Lipoleiomyomas of the Uterine Cervix: A New Series including the First Recurrent Case and the First Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Our Case Series
2.2. Systematic Literature Review
- Population: human patients with a diagnosis of LLM of the cervix;
- Intervention: any;
- Comparison: none;
- Outcomes: patients’ clinical outcomes (status at last follow-up, and survival and recurrence rates).
- Eligibility/inclusion criteria: studies reporting LLMs of the cervix in human patients.
- Exclusion criteria: unclear diagnosis; LLMs of other sites; non-analyzable results (aggregated data).
3. Results
3.1. Our Case Series
3.1.1. Case 1
3.1.2. Case 2
3.1.3. Case 3
3.1.4. Case 4
3.1.5. Case 5
3.2. Systematic Literature Review Results
3.2.1. Overview
- Akbulut et al. described 76 LLMs (2.9% of the uterine LLMs in their files) arising in the uterine corpus (69/76, 90.7%), cervix (5/76, 6.5%), retroperitoneum (one case), and broad ligament (one case) [15].
- Bolat et al. found 10 (1.4%) LLMs among 707 uterine leiomyomas; only 1/10 (10%) of these cases arose in the cervix [17].
- Wang et al. reported a series of 50 LLMs occurring in the cervix (seven cases, 14%), uterine corpus (43 cases, 86%), retroperitoneum (one case), and broad ligament (one case) [16].
3.2.2. Clinical Symptoms and Signs
3.2.3. Imaging
3.2.4. Treatment and Follow-Up
3.2.5. Gross Findings
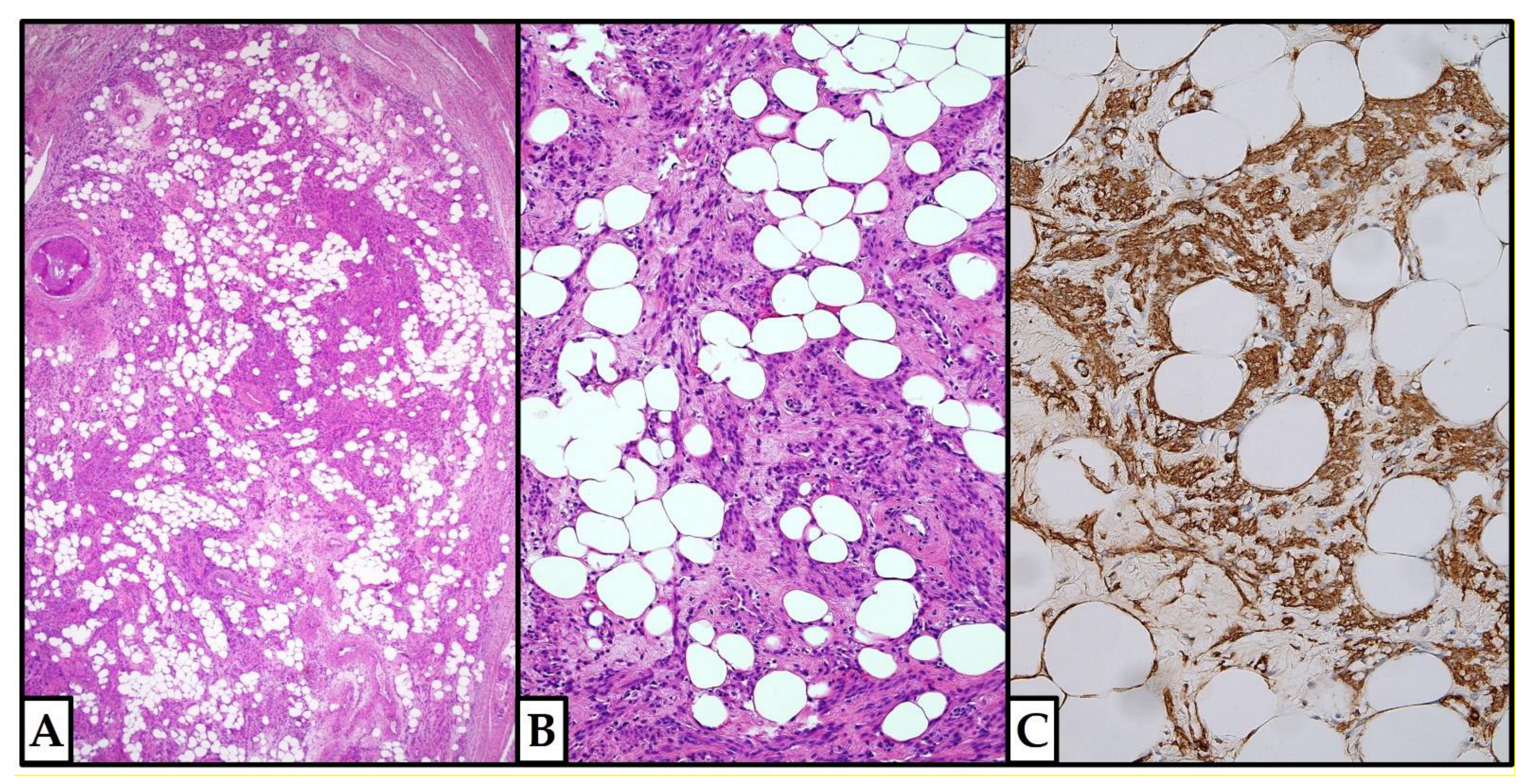
3.2.6. Histopathological Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. Female Genital Tumours: WHO Classification of Tumours, 5th ed.; IARC: Lyon, France, 2020.
- Solomon, L.A.; Schimp, V.L.; Ali-Fehmi, R.; Diamond, M.P.; Munkarah, A.R. Clinical update of smooth muscle tumors of the uterus. J. Minim. Invasive Gynecol. 2005, 12, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Alonso Pacheco, L.; Tinelli, A.; Haimovich, S.; Carugno, J.; Ghezzi, F.; Mazzon, I.; Bettocchi, S. Management of Asymptomatic Submucous Myomas in Women of Reproductive Age: A Consensus Statement from the Global Congress on Hysteroscopy Scientific Committee. J. Minim. Invasive Gynecol. 2019, 26, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Sapia, F.; Rapisarda, A.M.C.; Valenti, G.; Santangelo, F.; Rossetti, D.; Chiofalo, B.; Sarpietro, G.; La Rosa, V.L.; Triolo, O.; et al. Hysteroscopic morcellation of submucous myomas: A systematic review. Biomed. Res. Int. 2017, 2017, 6848250. [Google Scholar] [CrossRef] [PubMed]
- Tiltman, A.J. Leiomyomas of the uterine cervix: A study of frequency. Int. J. Gynecol. Pathol. 1998, 17, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Ciravolo, G.; Ferrari, F.; Zizioli, V.; Donarini, P.; Forte, S.; Sartori, E.; Odicino, F. Laparoscopic management of a large urethral leiomyoma. Int. Urogynecol. J. 2019, 30, 1211–1213. [Google Scholar] [CrossRef]
- Ferrari, F.; Forte, S.; Valenti, G.; Ardighieri, L.; Barra, F.; Esposito, V.; Sartori, E.; Odicino, F. Current Treatment Options for Cervical Leiomyomas: A Systematic Review of Literature. Medicina 2021, 57, 92. [Google Scholar] [CrossRef]
- Goia, M.; Disanto, M.G.; Ferraioli, D.; Palicelli, A. Uterine smooth muscle tumor of uncertain malignant potential. In Uterine Fibroids from Diagnosis to Treatment; Mitidieri, M., Danese, S., Picardo, E., Eds.; Nova Science Publishers, Inc.: New York, USA, 2021; pp. 135–146. [Google Scholar]
- Soleymani Majd, H.; Ferrari, F.; Gubbala, K.; Campanile, R.G.; Tozzi, R. Latest developments and techniques in gynaecological oncology surgery. Curr. Opin. Obstet. Gynecol. 2015, 27, 291–296. [Google Scholar] [CrossRef]
- Hu, J.; Tao, X.; Yin, L.; Shi, Y. Successful conservative treatment of cervical pregnancy with uterine artery embolization followed by curettage: A report of 19 cases. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 97–102. [Google Scholar] [CrossRef]
- Ko, J.S.; Suh, C.H.; Huang, H.; Zhuo, H.; Harmanli, O.; Zhang, Y. Association of Race/Ethnicity with Surgical Route and Perioperative Outcomes of Hysterectomy for Leiomyomas. J. Minim. Invasive Gynecol. 2021, 28, 1403–1410. [Google Scholar] [CrossRef]
- Wilke, S.; Benson, J.; Roller, L. Uterine lipoleiomyoma: Case report and review of the literature. Radiol. Case Rep. 2022, 17, 954–958. [Google Scholar] [CrossRef]
- Di Spiezio Sardo, A.; Gencarelli, A.; Vieira, M.D.C.; Riemma, G.; De Simone, T.; Carugno, J. Differentiating a rare uterine lipoleiomyoma from uterine perforation at hysteroscopy: A scary story. J. Minim. Invasive Gynecol. 2020, 27, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Chandawale, S.S.; Karia, K.M.; Agrawal, N.S.; Patil, A.A.; Shetty, A.B.; Kaur, M. Uterine lipoleiomyoma and lipoma: A rare unique case report with review of literature. Int. J. Appl. Basic Med. Res. 2018, 8, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, M.; Gündoğan, M.; Yörükoğlu, A. Clinical and pathological features of lipoleiomyoma of the uterine corpus: A review of 76 cases. Balk. Med. J. 2014, 31, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kumar, D.; Seidman, J.D. Uterine lipoleiomyomas: A clinicopathologic study of 50 cases. Int. J. Gynecol. Pathol. 2006, 25, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Bolat, F.; Kayaselçuk, F.; Canpolat, T.; Erkanli, S.; Tuncer, I. Histogenesis of lipomatous component in uterine lipoleiomyomas. Turk. J. Pathol. 2007, 23, 82–86. [Google Scholar]
- Mihmanlı, V.; Atik, A.E. Cervical Lipoleiomyoma: Case Report. J. Acad. Res. Med. 2020, 10, 189–191. [Google Scholar] [CrossRef]
- Agrawal, P.; Agrawal, R.; Lobo, A. A case of large cervical lipoleiomyoma simulating malignancy: An intraoperative dilemma. Int. J. Reprod. Contracept. Obstet. Gynecol. 2020, 9, 5147–5149. [Google Scholar] [CrossRef]
- Ravikanth, R.; Kamalasekar, K. A Rare Presentation of Cervical Lipoleiomyoma with Hematometra. Gynecol. Minim. Invasive Ther. 2020, 9, 49–50. [Google Scholar] [CrossRef]
- Rathore, R.; Anand, P.; Butti, A.K.; Sharma, R.; Sarin, N. Giant endocervical lipoleiomyoma of cervix in a young female, presenting with prolapse—An unusual presentation. Int. J. Curr. Res. 2018, 10, 66528–66532. [Google Scholar]
- Bannur, H.; Suranagi, V.; Davanageri, R. Subserosal lipoleiomyoma of the cervix in a postmenopausal woman: A rare case report. J. Sci. Soc. 2017, 44, 163–164. [Google Scholar] [CrossRef]
- Şengiz Erhan, S.; Hallaç Keser, S.; Soylu Boy, F.N.; Çom, C. Lipoleiomyoma of the Uterine Cervix: A Case Report. Okmeydanı Tıp Derg. 2017, 33, 50–53. [Google Scholar] [CrossRef]
- Ye, X.; Xu, R.; Yan, L.; Xu, X.; Chen, X. Comparative analysis of ultrasonographic and pathologic findings of uterine lipoleiomyoma. Chin. J. Interv Imaging Ther. 2016, 13, 627–631. [Google Scholar]
- Adaikkalam, J. Lipoleiomyoma of Cervix. J. Clin. Diagn. Res. 2016, 10, EJ01–EJ02. [Google Scholar] [CrossRef] [PubMed]
- Terada, T. Giant subserosal lipoleiomyomas of the uterine cervix and corpus: A report of 2 cases. Appl. Immunohistochem. Mol. Morphol. 2015, 23, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nagarajan, K.; Srinivasamurthy, B.C.; Jena, S.K.; Sasmal, P.K. Giant Plexiform Lipoleiomyoma of the Broad Ligament with Extensive Cystic Degeneration in a Reproductive-Age Female. J. Gynecol. Surg. 2015, 31, 107–114. [Google Scholar] [CrossRef]
- Barnard, E.P.; Bakkum-Gamez, J.N.; Hopkins, M.R.; Occhino, J.A. Cervical Lipoleiomyoma: Transvaginal Approach for Excision. J. Gynecol. Surg. 2015, 31, 370–372. [Google Scholar] [CrossRef]
- El-Agwany, A.S. Lipoleiomyoma of the uterine cervix: An unusual variant of uterine leiomyoma. Egypt. J. Radiol. Nucl. Med. 2015, 46, 211–213. [Google Scholar] [CrossRef]
- Sharma, S.; Ahluwalia, C.; Mandal, A.K. A rare incidental case of lipoleiomyoma cervix. Asian Pac. J. Health Sci. 2015, 2, 186–190. [Google Scholar] [CrossRef]
- Mandal, R.; Mondal, K.; Pramanik, P. Gigantic lipoleiomyoma of cervix with extensive hyaline degeneration: A case report with review of literature. Ann. Pathol. Lab. Med. 2015, 2, C183–C186. [Google Scholar]
- Kalyankar, V.; Kalyankar, B. Rare case of cervical lipoleiomyoma. J. Evol. Med. Dent. Sci. 2014, 3, 5529–5534. [Google Scholar] [CrossRef]
- Goyal, P.; Agrawal, D.; Ghosh, S.; Sehgal, S.; Kumar, A.; Singh, S. Giant lipoleiomyoma of the cervix mimicking ovarian cancer in a premenopausal woman: A case report and literature Review. J. Gynecol. Surg. 2014, 30, 24–27. [Google Scholar] [CrossRef]
- Chaudhari, J.; Kothari, K.; Gupta, A.S.; Dwivedi, J. Cervical Lipoleiomyoma. J. Postgrad. Gynecol. Obstet. 2014, 1, 9. [Google Scholar]
- Fagouri, H.; Hafidi, M.R.; Guelzim, K.; Hakimi, I.; Kouach, J.; Moussaoui, D.R.; Dehayni, M. Lipoleiomyoma of the uterine cervix (about an observation). Int. J. Sci. Technol. Res. 2014, 3, 449–450. [Google Scholar]
- Agrawal, D.; Fotedar, S.; Daral, R.; Kumar, K. Cervical lipoleiomyoma in premenopausal woman: A rare. Ann. Pathol. Lab. Med. 2014, 1, C14–C17. [Google Scholar]
- Terada, T. Huge lipoleiomyoma of the uterine cervix. Arch. Gynecol. Obstet. 2011, 283, 1169–1171. [Google Scholar] [CrossRef] [PubMed]
- Walid, M.S.; Heaton, R.L. Case report of a cervical lipoleiomyoma with an incidentally discovered ovarian granulosa cell tumor—Imaging and minimal-invasive surgical procedure. Ger. Med. Sci. 2010, 8, Doc26. [Google Scholar] [CrossRef] [PubMed]
- Mohan, H.; Bhutani, A.; Punia, R.P.S. Lipoleiomyoma of uterus: Report of 8 cases. J. Obstet. Gynaecol. India 2002, 52, 76–78. [Google Scholar]
- Shintaku, M. Lipoleiomyomatous tumors of the uterus: A heterogeneous group? Histopathological study of five cases. Pathol. Int. 1996, 46, 498–502. [Google Scholar] [CrossRef]
- Volpe, R.; Canzonieri, V.; Gloghini, A.; Carbone, A. “Lipoleiomyoma with metaplastic cartilage” (benign mesenchymoma) of the uterine cervix. Pathol. Res. Pract. 1992, 188, 799–803. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, J.H. A case report of pelviscopic resection of lipoleiomyoma originating from the uterine cervix in a postmenopausal woman. Medicine 2022, 101, e30665. [Google Scholar] [CrossRef]
- Doldan, A.; Otis, C.N.; Pantanowitz, L. Adipose Tissue: A Normal Constituent of the Uterine Cervical Stroma. Int. J. Gynecol. Pathol. 2009, 28, 396–400. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.A.; Pertence, A.P.; de Souza, M.A. Heterotopic adipose tissue in the uterine cervix. Rev. Hosp. Clin. Fac. Med. Sao Paulo 1998, 53, 149–151. [Google Scholar] [PubMed]
- Takeuchi, K.; Murata, K.; Funaki, K.; Fujita, I.; Hayakawa, Y.; Kitazawa, S. Liposarcoma of the uterine cervix: Case report. Eur. J. Gynaecol. Oncol. 2000, 21, 290–291. [Google Scholar] [PubMed]
- Fadare, O. Uncommon sarcomas of the uterine cervix: A review of selected entities. Diagn Pathol. 2006, 1, 30. [Google Scholar] [CrossRef][Green Version]
- Tandon, B.; Hagemann, I.S.; Maluf, H.M.; Pfeifer, J.D.; Al-Kateb, H. Association of Li-Fraumeni Syndrome With Small Cell Carcinoma of the Ovary, Hypercalcemic Type and Concurrent Pleomorphic Liposarcoma of the Cervix. Int. J. Gynecol. Pathol. 2017, 36, 593–599. [Google Scholar] [CrossRef]
- Zaman, M.U.; Fatima, N.; Memon, W.A.; Zaman, A.; Zaman, S. Pure Uterine Lipoma on 18FDG PET/CT: Rare But Easy to Diagnose. Cureus 2019, 11, e4334. [Google Scholar] [CrossRef]
- Alfarra, K.S.; Aldhamer, A.A.; Aldubaib, H.S.; Majoun, M.A.; Alrammah, A.S.; Alshehri, F.S.; Mughallis, H.M.; Almalki, A.J.; Basakran, G.G.; Alayed, A.M.; et al. Pure Uterine Lipoma: A Report of a Rare Entity. Cureus 2021, 13, e20444. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Nakai, Y.; Gonoi, W.; Kurokawa, R.; Okimoto, N.; Sakamoto, N.; Fukuchi, H.; Kobayashi, H.; Makise, N.; Abe, O. Fat-forming solitary fibrous tumor of the sacrum: A case report and literature review. Radiol. Case Rep. 2021, 16, 1874–1877. [Google Scholar] [CrossRef]
- Devins, K.M.; Young, R.H.; Croce, S.; Burandt, E.; Bennett, J.A.; Pesci, A.; Zannoni, G.F.; Ip, P.P.C.; Nielsen, G.P.; Oliva, E. Solitary Fibrous Tumors of the Female Genital Tract: A Study of 27 Cases Emphasizing Nonvulvar Locations, Variant Histology, and Prognostic Factors. Am. J. Surg. Pathol. 2022, 46, 363–375. [Google Scholar] [CrossRef]
- Ardighieri, L.; Palicelli, A.; Ferrari, F.; Ragnoli, M.; Ghini, I.; Bugatti, M.; Bercich, L.; Sartori, E.; Odicino, F.E. Risk Assessment in Solitary Fibrous Tumor of the Uterine Corpus: Report of a Case and Systematic Review of the Literature. Int. J. Surg. Pathol. 2022, 30, 177–183. [Google Scholar] [CrossRef]
- Hsiao, S.-M.; Lin, H.-H.; Peng, F.-S.; Jen, P.-J.; Hsiao, C.-F.; Tu, F.-C. Comparison of robot-assisted laparoscopic myomectomy and traditional laparoscopic myomectomy. J. Obstet. Gynaecol Res 2013, 39, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Del Priore, G.; Klapper, A.S.; Gurshumov, E.; Vargas, M.M.; Ungar, L.; Smith, J.R. Rescue radical trachelectomy for preservation of fertility in benign disease. Fertil. Steril. 2010, 94, 1910.e5–1910.e7. [Google Scholar] [CrossRef] [PubMed]
- Mandato, V.D.; Torricelli, F.; Mastrofilippo, V.; Palicelli, A.; Ciarlini, G.; Pirillo, D.; Annunziata, G.; Aguzzoli, L. Accuracy of preoperative endometrial biopsy and intraoperative frozen section in predicting the final pathological diagnosis of endometrial cancer. Surg. Oncol. 2020, 35, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Mandato, V.D.; Palicelli, A.; Torricelli, F.; Mastrofilippo, V.; Leone, C.; Dicarlo, V.; Tafuni, A.; Santandrea, G.; Annunziata, G.; Generali, M.; et al. Should Endometrial Cancer Treatment Be Centralized? Biology 2022, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Peker, N.; Gündoğan, S.; Şendağ, F. Laparoscopic Management of Huge Cervical Myoma. J. Minim. Invasive Gynecol. 2017, 24, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Garzon-Lopez, O.; Garzón-Lopez, F.; Gomez-Ponce, H.; Morgan-Ortiz, F. Laparoscopic Management of a Huge Retro-Cervical Myoma. J. Minim. Invasive Gynecol. 2015, 22, S218. [Google Scholar] [CrossRef]
- Chang, W.-C.; Chen, S.; Huang, S.-C.; Chang, D.-Y.; Chou, L.-Y.; Sheu, B.-C. Strategy of cervical myomectomy under laparoscopy. Fertil. Steril. 2010, 94, 2710–2715. [Google Scholar] [CrossRef]
- Fletcher, H.; Frederick, J.; Hardie, M.; Simeon, D. A Randomized Comparison of Vasopressin and Tourniquet as Hemostatic Agents during Myomectomy. Obstet. Gynecol. 1996, 87, 1014–1018. [Google Scholar] [CrossRef]
- Liu, W.-M.; Wang, P.-H.; Chou, C.-S.; Tang, W.-L.; Wang, I.-T.; Tzeng, C.-R. Efficacy of combined laparoscopic uterine artery occlusion and myomectomy via minilaparotomy in the treatment of recurrent uterine myomas. Fertil. Steril. 2007, 87, 356–361. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, W.; Dai, H.; Hu, L.; Qu, X.; Kang, L. Laparoscopic Uterine Artery Occlusion Combined with Myomectomy for Uterine Myomas. J. Minim. Invasive Gynecol. 2008, 15, 346–349. [Google Scholar] [CrossRef]
- Mittal, K.R.; Chen, F.; Wei, J.J.; Rijhvani, K.; Kurvathi, R.; Streck, D.; Dermody, J.; Toruner, G.A. Molecular and immunohistochemical evidence for the origin of uterine leiomyosarcomas from associated leiomyoma and symplastic leiomyoma-like areas. Mod. Pathol. 2009, 22, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, C.C.; Adler, M.T.; Chohan, L. Vaginal Myomectomy in Pregnancy: A Report of Two Cases. South. Med. J. 2010, 103, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Koyama, K.; Imoto, S.; Mori, M.; Nakano, T.; Nakamura, H. Temporary endovascular balloon occlusion of the bilateral internal iliac arteries to control hemorrhage during laparoscopic-assisted vaginal hysterectomy for cervical myoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Diakosavvas, M.; Angelou, K.; Fasoulakis, Z.; Kathopoulis, N.; Zacharakis, D.; Blontzos, N.; Antsaklis, P.; Haidopoulos, D.; Daskalakis, G.; Rodolakis, A.; et al. Myomectomy during pregnancy; diagnostical dilemmas: Two case reports and a systematic review of the literature. J. Obstet. Gynaecol. 2022, 42, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowski, J.; Wozniak, S.; Szkodziak, P.; Krzyzanowski, A.; Wojciech, W.; Paszkowski, T. Minimally invasive treatment options for uterine fibroids—State-of-the art 2021. Ginekol. Pol. 2022, 93, 242–247. [Google Scholar] [CrossRef]
- Cavaliere, A.F.; Vidiri, A.; Gueli Alletti, S.; Fagotti, A.; La Milia, M.C.; Perossini, S.; Restaino, S.; Vizzielli, G.; Lanzone, A.; Scambia, G. Surgical Treatment of “Large Uterine Masses” in Pregnancy: A Single-Center Experience. Int. J. Environ. Res. Public Health. 2021, 18, 12139. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Y.; You, M.; Chen, L.; Sun, F. Observation of pregnancy outcomes in patients with hysteroscopic resection on submucous myomas. J. Obstet. Gynaecol. Res. 2022, 48, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Keriakos, R.; Maher, M. Management of Cervical Fibroid during the Reproductive Period. Case Rep. Obstet. Gynecol. 2013, 2013, 984030. [Google Scholar] [CrossRef] [PubMed]
- Palicelli, A.; Giaccherini, L.; Zanelli, M.; Bonasoni, M.; Gelli, M.; Bisagni, A.; Zanetti, E.; De Marco, L.; Torricelli, F.; Manzotti, G.; et al. How Can We Treat Vulvar Carcinoma in Pregnancy? A Systematic Review of the Literature. Cancers 2021, 13, 836. [Google Scholar] [CrossRef]
- D’Agostino, C.; Surico, D.; Monga, G.; Palicelli, A. Pregnancy-related decidualization of subcutaneous endometriosis occurring in a post-caesarean section scar: Case study and review of the literature. Pathol.-Res. Pract. 2019, 215, 828–831. [Google Scholar] [CrossRef]
- Higuchi, Y.; Okuda, K.; Nakamura, Y.; Hayashi, A.; Hayashi, M.; Fujiyama, F.; Yoshida, Y.; Yamashita, Y.; Terai, Y.; Kamegai, H.; et al. Efficacy and safety of bipolar electrode grasping forceps for laparoscopic myomectomy in uterine cervical myoma. Asian J. Endosc. Surg. 2012, 5, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Koyama, K.; Imoto, S.; Mori, M.; Sakai, K.; Nakamura, H. Temporary endovascular balloon occlusion of the bilateral internal iliac arteries for control of hemorrhage during laparoscopic-assisted myomectomy in a nulligravida with a large cervical myoma. Fertil. Steril. 2009, 91, 935.e5–935.e9. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Hu, W. Cervical leiomyomas in pregnancy: Report of 17 cases. Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Gurung, G.; Rana, A.; Bahadur Rana Magar, D. Utero-vaginal prolapse due to portio vaginal fibroma. J. Obstet. Gynaecol. Res. 2003, 29, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Oruç, S.; Karaer, O.; Kurtul, O. Coexistence of a prolapsed, pedunculated cervical myoma and pregnancy complications: A case report. J. Reprod. Med. 2004, 49, 575–577. [Google Scholar]
- González González, V.; Herráez Moreta, A.; Mayoral Triana, A.; Riolobos Sierra, L.; Cristóbal García, I.; Izquierdo Méndez, N. Prolapsed cervical myoma during pregnancy. Eur J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 150–154. [Google Scholar] [CrossRef]
- Gandhi, A.; Dugad, H.; Shah, Y. A rare presentation of cervical fibroid in pregnancy. Ann. Afr. Med. 2014, 13, 88–90. [Google Scholar] [CrossRef]
- Lohle, P.N.M.; Boekkooi, P.F.; Fiedeldeij, C.A.; Berden, H.J.J.M.; de Jong, W.; Reekers, J.A.; Franx, A.; van Rooij, W.J.J. Selective Embolisation of a Heavily Bleeding Cervical Fibroid in a Pregnant Woman. Cardiovasc. Interv. Radiol. 2015, 38, 1649–1653. [Google Scholar] [CrossRef]
- Kamra, H.T.; Dantkale, S.S.; Birla, K.; Sakinlawar, P.W.; Narkhede, R.R. Myxoid leiomyoma of cervix. J. Clin. Diagn. Res. 2013, 7, 2956–2957. [Google Scholar] [CrossRef]
- Scott, R.B.; Spence, J.M., Jr. Delivering submucous myoma complicating pregnancy. Am J. Obstet. Gynecol. 1951, 62, 447–449. [Google Scholar] [CrossRef]
- Rozanska-Waledziak, A.; Kacperczyk-Bartnik, J.; Bartnik, P.; Waledziak, M.; Czajkowski, K. A successful vaginal myomectomy of cervical leiomyoma in early pregnancy. Ginekol. Pol. 2021, 92, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Holub, Z.; Mara, M.; Kuzel, D.; Jabor, A.; Maskova, J.; Eim, J. Pregnancy outcomes after uterine artery occlusion: Prospective multicentric study. Fertil. Steril. 2008, 90, 1886–1891. [Google Scholar] [CrossRef] [PubMed]
- DeMeritt, J.S.; Wajswol, E.; Wattamwar, A. Pregnancy after Superselective Embolization of the Cervicovaginal Arteries for a Bleeding Cervical Fibroid. J. Vasc. Interv. Radiol. 2019, 30, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, J.F.; Pijnenborg, J.M.; Boomsma, C.M.; Verberg, M.F.; Geomini, P.M.; Bongers, M.Y. Parasitic myoma after laparoscopic morcellation: A systematic review of the literature. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Leren, V.; Langebrekke, A.; Qvigstad, E. Parasitic leiomyomas after laparoscopic surgery with morcellation. Acta Obstet. Gynecol. Scand. 2012, 91, 1233–1236. [Google Scholar] [CrossRef]
- Ghorbani, H.; Ranaee, M.; Vosough, Z. Two Rare Cases of Uterine Leiomyosarcomas Originating from Submucosal Leiomyomas Proved by Their Immunohistochemistry Profiles. Int. J. Fertil. Steril. 2020, 14, 256–259. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kusunoki, S.; Hirayama, T.; Fujino, K.; Terao, Y.; Itakura, A. Case of leiomyosarcoma arising from subserosal leiomyoma. J. Obstet. Gynaecol. Res. 2019, 45, 1944–1947. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.G.; Dal Cin, P.; Ganguly, A.; Campbell, S.; Imai, Y.; Rosenberg, A.E.; Oliva, E. Liposarcoma arising in uterine lipoleiomyoma: A report of 3 cases and review of the literature. Am. J. Surg. Pathol. 2011, 35, 221–227. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Update: Perform Only Contained Morcellation When Laparoscopic Power Morcellation Is Appropriate; FDA Safety Communication; FDA: Silver Spring, MD, USA, 2020.
- Mandato, V.D.; Torricelli, F.; Pirillo, D.; Aguzzoli, L.; Abrate, M.; Palomba, S.; La Sala, G.B. Impact of the Food and Drug Administration Safety Communication on the Use of Power Morcellator in Daily Clinical Practice: An Italian Survey. J. Minim. Invasive Gynecol. 2016, 23, 206–214. [Google Scholar] [CrossRef]
- Ameer, M.A.; Fagan, S.E.; Sosa-Stanley, J.N.; Peterson, D.C. Anatomy, Abdomen and Pelvis, Uterus; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Chen, C.J.; Thompson, H. Uterine Prolapse; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Jeon, M.J. Surgical decision making for symptomatic pelvic organ prolapse: Evidence-based approach. Obstet. Gynecol. Sci. 2019, 62, 307–312. [Google Scholar] [CrossRef]
- Doshani, A.; Teo, R.E.; Mayne, C.J.; Tincello, D.G. Uterine prolapse. BMJ 2007, 335, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Gutman, R.E.; Ford, D.E.; Quiroz, L.H.; Shippey, S.H.; Handa, V.L. Is there a pelvic organ prolapse threshold that predicts pelvic floor symptoms? Am J. Obstet. Gynecol. 2008, 199, 683.e1–683.e7. [Google Scholar] [CrossRef] [PubMed]
- Parvathavarthini, K.; Vanusha, A. Clinical epidemiological study of uterine prolapse. Int. J. Reprod. Contracept. Obstet. Gynecol. 2019, 8, 79–85. [Google Scholar]
- Carley, M.E.; Schaffer, J. Urinary incontinence and pelvic organ prolapse in women with Marfan or Ehlers Danlos syndrome. Am J. Obstet. Gynecol. 2000, 182, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Paladini, D.; Di Spiezio Sardo, A.; Mandato, V.D.; Guerra, G.; Bifulco, G.; Mauriello, S.; Nappi, C. Association of cutis laxa and genital prolapse: A case report. Int. Urogynecol. J. 2007, 18, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Onowhakpor, E.A.; Omo-Aghoja, L.O.; Akani, C.I.; Feyi-Waboso, P. Prevalence and determinants of utero-vaginal prolapse in southern Nigeria. Niger. Med. J. 2009, 50, 29–32. [Google Scholar]
- Turhan, N.; Simavli, S.; Kaygusuz, I.; Kasap, B. Totally inverted cervix due to a huge prolapsed cervical myoma simulating chronic non-puerperal uterine inversion. Int. J. Surg. Case Rep. 2014, 5, 513–515. [Google Scholar] [CrossRef]
- Abe, S. Prolapse of uterus caused by myoma of the uterus with cervical tumor. Case reports. J. Jpn. Obstet. Gynecol. Soc. 1961, 13, 1255–1258. [Google Scholar]
- Ben-Baruch, G.; Schiff, E.; Menashe, Y.; Menczer, J. Immediate and late outcome of vaginal myomectomy for prolapsed pedunculated submucous myoma. Obstet. Gynecol. 1988, 72, 858–861. [Google Scholar] [CrossRef]
- Okonkwo, J.E.N.; Obiechina, N.J.A.; Obionu, N. Incidence of pelvic organ prolapse in nigerian women. J. Natl. Med. Assoc. 2003, 95, 132–136. [Google Scholar]
- Milsom, I.; Altman, D.; Cartwright, R.; Lapitan, M.C.; Nelson, R.; Sjöström, S.; Tikkinen, K.A.O. Epidemiology of urinary incontinence (UI) and other lower urinary tract symptoms (LUTS), pelvic organ prolapse (POP) and anal (AI) incontinence. In Incontinence, 6th ed.; Abrams, P., Cardozo, L., Wagg, A., Wein, A., Eds.; ICS Publishing: Tokyo, Japan, 2017; pp. 61–67. [Google Scholar]




| Authors | Age | Size (cm) | Clinical Features | Site | Therapy | %Fat | Follow-Up |
|---|---|---|---|---|---|---|---|
| Palicelli et al., 2022: case 1 | 43 | 2.1 | P6402, VB, uterine prolapse (progressing to 3rd degree), moderate cysto-rectocele, UD | A | TVH (mor) + BSO + PFR | 5–10% | NED, 12 mo |
| Palicelli et al., 2022: case 2 | 43 | 0.8 | P3003, BS (sterilization, 5 years before) | NR | TAH + adhesiolysis | 45% | NED, 6 years |
| Palicelli et al., 2022: case 3 | 42 | 3.8 | Pr, cystocele (2nd-3rd degree) | Sm | EXC | 25% | NED, 17 years |
| Palicelli et al., 2022: case 4 | 40 | 2; 5 (REC) | Pr/Pe | Sm | Partial EXC | <10% | REC, 2 years; NED, 13 years after REC |
| Palicelli et al., 2022: case 5 | 44 | 1 | Pr/Pe. Concomitant right ovarian Sertoli cell tumor | NR | TH + BSO | 5% | NR |
| Kim et al., 2022 [42] | 55 | 5.8 | G2P2, asymptomatic, normal BMI (23.3), suddenly increasing cervical mass after 4 years of follow-up | NR | Pelviscopic EXC + BS | NR (significant component, 20%?) | NED, 3 years |
| Mihmanli et al., 2020 [18] | 39 | 8.3 | G2P2, inguinal pain (increased during sexual intercourse), Pr | NR | TH + BS | NR | NR |
| Agrawal et al., 2020 [19] | 45 | 13 | G2P2, VB, PP, Pr, UD (6 mo), treated hypothyroidism (5 years) | A | TAH (mor) + LSO + RS | NR | NR |
| Ravikanth et al., 2020 [20] | 68 | 4.3 | VB, PP (2 we) | A | NR | NR | NR |
| Rathore et al., 2018 [21] | 37 | 13 | G2P2L2A1, VB (3 mo), PP (1 mo), Pr (1 we) | P | TAH + USO | <50% (#) | NED, 3 mo |
| Bannur et al., 2017 [22] | 58 | 6 | UD (22 we), Pr (6 mo), 3rd degree vaginal prolapse | P, Is, Su | VH + PFR | NR | NR |
| Şengiz Erhan et al., 2017 [23] | 53 | 2 | PP (3 mo) | R, In | TAH + BSO | NR | NR |
| Ye et al., 2016: case 3 [24] | 44 | 6.5 | NR | NR | NR | 35% | NR |
| Adaikkalam et al., 2016 [25] | 39 | 8 | Multiparous, UD (1 we), Pr (3 mo) | P | VH + PFR | NR | NR |
| Terada T., 2015: case 1 [26] | 47 | 10 | VB | Is, Su | SH | 50% | NED, 5 mo |
| Singh et al., 2015 [27] | 37 | 9 | Tubal sterilization | R, In | TAH + BSO + HR | NR | NED, 6 we |
| Barnard et al., 2015 [28] | 50 | 4 | Multiparous, LR-IUD (5 mo), Pr (3 mo), rectocele | A | EXC | NR | NR |
| El-Agwany AS, 2015 [29] | 35 | 5 | G2P2, VB, PP (3 mo) | A | SM | NR | NED |
| Sharma et al., 2015 [30] | 39 | 3 | VB, PP, Pr, UD (4 mo) | P, In | TAH + BSO | NR (°) | NED |
| Mandal et al., 2015 [31] | 48 | 19 | P2 + 0, PP (4 mo) | Su | TAH + BSO | <50% (#) ($) | NED, 6 mo |
| Kalyankar et al., 2014 [32] | 35 | 15 | P3L3, salpingectomy (12 years before), PP, VD (4 mo) | P | TAH + RSO | NR | NR |
| Goyal et al., 2014 [33] | 40 | 20 | G3P3, PP, Dysp (45 days), Pe | P, Su | TAH + BSO + Om | NR | NR |
| Chaudhari et al., 2014 [34] | 40 | NR | UD (2 mo), Pr (6 mo) | WC (*) | TAH | NR | NR |
| Fagouri et al., 2014 [35] | 39 | 6 | G4P4, VB (3 mo) | P, Is | TAH + BSO | NR | NED |
| Agrawal et al., 2014 [36] | 38 | 5 | P3G3A0, salpingectomy (15 years before), VB (3.5 mo), Pr | WC | TAH + RSO | NR | NR |
| Terada T., 2011 [37] | 73 | 18 | PP, lower abdominal mass | NR | SH + BSO | 40% | NED, 36 mo |
| Walid et al., 2010 [38] | 48 | 5.5 | G1P1, slowly growing cervical mass | Is, Par | TAH + BSO | NR | NR |
| Mohan et al., 2002: case 3 [39] | 38 | 2.5 | VB | NR | TAH | focal | NR |
| Shintaku M, 1996: case 4 [40] | 74 | 3.5 | Uterine prolapse, cystocele | In | VH | <50% (#) | NED |
| Shintaku M, 1996: case 5 [40] | 60 | 4.2 | Low abdominal distension (due to ovarian tumor), Pe | R, Su | SH + BSO | <50% (#) | NED |
| Volpe et al., 1992 [41] | 51 | 15 | Multiparous, VB (10 mo) | P | TAH + BSO | <50% (#) (@) | NED, 5 mo |
| Marker | Smooth Muscle Cells | Adipocytes |
|---|---|---|
| Vimentin [26,30,37,41] | 100% (4/4) | 100% (3/3) |
| Desmin [19,23,26,27,30,37,40,41] | 89% (8/9) | 0% (0/2) |
| Smooth muscle actin [19,23,26,27,30,37,40] | 100% (10/10) | 0% (0/2) |
| h-caldesmon [19] | 100% (1/1) | |
| Estrogen receptor [26,30] | 100% (1/1) | 50% (1/2) |
| Progesterone receptor [26,30] | 100% (1/1) | 50% (1/2) |
| pan-cytokeratins (AE1/3 and CAM5.2) [26,37] | 0% (0/2) | 0% (0/2) |
| S-100 [19,23,26,37,40,41] | 0% (0/2) | 100% (7/7) |
| HMB-45 [26,37] | 0% (0/2) | 0% (0/2) |
| p53 [26,37] | 0% (0/2) | 0% (0/2) |
| MDM2 [26,37] | 0% (0/2) | 0% (0/2) |
| CDK4 [26,37] | 0% (0/2) | 0% (0/2) |
| CD34 [37] | 0% (0/1) | 0% (0/2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palicelli, A.; Ardighieri, L.; Broggi, G.; Caltabiano, R.; Melli, B.; Gelli, M.C.; Zanelli, M.; Bonasoni, M.P.; Asaturova, A.; Zizzo, M.; et al. Lipoleiomyomas of the Uterine Cervix: A New Series including the First Recurrent Case and the First Systematic Literature Review. J. Pers. Med. 2022, 12, 1852. https://doi.org/10.3390/jpm12111852
Palicelli A, Ardighieri L, Broggi G, Caltabiano R, Melli B, Gelli MC, Zanelli M, Bonasoni MP, Asaturova A, Zizzo M, et al. Lipoleiomyomas of the Uterine Cervix: A New Series including the First Recurrent Case and the First Systematic Literature Review. Journal of Personalized Medicine. 2022; 12(11):1852. https://doi.org/10.3390/jpm12111852
Chicago/Turabian StylePalicelli, Andrea, Laura Ardighieri, Giuseppe Broggi, Rosario Caltabiano, Beatrice Melli, Maria Carolina Gelli, Magda Zanelli, Maria Paola Bonasoni, Aleksandra Asaturova, Maurizio Zizzo, and et al. 2022. "Lipoleiomyomas of the Uterine Cervix: A New Series including the First Recurrent Case and the First Systematic Literature Review" Journal of Personalized Medicine 12, no. 11: 1852. https://doi.org/10.3390/jpm12111852
APA StylePalicelli, A., Ardighieri, L., Broggi, G., Caltabiano, R., Melli, B., Gelli, M. C., Zanelli, M., Bonasoni, M. P., Asaturova, A., Zizzo, M., Aguzzoli, L., Baraldi, R., & Mandato, V. D. (2022). Lipoleiomyomas of the Uterine Cervix: A New Series including the First Recurrent Case and the First Systematic Literature Review. Journal of Personalized Medicine, 12(11), 1852. https://doi.org/10.3390/jpm12111852











