer treated with anthracyclines in adjuvant setting: A 10-year single institution experience. Am. J. Cardiovasc. Drugs 2008, 8, 257–263. [Google Scholar] [CrossRef]
- Tebbi, C.K.; London, W.B.; Friedman, D.; Villaluna, D.; De Alarcon, P.A.; Constine, L.S.; Mendenhall, N.P.; Sposto, R.; Chauvenet, A.; Schwartz, C.L. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J. Clin. Oncol. 2007, 25, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Salzer, W.L.; Devidas, M.; Carroll, W.L.; Winick, N.; Pullen, J.; Hunger, S.P.; Camitta, B.A. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: A report from the children’s oncology group. Leukemia 2010, 24, 355–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.R.; Sharma, A.; Lytwyn, M.; Bohonis, S.; Thliveris, J.; Singal, P.K.; Jassal, D.S. The cardioprotective role of probucol against anthracycline and trastuzumab-mediated cardiotoxicity. J. Am. Soc. Echocardiogr. 2011, 24, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Tikoo, K.; Sane, M.S.; Gupta, C. Tannic acid ameliorates doxorubicin-induced cardiotoxicity and potentiates its anti-cancer activity: Potential role of tannins in cancer chemotherapy. Toxicol. Appl. Pharm. 2011, 251, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.; Barshack, I.; Luboshits, G.; Wexler, D.; Deutsch, V.; Keren, G.; George, J. Erythropoietin improves myocardial performance in doxorubicin-induced cardiomyopathy. Eur. Heart J. 2006, 27, 1876–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayza, M.A.; Zewdie, K.A.; Tesfaye, B.A.; Wondafrash, D.Z.; Berhe, A.H. The Role of Antioxidants in Ameliorating Cyclophosphamide-Induced Cardiotoxicity. Oxid. Med. Cell Longev. 2020, 2020, 4965171. [Google Scholar] [CrossRef]
- Mythili, Y.; Sudharsan, P.T.; Selvakumar, E.; Varalakshmi, P. Protective effect of DL-alpha-lipoic acid on cyclophosphamide induced oxidative cardiac injury. Chem. Biol. Interact. 2004, 151, 13–19. [Google Scholar] [CrossRef]
- Mythili, Y.; Sudharsan, P.T.; Sudhahar, V.; Varalakshmi, P. Protective effect of DL-alpha-lipoic acid on cyclophosphamide induced hyperlipidemic cardiomyopathy. Eur. J. Pharm. 2006, 543, 92–96. [Google Scholar] [CrossRef]
- Mythili, Y.; Sudharsan, P.T.; Amudha, G.; Varalakshmi, P. Effect of DL-alpha-lipoic acid on cyclophosphamide induced lysosomal changes in oxidative cardiotoxicity. Life Sci. 2007, 80, 1993–1998. [Google Scholar] [CrossRef]
- Gunes, S.; Sahinturk, V.; Karasati, P.; Sahin, I.K.; Ayhanci, A. Cardioprotective Effect of Selenium against Cyclophosphamide-Induced Cardiotoxicity in Rats. Biol. Trace Elem. Res. 2017, 177, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.H.; El Kiki, S.M.; Hasan, H.F. Protective effect of N-acetylcysteine on cyclophosphamide-induced cardiotoxicity in rats. Env. Toxicol. Pharm. 2015, 40, 417–422. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, A.A.; Abdelzaher, W.Y.; Gad, A.A.; Abdel-Gaber, S.A. Purine versus non-purine xanthine oxidase inhibitors against cyclophosphamide-induced cardiac and bone marrow toxicity in rats. Hum. Exp. Toxicol. 2020, 39, 249–261. [Google Scholar] [CrossRef]
- Horinaka, S. Use of nicorandil in cardiovascular disease and its optimization. Drugs 2011, 71, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Refaie, M.M.M.; Shehata, S.; El-Hussieny, M.; Abdelraheem, W.M.; Bayoumi, A.M.A. Role of ATP-Sensitive Potassium Channel (KATP) and eNOS in Mediating the Protective Effect of Nicorandil in Cyclophosphamide-Induced Cardiotoxicity. Cardiovasc. Toxicol. 2020, 20, 71–81. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; He, R.; Oerther, S.; Zhou, W.; Vosough, M.; Hassan, M. Cardiovascular Complications in Hematopoietic Stem Cell Transplanted Patients. J. Pers. Med. 2022, 12, 1797. https://doi.org/10.3390/jpm12111797
Zhao Y, He R, Oerther S, Zhou W, Vosough M, Hassan M. Cardiovascular Complications in Hematopoietic Stem Cell Transplanted Patients. Journal of Personalized Medicine. 2022; 12(11):1797. https://doi.org/10.3390/jpm12111797
Chicago/Turabian StyleZhao, Ying, Rui He, Sandra Oerther, Weiying Zhou, Massoud Vosough, and Moustapha Hassan. 2022. "Cardiovascular Complications in Hematopoietic Stem Cell Transplanted Patients" Journal of Personalized Medicine 12, no. 11: 1797. https://doi.org/10.3390/jpm12111797
APA StyleZhao, Y., He, R., Oerther, S., Zhou, W., Vosough, M., & Hassan, M. (2022). Cardiovascular Complications in Hematopoietic Stem Cell Transplanted Patients. Journal of Personalized Medicine, 12(11), 1797. https://doi.org/10.3390/jpm12111797






