Degenerative Disc Disease of the Spine: From Anatomy to Pathophysiology and Radiological Appearance, with Morphological and Functional Considerations
Abstract
:1. Introduction
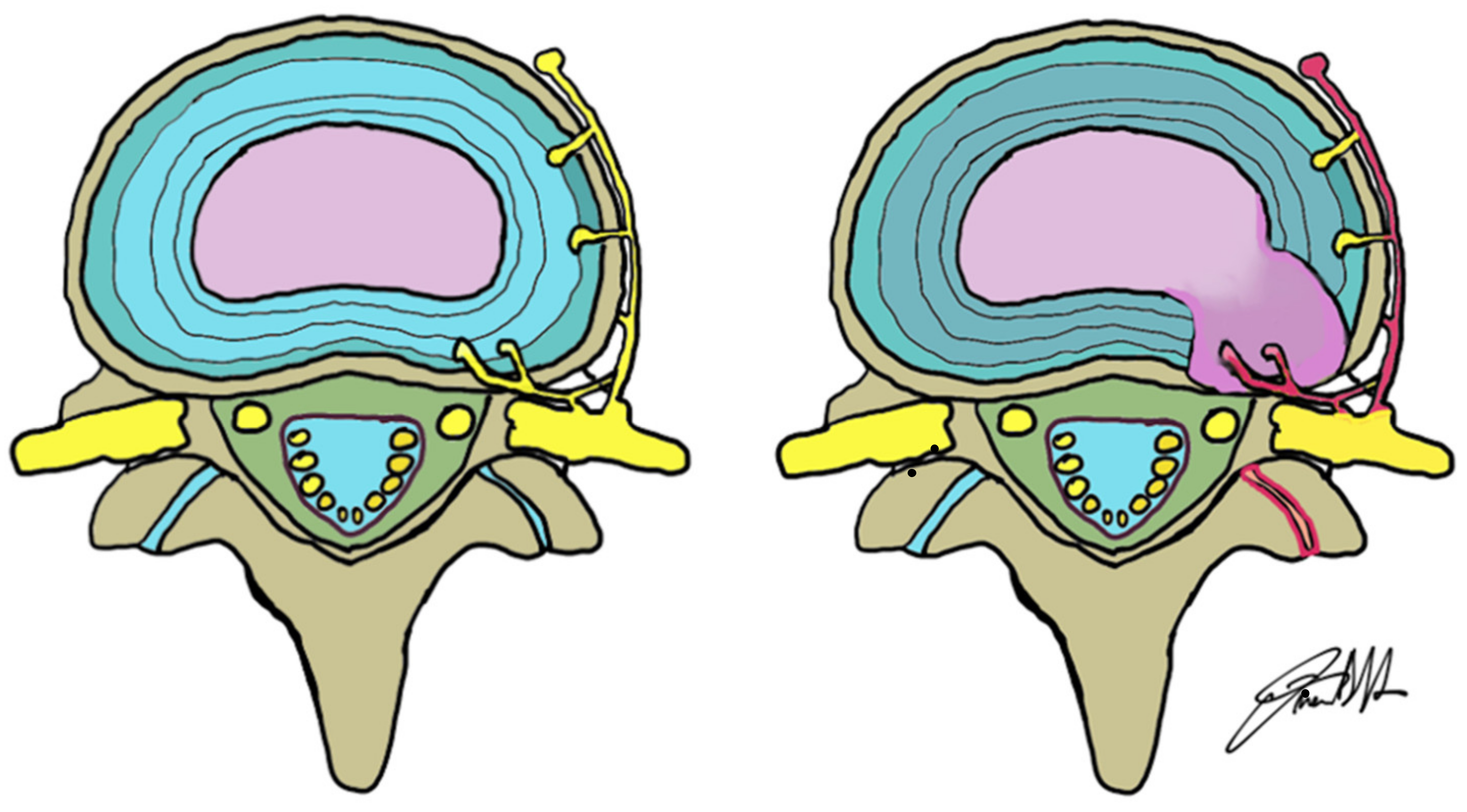
2. Anatomical Considerations: The Normal Disc and the Discovertebral Complex
3. Pathophysiology of Degenerative Disc Disease
4. Radiological Considerations
5. Nomenclature
- Aging disc: aging effects show loss of water content from the nucleus, which is an alteration that occurs before MRI changes consistent with the progressive loss of water content and the increase in collagen and aggregating proteoglycans [49].
- Disc degeneration refers to various alterations made in any of the following ways: desiccation, cleft formation, fibrosis, gaseous or mucinous degradation of the nucleus, fissuring, loss of integrity of the anulus, defects in and/or sclerosis of the end plates and to the presence of osteophytes at the vertebral apophyses. Imaging features of disc degeneration include morphological alterations such as disc space narrowing and peridiscal osteophytes. Various grading systems referring, respectively, to MRI disc modifications (Pfirrmann classification) [49], MRI changes of vertebral end plate, and subchondral bone marrow features (Modic classification) [16] can be used to assess those kinds of changes. (Table 3)
- Degenerative disc disease is a clinical condition evidenced by disc degeneration and symptoms associated with degenerative changes. A causal relationship between disc degeneration and symptoms such as low back pain is sometimes difficult to establish. However, the term “Degenerative disc disease” suggests an illness and the term should be considered as a nonstandard one when used instead of “degenerated disc” or “disc degeneration” for describing imaging features of the degenerative spine.
- Dark disc or black disc is a colloquial, nonstandard term used to describe a dehydrated disc. On MRI imaging, the disc loses its central high T2 signal intensity and it appears dark as a consequence of the dehydration of the nucleus.
- Disc height is defined as the distance between vertebral endplates on adjacent vertebrae. With degenerative disc disease, the intervertebral disc shrinks in height. In order to quantify the degree of such kind of modification, disc height should be measured at the center of the intervertebral disc, not at its periphery. If disc height is measured at the posterior or anterior margin of the disc on sagittal sections, this should be specified.
- Desiccated disc refers to a disc with reduced water content, predominantly of nuclear tissues. On MRI Imaging, the intervertebral disc shows decreased signal intensity on T2-weighted images (dark disc) as a consequence of the loss of water content and changes in the concentration of hydrophilic glycosaminoglycans.
- Vacuum disc refers to a degenerated intervertebral disc characterized by the presence of gas, predominantly nitrogen, within the disc space.
- Dallas classification [54] is a grading scale used to quantify the extent of anular fissuring seen on postdiscography CT imaging. According to the discogram description, grade 0 refers to a normal disc, grades 1, 2 and 3 describe a leakage of contrast into the inner one-third, the inner two-thirds and through the entire thickness of the anulus, respectively. In grade 4, the contrast media extends circumferentially; grade 5 is characterized by an overt contrast extravasation into the epidural space.
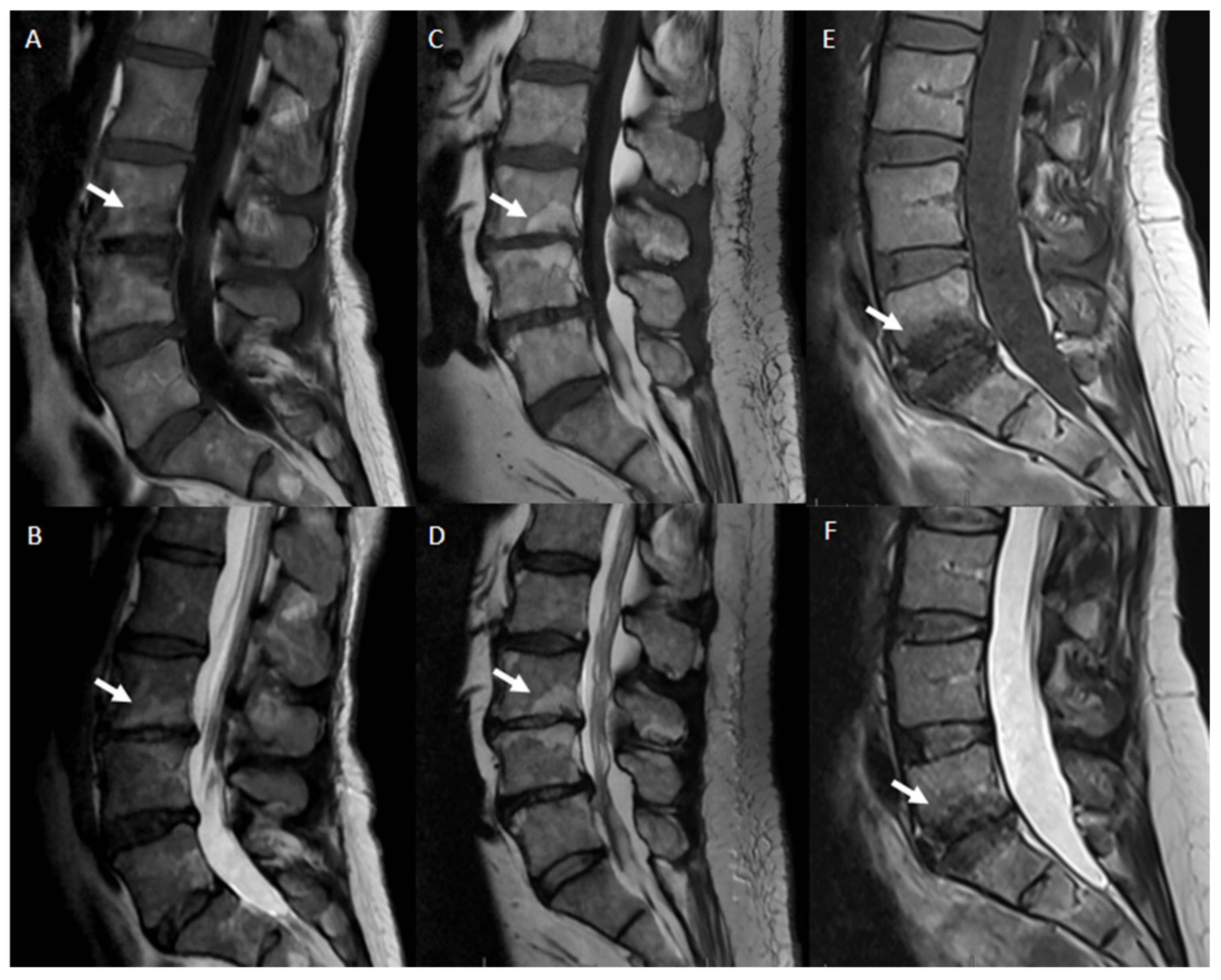
- Modic classification [16] is a classification for vertebral end plates and adjacent vertebral bodies MRI signal modifications secondary to disc inflammation and degenerative disc disease. Modic type 1 refers to decreased signal intensity on T1-weighted images and increased signal intensity on T2-weighted images. Such modifications may be chronic or acute and reflect the penetration of the end plate by fibrovascular tissue, inflammatory changes, and edema. Modic type II refers to increased signal intensity on T1-weighted images and isointense or increased signal intensity on T2-weighted images, indicating replacement of normal bone marrow by fat. Modic type III refers to decreased signal intensity on both T1- and T2-weighted images, indicating reactive osteosclerosis [55] (Figure 4).
6. Functional–Morphological Correlation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Brunori, A.; De Caro, G.M.; Giuffrè, R. Surgery of lumbar disk hernia: Historical perspective. Ann. Ital. Chir. 1998, 69, 285–293. [Google Scholar] [PubMed]
- Chedid, K.J.; Chedid, M.K. The “tract” of history in the treatment of lumbar degenerative disc disease. Neurosurg. Focus 2004, 16, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Benini, A.; Bonar, S.K. Andreas Vesalius. Spine 1996, 21, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, R.V.; Hadley, M.N. History of Surgery for Ruptured Disk. Neurosurg. Clin. N. Am. 2001, 12, 173–179. [Google Scholar] [CrossRef]
- Weber, K.T.; Jacobsen, T.D.; Maidhof, R.; Virojanapa, J.; Overby, C.; Bloom, O.; Quraishi, S.; Levine, M.; Chahine, N.O. Developments in intervertebral disc disease research: Pathophysiology, mechanobiology, and therapeutics. Curr. Rev. Musculoskelet. Med. 2015, 8, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Shah, B.S.; Burt, K.G.; Jacobsen, T.; Fernandes, T.D.; Alipui, D.O.; Weber, K.T.; Levine, M.; Chavan, S.S.; Yang, H.; Tracey, K.J.; et al. High mobility group box-1 induces pro-inflammatory signaling in human nucleus pulposus cells via toll-like receptor 4-dependent pathway. J. Orthop. Res. 2018, 37, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-J.; Yu, X.-H.; Wang, C.; Yang, W.; He, W.-S.; Zhang, S.-J.; Yan, Y.-G.; Zhang, J. MMPs and ADAMTSs in intervertebral disc degeneration. Clin. Chim. Acta 2015, 448, 238–246. [Google Scholar] [CrossRef]
- Kalichman, L.; Hunter, D.J. The genetics of intervertebral disc degeneration. Associated genes. Jt. Bone Spine 2008, 75, 388–396. [Google Scholar] [CrossRef]
- Sadowska, A.; Touli, E.; Hitzl, W.; Greutert, H.; Ferguson, S.J.; Wuertz-Kozak, K.; Hausmann, O.N. Inflammaging in cervical and lumbar degenerated intervertebral discs: Analysis of proinflammatory cytokine and TRP channel expression. Eur. Spine J. 2017, 27, 564–577. [Google Scholar] [CrossRef] [Green Version]
- Cazzanelli, P.; Wuertz-Kozak, K. MicroRNAs in Intervertebral Disc Degeneration, Apoptosis, Inflammation, and Mechanobiology. Int. J. Mol. Sci. 2020, 21, 3601. [Google Scholar] [CrossRef]
- De Luca, P.; De Girolamo, L.; Kouroupis, D.; Castagnetta, M.; Orfei, C.P.; Coviello, D.; Coco, S.; Correa, D.; Brayda-Bruno, M.; Colombini, A.; et al. Intervertebral disc and endplate cells response to IL-1β inflammatory cell priming and identification of molecular targets of tissue degeneration. Eur. Cells Mater. 2020, 39, 227–248. [Google Scholar] [CrossRef]
- Wang, Y.; Che, M.; Xin, J.; Zheng, Z.; Li, J.; Zhang, S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed. Pharmacother. 2020, 131, 110660. [Google Scholar] [CrossRef]
- Bisson, D.; Mannarino, M.; Racine, R.; Haglund, L. For whom the disc tolls: Intervertebral disc degeneration, back pain and toll-like receptors. Eur. Cells Mater. 2021, 41, 355–369. [Google Scholar] [CrossRef]
- Krock, E.; Currie, J.B.; Weber, M.H.; Ouellet, J.A.; Stone, L.S.; Rosenzweig, D.H.; Haglund, L. Nerve Growth Factor Is Regulated by Toll-Like Receptor 2 in Human Intervertebral Discs. J. Biol. Chem. 2016, 291, 3541–3551. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, Y. Genetic background of degenerative disc disease in the lumbar spine. Spine Surg. Relat. Res. 2018, 2, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Modic, M.T.; Ross, J.S. Lumbar Degenerative Disk Disease. Radiology 2007, 245, 43–61. [Google Scholar] [CrossRef]
- Hadjipavlou, A.G.; Tzermiadianos, M.N.; Bogduk, N.; Zindrick, M. The pathophysiology of disc degeneration. J. Bone Jt. Surg. Br. Vol. 2008, 90, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Fujita, N.; Ishihara, S.; Michikawa, T.; Azuma, K.; Suzuki, S.; Tsuji, O.; Nagoshi, N.; Okada, E.; Yagi, M.; Tsuji, T.; et al. Potential association of metabolic and musculoskeletal disorders with lumbar intervertebral disc degeneration: Cross-sectional study using medical checkup data. J. Orthop. Sci. 2020, 25, 384–388. [Google Scholar] [CrossRef]
- Teraguchi, M.; Yoshimura, N.; Hashizume, H.; Yamada, H.; Oka, H.; Minamide, A.; Nagata, K.; Ishimoto, Y.; Kagotani, R.; Kawaguchi, H.; et al. Progression, incidence, and risk factors for intervertebral disc degeneration in a longitudinal population-based cohort: The Wakayama Spine Study. Osteoarthr. Cartil. 2017, 25, 1122–1131. [Google Scholar] [CrossRef] [Green Version]
- Kirnaz, S.; Capadona, C.; Lintz, M.; Kim, B.; Yerden, R.; Goldberg, J.L.; Medary, B.; Sommer, F.; McGrath, L.B.; Bonassar, L.J.; et al. Pathomechanism and Biomechanics of Degenerative Disc Disease: Features of Healthy and Degenerated Discs. Int. J. Spine Surg. 2021, 15, 10–25. [Google Scholar] [CrossRef]
- Dowdell, J.; Erwin, M.; Choma, T.; Vaccaro, A.; Iatridis, J.; Cho, S.K. Intervertebral Disk Degeneration and Repair. Neurosurgery 2017, 80, S46–S54. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Wang, Z.; Chen, J.; Zhang, Z.; Qian, H.; Chen, Y. Latent infection of low-virulence anaerobic bacteria in degenerated lumbar intervertebral discs. BMC Musculoskelet. Disord. 2018, 19, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Jiao, Y.; Yuan, Y.; Zhou, Z.; Zheng, Y.; Xiao, J.; Li, C.; Chen, Z.; Cao, P. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell apoptosis via the TLR2/JNK/mitochondrial-mediated pathway. Emerg. Microbes Infect. 2018, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altun, I. Cytokine profile in degenerated painful intervertebral disc: Variability with respect to duration of symptoms and type of disease. Spine J. 2016, 16, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lv, F.-J. Symptomatic versus Asymptomatic Intervertebral Disc Degeneration: Is Inflammation the Key? Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 13–21. [Google Scholar] [CrossRef]
- Khan, A.N.; Jacobsen, H.E.; Khan, J.; Filippi, C.G.; Levine, M.; Lehman, R.A.; Riew, K.D.; Lenke, L.G.; Chahine, N.O. Inflammatory biomarkers of low back pain and disc degeneration: A review. Ann. N. Y. Acad. Sci. 2017, 1410, 68–84. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2013, 10, 44–56. [Google Scholar] [CrossRef]
- Teixeira, G.Q.; Yong, Z.; Goncalves, R.M.; Kuhn, A.; Riegger, J.; Brisby, H.; Henriksson, H.B.; Ruf, M.; Nerlich, A.; Mauer, U.M.; et al. Terminal complement complex formation is associated with intervertebral disc degeneration. Eur. Spine J. 2021, 30, 217–226. [Google Scholar] [CrossRef]
- Horner, H.A.; Urban, J.P.G. 2001 Volvo Award Winner in Basic Science Studies: Effect of Nutrient Supply on the Viability of Cells from the Nucleus Pulposus of the Intervertebral Disc. Spine 2001, 26, 2543–2549. [Google Scholar] [CrossRef]
- Navone, S.E.; Marfia, G.; Giannoni, A.; Beretta, M.; Guarnaccia, L.; Gualtierotti, R.; Nicoli, D.; Rampini, P.; Campanella, R. Inflammatory mediators and signalling pathways controlling intervertebral disc degeneration. Histol. Histopathol. 2017, 32, 523–542. [Google Scholar] [CrossRef]
- Feng, C.; Liu, H.; Yang, M.; Zhang, Y.; Huang, B.; Zhou, Y. Disc cell senescence in intervertebral disc degeneration: Causes and molecular pathways. Cell Cycle 2016, 15, 1674–1684. [Google Scholar] [CrossRef] [Green Version]
- Vo, N.V.; Hartman, R.A.; Yurube, T.; Jacobs, L.J.; Sowa, G.A.; Kang, J.D. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013, 13, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Desmoulin, G.T.; Pradhan, V.; Milner, T.E. Mechanical Aspects of Intervertebral Disc Injury and Implications on Biomechanics. Spine 2020, 45, E457–E464. [Google Scholar] [CrossRef]
- Hughes, S.P.F.; Freemont, A.J.; Hukins, D.W.L.; McGregor, A.H.; Roberts, S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J. Bone Jt. Surg. Br. Vol. 2012, 94, 1298–1304. [Google Scholar] [CrossRef] [Green Version]
- Freemont, A.; Peacock, T.; Goupille, P.; Hoyland, J.; O’Brien, J.; Jayson, M. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997, 350, 178–181. [Google Scholar] [CrossRef]
- Kim, H.S.; Wu, P.H.; Jang, I.-T. Lumbar Degenerative Disease Part 1: Anatomy and Pathophysiology of Intervertebral Discogenic Pain and Radiofrequency Ablation of Basivertebral and Sinuvertebral Nerve Treatment for Chronic Discogenic Back Pain: A Prospective Case Series and Review of Literature. Int. J. Mol. Sci. 2020, 21, 1483. [Google Scholar] [CrossRef] [Green Version]
- Ohtori, S.; Miyagi, M.; Inoue, G. Sensory nerve ingrowth, cytokines, and instability of discogenic low back pain: A review. Spine Surg. Relat. Res. 2018, 2, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Lama, P.; Le Maitre, C.L.; Harding, I.J.; Dolan, P.; Adams, M.A. Nerves and blood vessels in degenerated intervertebral discs are confined to physically disrupted tissue. J. Anat. 2018, 233, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Silagi, E.S.; Shapiro, I.M.; Risbud, M.V. Glycosaminoglycan synthesis in the nucleus pulposus: Dysregulation and the pathogenesis of disc degeneration. Matrix Biol. 2018, 71–72, 368–379. [Google Scholar] [CrossRef]
- Purmessur, D.; Freemont, A.J.; Hoyland, J. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res. Ther. 2008, 10, R99. [Google Scholar] [CrossRef]
- Clarençon, F.; Law-Ye, B.; Bienvenot, P.; Cormier, E.; Chiras, J. The Degenerative Spine. Magn. Reson. Imaging Clin. N. Am. 2016, 24, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.P.Y.; Luk, K.D.K. The relevance of high-intensity zones in degenerative disc disease. Int. Orthop. 2018, 43, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Colosimo, C.; Gaudino, S.; Alexandre, A.M. Imaging in Degenerative Spine Pathology. In Advances in Minimally Invasive Surgery and Therapy for Spine and Nerves; Springer: Vienna, Austria, 2011; Volume 108, pp. 9–15. [Google Scholar] [CrossRef]
- Jones, K.M.; Unger, E.C.; Granstrom, P.; Seeger, J.F.; Carmody, R.F.; Yoshino, M. Bone marrow imaging using STIR at 0.5 and 1.5T. Magn. Reson. Imaging 1992, 10, 169–176. [Google Scholar] [CrossRef]
- Michelini, G.; Corridore, A.; Torlone, S.; Bruno, F.; Marsecano, C.; Capasso, R.; Caranci, F.; Barile, A.; Masciocchi, C.; Splendiani, A. Dynamic MRI in the evaluation of the spine: State of the art. Acta Biomed. Atenei Parm. 2018, 89, 89–101. [Google Scholar] [CrossRef]
- Neeson, D.; Roberts, D. Imaging the spine. Surgery 2021, 39, 371–382. [Google Scholar] [CrossRef]
- Thompson, J.P.; Pearce, R.H.; Schechter, M.T.; Adams, M.E.; Tsang, I.K.Y.; Bishop, P.B. Preliminary Evaluation of a Scheme for Grading the Gross Morphology of the Human Intervertebral Disc. Spine 1990, 15, 411–415. [Google Scholar] [CrossRef]
- Parizel, P.; Van Hoyweghen, A.; Bali, A.; Van Goethem, J.; Hauwe, L.V.D. The Degenerative Spine. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 787–808. [Google Scholar] [CrossRef]
- Pfirrmann, C.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic Resonance Classification of Lumbar Intervertebral Disc Degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef]
- Griffith, J.F.; Wang, Y.-X.; Antonio, G.E.; Choi, K.C.; Yu, A.; Ahuja, A.T.; Leung, P.C. Modified Pfirrmann Grading System for Lumbar Intervertebral Disc Degeneration. Spine 2007, 32, E708–E712. [Google Scholar] [CrossRef]
- Brinjikji, W.; Diehn, F.E.; Jarvik, J.G.; Carr, C.M.; Kallmes, D.F.; Murad, M.H.; Luetmer, P.H. MRI Findings of Disc Degeneration are More Prevalent in Adults with Low Back Pain than in Asymptomatic Controls: A Systematic Review and Meta-Analysis. Am. J. Neuroradiol. 2015, 36, 2394–2399. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Liu, X.; Chen, D.-J.; Lai, Q.; Tang, B.; Zhang, B.; Dai, M.; Wan, Z. Evaluation of MRI and CT parameters to analyze the correlation between disc and facet joint degeneration in the lumbar three-joint complex. Medicine 2019, 98, e17336. [Google Scholar] [CrossRef]
- Fardon, D.F.; Williams, A.L.; Dohring, E.J.; Murtagh, F.R.; Rothman, S.L.G.; Sze, G.K. Lumbar disc nomenclature: Version 2.0. Spine J. 2014, 14, 2525–2545. [Google Scholar] [CrossRef] [Green Version]
- Sachs, B.L.; Vanharanta, H.; Spivey, M.A.; Guyer, R.D.; Videman, T.; Rashbaum, R.F.; Johnson, R.G.; Hochschuler, S.H.; Mooney, V. Dallas Discogram Description A New Classification of CT/Discography in Low-back Disorders. Spine 1987, 12, 287–294. [Google Scholar] [CrossRef]
- Quattrocchi, C.C.; Alexandre, A.M.; Della Pepa, G.M.; Altavilla, R.; Zobel, B.B. Modic Changes: Acta Neurochirurgica Supplementum; Springer: Vienna, Austria, 2010; pp. 49–53. [Google Scholar] [CrossRef]
- Virk, S.; Meyers, K.N.; Lafage, V.; Maher, S.A.; Chen, T. Analysis of the influence of species, intervertebral disc height and Pfirrmann classification on failure load of an injured disc using a novel disc herniation model. Spine J. 2020, 21, 698–707. [Google Scholar] [CrossRef]
- Yang, B.; O’Connell, G.D. GAG content, fiber stiffness, and fiber angle affect swelling-based residual stress in the intact annulus fibrosus. Biomech. Model. Mechanobiol. 2018, 18, 617–630. [Google Scholar] [CrossRef]
- Naresh-Babu, J.; Neelima, G.; Begum, S.R.; Siva-Leela, V. Diffusion characteristics of human annulus fibrosus—A study documenting the dependence of annulus fibrosus on end plate for diffusion. Spine J. 2016, 16, 1007–1014. [Google Scholar] [CrossRef]
- McDonnell, E.E.; Buckley, C.T. Investigating the physiological relevance of ex vivo disc organ culture nutrient microenvironments using in silico modeling and experimental validation. JOR Spine 2021, 4, e1141. [Google Scholar] [CrossRef]
- Bell, K.M.; Yan, Y.; Hartman, R.A.; Lee, J.Y. Influence of follower load application on moment-rotation parameters and intradiscal pressure in the cervical spine. J. Biomech. 2018, 76, 167–172. [Google Scholar] [CrossRef]
- Kang, S.; Chang, M.C.; Kim, H.; Kim, J.; Jang, Y.; Park, D.; Hwang, J.-M. The Effects of Paraspinal Muscle Volume on Physiological Load on the Lumbar Vertebral Column. Spine 2021, 46, E1015–E1021. [Google Scholar] [CrossRef]
- Ghezelbash, F.; Eskandari, A.H.; Shirazi-Adl, A.; Kazempour, M.; Tavakoli, J.; Baghani, M.; Costi, J.J. Modeling of human intervertebral disc annulus fibrosus with complex multi-fiber networks. Acta Biomater. 2021, 123, 208–221. [Google Scholar] [CrossRef]
- Pope, M.H. Biomechanics of the Lumbar Spine. Ann. Med. 1989, 21, 347–351. [Google Scholar] [CrossRef]
- Lomelí-Rivas, A.; E Larrinúa-Betancourt, J. Biomechanics of the lumbar spine: A clinical approach. Acta Ortop. Mex. 2019, 33, 185–191. [Google Scholar] [PubMed]
- Galbusera, F.; van Rijsbergen, M.; Ito, K.; Huyghe, J.M.; Brayda-Bruno, M.; Wilke, H.-J. Ageing and degenerative changes of the intervertebral disc and their impact on spinal flexibility. Eur. Spine J. 2014, 23, 324–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergroesen, P.-P.A.; Emanuel, K.S.; Peeters, M.; Kingma, I.; Smit, T.H. Are axial intervertebral disc biomechanics determined by osmosis? J. Biomech. 2018, 70, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Lombardi, G.; Corsi, M.M.; Banfi, G. Pathophysiology of the human intervertebral disc. Int. J. Biochem. Cell Biol. 2008, 40, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, C.L.; Boesen, M.; Fournier, G.L.; Bliddal, H.; Hansen, P.; Hansen, B.B. Positional changes in lumbar disc herniation during standing or lumbar extension: A cross-sectional weight-bearing MRI study. Eur. Radiol. 2020, 31, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Ghezelbash, F.; Shirazi-Adl, A.; Baghani, M.; Eskandari, A.H. On the modeling of human intervertebral disc annulus fibrosus: Elastic, permanent deformation and failure responses. J. Biomech. 2020, 102, 109463. [Google Scholar] [CrossRef]
- Cai, X.; Sun, M.; Huang, Y.; Liu, Z.; Be, C.L.; Du, C.; Yang, Q.; Be, X.C.; Be, M.S.; Be, Z.L.; et al. Biomechanical Effect of L4–L5 Intervertebral Disc Degeneration on the Lower Lumbar Spine: A Finite Element Study. Orthop. Surg. 2020, 12, 917–930. [Google Scholar] [CrossRef]
- Newell, N.; Carpanen, D.; Evans, J.H.; Pearcy, M.; Masouros, S.D. Mechanical Function of the Nucleus Pulposus of the Intervertebral Disc Under High Rates of Loading. Spine 2019, 44, 1035–1041. [Google Scholar] [CrossRef]
- Schmidt, H.; Shirazi-Adl, A. Temporal and spatial variations of pressure within intervertebral disc nuclei. J. Mech. Behav. Biomed. Mater. 2018, 79, 309–313. [Google Scholar] [CrossRef]
- Mörl, F.; Günther, M.; Riede, J.M.; Hammer, M.; Schmitt, S. Loads distributed in vivo among vertebrae, muscles, spinal ligaments, and intervertebral discs in a passively flexed lumbar spine. Biomech. Model. Mechanobiol. 2020, 19, 2015–2047. [Google Scholar] [CrossRef]
- Zhou, M.; Lim, S.; O’Connell, G.D. A Robust Multiscale and Multiphasic Structure-Based Modeling Framework for the Intervertebral Disc. Front. Bioeng. Biotechnol. 2021, 9, 685799. [Google Scholar] [CrossRef]
- Paul, C.P.L.; Emanuel, K.S.; Kingma, I.; van der Veen, A.J.; Holewijn, R.M.; Vergroesen, P.-P.A.; van de Ven, P.M.; Mullender, M.G.; Helder, M.N.; Smit, T.H. Changes in Intervertebral Disk Mechanical Behavior During Early Degeneration. J. Biomech. Eng. 2018, 140, 091008. [Google Scholar] [CrossRef]



| Grade | Nucleus | Anulus | Endplate | Vertebral Body |
|---|---|---|---|---|
| I | Bulging gel | Discrete fibrous lamellas | Hyaline, uniformly thick | Margins rounded |
| II | White fibrous tissue peripherally | Mucinous material between lamellas | Thickness irregular | Margins pointed |
| III | Consolidated fibrous tissue | Extensive mucinous infiltration; loss of anular demarcation | Focal defects in cartilage | Early chondrophytes or osteophytes at margins |
| IV | Horizontal clefts parallel to endplate | Focal disruptions | Fibro-cartilage extending from subchondral bone, irregularity and focal sclerosis in subchondral bone | Osteophytes less than 2 mm |
| V | Clefts extend through nucleus and annulus | - | Diffuse sclerosis | Osteophytes greater than 2 mm |
| Grade | Structure | Distinction of Nucleus and Anulus | Signal Intensity | Height of Intervertebral Disc |
|---|---|---|---|---|
| I | Homogeneous, bright white | Clear | T2-w Hyperintense, isointense to cerebrospinal fluid | Normal |
| II | Inhomogeneous with or without horizontal bands | Clear | T2-w Hyperintense, isointense to cerebrospinal fluid | Normal |
| III | Inhomogeneous, gray | Unclear | Intermediate | Normal to slightly decreased |
| IV | Inhomogeneous, gray to black | Lost | Intermediate to hypointense | Normal to moderately decreased |
| V | Inhomogeneous, black | Lost | hypointense | Collapsed disc space |
| Type | T1 | T2 | Histopathology |
|---|---|---|---|
| 1 | Hypointense | Hyperintense | Bone marrow edema |
| 2 | Hyperintense | Hypointense | Fatty replacement |
| 3 | Hypointense | Hypointense | Sclerosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarcia, L.; Pileggi, M.; Camilli, A.; Romi, A.; Bartolo, A.; Giubbolini, F.; Valente, I.; Garignano, G.; D’Argento, F.; Pedicelli, A.; et al. Degenerative Disc Disease of the Spine: From Anatomy to Pathophysiology and Radiological Appearance, with Morphological and Functional Considerations. J. Pers. Med. 2022, 12, 1810. https://doi.org/10.3390/jpm12111810
Scarcia L, Pileggi M, Camilli A, Romi A, Bartolo A, Giubbolini F, Valente I, Garignano G, D’Argento F, Pedicelli A, et al. Degenerative Disc Disease of the Spine: From Anatomy to Pathophysiology and Radiological Appearance, with Morphological and Functional Considerations. Journal of Personalized Medicine. 2022; 12(11):1810. https://doi.org/10.3390/jpm12111810
Chicago/Turabian StyleScarcia, Luca, Marco Pileggi, Arianna Camilli, Andrea Romi, Andrea Bartolo, Francesca Giubbolini, Iacopo Valente, Giuseppe Garignano, Francesco D’Argento, Alessandro Pedicelli, and et al. 2022. "Degenerative Disc Disease of the Spine: From Anatomy to Pathophysiology and Radiological Appearance, with Morphological and Functional Considerations" Journal of Personalized Medicine 12, no. 11: 1810. https://doi.org/10.3390/jpm12111810





