Nontraumatic Spinal Cord Injury: Epidemiology, Etiology and Management
Abstract
:1. Epidemiology
2. Etiologies of Nontraumatic Sci
2.1. Congenital/Genetic Disorders
Congenital
2.2. Genetic Disorders
2.2.1. Hereditary Spastic Paraplegia (HSP)
2.2.2. Friedreich’s Ataxia
2.2.3. Spinal Muscular Atrophy
2.3. Acquired Ntsci
Degenerative Spine Disease
2.4. Metabolic
Subacute Combined Degeneration (Vitamin B12 Deficiency)
2.5. Vascular Disorders
2.5.1. Hemorrhage
2.5.2. Vascular Malformations
2.5.3. Ischemia
2.6. Inflammatory/Autoimmune Diseases
2.6.1. Acute Transverse Myelitis
2.6.2. Acquired Demyelinating Disorders
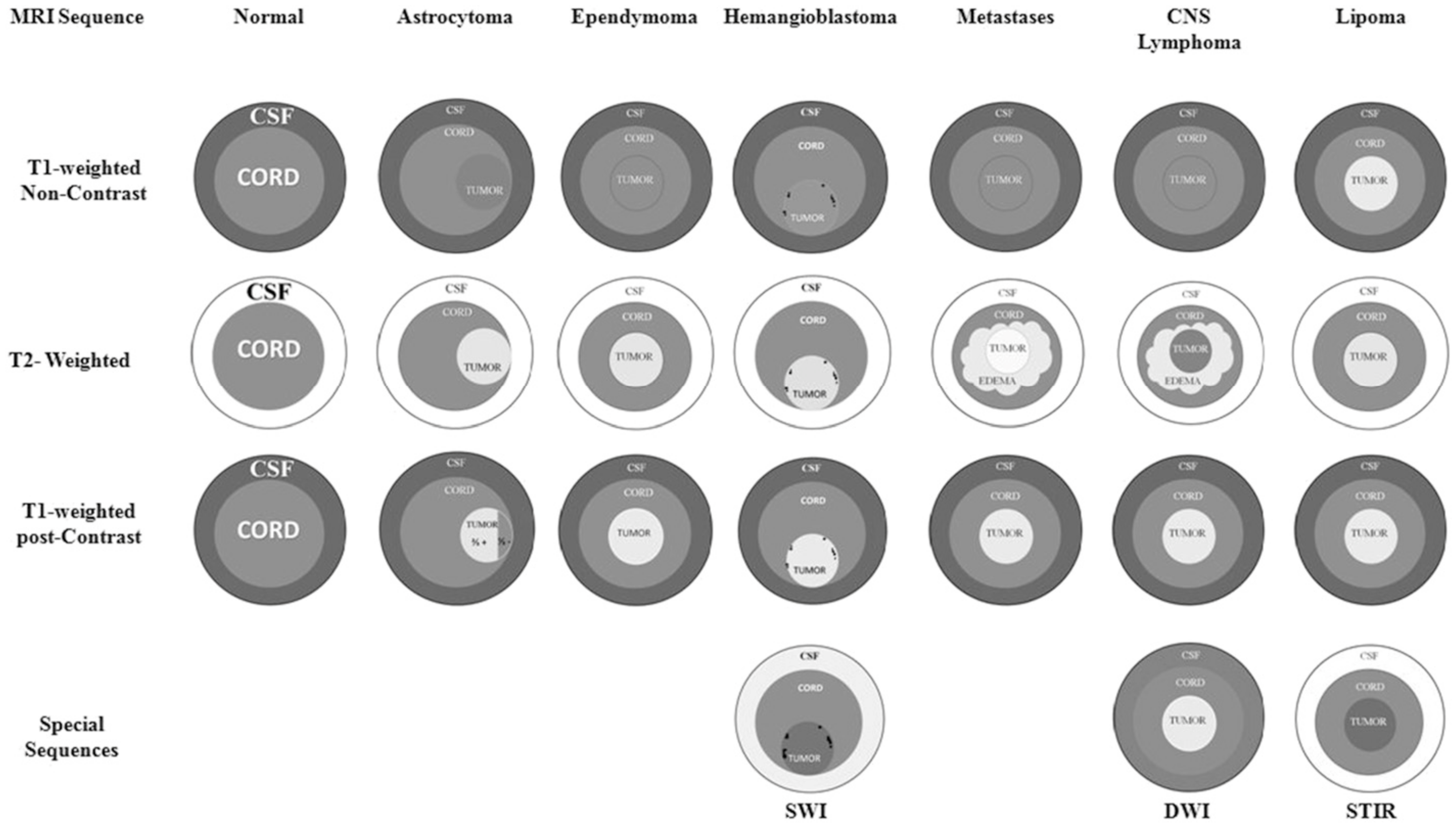
2.6.3. Neoplasms
3. Benign Neoplasms
4. Malignant Neoplasms
4.1. Infection
4.1.1. Spinal Epidural Abscesses
4.1.2. Viral Infections
4.1.3. Neurosyphilis
4.2. Miscellaneous
4.2.1. Amyotrophic Lateral Sclerosis
4.2.2. Primary Lateral Sclerosis
4.3. Mimics
4.3.1. Neurological/Non-neurological Disorders
4.3.2. Assessment and Classification of Ntsci
4.3.3. Ntsci Comorbidities
4.3.4. Rehabilitation Outcomes & Admission
4.4. Nontraumatic Sci Specific Challenges
4.4.1. Neurological Prognosis and Survival
4.4.2. Participation in Rehabilitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, N.B.; Ayers, G.D.; Peterson, E.N.; Harris, M.B.; Morse, L.; O’Connor, K.C.; Garshick, E. Traumatic spinal cord injury in the United States, 1993–2012. Jama 2015, 313, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Lasfargues, J.E.; Custis, D.; Morrone, F.; Carswell, J.; Nguyen, T. A model for estimating spinal cord injury prevalence in the United States. Paraplegia 1995, 33, 62–68. [Google Scholar] [CrossRef] [PubMed]
- National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance. J. Spinal Cord Med. 2013, 36, 1–2. [Google Scholar] [CrossRef]
- New, P.W.; Cripps, R.A.; Bonne Lee, B. Global maps of non-traumatic spinal cord injury epidemiology: Towards a living data repository. Spinal Cord 2014, 52, 97–109. [Google Scholar] [CrossRef]
- New, P.W.; Eriks-Hoogland, I.; Scivoletto, G.; Reeves, R.K.; Townson, A.; Marshall, R.; Rathore, F.A. Important Clinical Rehabilitation Principles Unique to People with Non-traumatic Spinal Cord Dysfunction. Top. Spinal Cord Inj. Rehabil. 2017, 23, 299–312. [Google Scholar] [CrossRef]
- New, P.W.; Marshall, R. International Spinal Cord Injury Data Sets for non-traumatic spinal cord injury. Spinal Cord 2014, 52, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- New, P.W.; Lee, B.B.; Cripps, R.; Vogel, L.C.; Scheinberg, A.; Waugh, M.C. Global mapping for the epidemiology of paediatric spinal cord damage: Towards a living data repository. Spinal Cord 2019, 57, 183–197. [Google Scholar] [CrossRef]
- New, P.W.; Simmonds, F.; Stevermuer, T. Comparison of patients managed in specialised spinal rehabilitation units with those managed in non-specialised rehabilitation units. Spinal Cord 2011, 49, 909–916. [Google Scholar] [CrossRef] [Green Version]
- Scivoletto, G.; Farchi, S.; Laurenza, L.; Molinari, M. Traumatic and non-traumatic spinal cord lesions: An Italian comparison of neurological and functional outcomes. Spinal Cord 2011, 49, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.S.; Stewart, M.E.; Davies, B.M.; Kotter, M.R.N. The Prevalence of Asymptomatic and Symptomatic Spinal Cord Compression on Magnetic Resonance Imaging: A Systematic Review and Meta-analysis. Global Spine J. 2021, 11, 597–607. [Google Scholar] [CrossRef]
- New, P.W.; Biering-Sorensen, F. Review of the History of Non-traumatic Spinal Cord Dysfunction. Top. Spinal Cord Inj. Rehabil. 2017, 23, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Alito, B.; Filardi, V.; Fama, F.; Bruschetta, D.; Ruggeri, C.; Basile, G.; Stancanelli, L.; D’Amico, C.; Bianconi, S.; Tisano, A. Traumatic and non-traumatic spinal cord injury: Demographic characteristics, neurological and functional outcomes. A 7-year single centre experience. J. Orthop. 2021, 28, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.; Datta, N.; Jimsheleishvili, G.; Gater, D.R. Pathophysiology, Classification and Comorbidities after Traumatic Spinal Cord Injury. J. Pers. Med. 2022, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- From the American Association of Neurological Surgeons (AANS); American Society of Neuroradiology (ASNR); Cardiovascular and Interventional Radiology Society of Europe (CIRSE); Cannadian International Radiology Assciation (CIRA); Congress of Neurological Surgeons (CNS); European Society of Minimally Invasive Neurological Therapy (ESMINT); European Society of Neuroradiology (ESNR); European Stroke Organization (ESO); Society for NeuroInterventional Surgery (SNIS); World Stroke Organization(WSO); et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int J Stroke 2018, 13, 612–632. [Google Scholar]
- Copp, A.J.; Adzick, N.S.; Chitty, L.S.; Fletcher, J.M.; Holmbeck, G.N.; Shaw, G.M. Spina bifida. Nat. Rev. Dis. Primers 2015, 1, 15007. [Google Scholar] [CrossRef] [Green Version]
- Hopson, B.; Rocque, B.G.; Joseph, D.B.; Powell, D.; McLain, A.B.J.; Davis, R.D.; Wilson, T.S.; Conklin, M.J.; Blount, J.P. The development of a lifetime care model in comprehensive spina bifida care. J. Pediatr. Rehabil. Med. 2018, 11, 323–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, A.M.; Herdt, M. Characteristics and first-year mortality, by lesion level, among infants with spina bifida in the New York State Birth Defects Registry, 2008–2017. Birth Defects Res. 2022, 114, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Gober, J.; Thomas, S.P.; Gater, D.R. Pediatric Spina Bifida and Spinal Cord Injury. J. Pers. Med. 2022, 12, 985. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.T.; Isenburg, J.L.; Canfield, M.A.; Meyer, R.E.; Correa, A.; Alverson, C.J.; Lupo, P.J.; Riehle-Colarusso, T.; Cho, S.J.; Aggarwal, D.; et al. National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. 2019, 111, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Ishii, K.; Watanabe, K.; Tsuji, T.; Matsumoto, M.; Toyama, Y.; Chiba, K. Clinical Significance and Prognosis of Idiopathic Syringomyelia. Clin. Spine Surg. 2009, 22, 372–375. [Google Scholar] [CrossRef]
- Fink, J.K. Hereditary spastic paraplegia: Clinical principles and genetic advances. Semin. Neurol. 2014, 34, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.E. Friedreich’s ataxia: A clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain 1981, 104, 589–620. [Google Scholar] [CrossRef]
- Doğan-Aslan, M.; Büyükvural-Şen, S.; Nakipoğlu-Yüzer, G.F.; Özgirgin, N. Demographic and clinical features and rehabilitation outcomes of patients with Friedreich ataxia: A retrospective study. Turk. J. Phys. Med. Rehabil. 2018, 64, 230–238. [Google Scholar] [CrossRef]
- Finkel, R.S.; Chiriboga, C.A.; Vajsar, J.; Day, J.W.; Montes, J.; De Vivo, D.C.; Bishop, K.M.; Foster, R.; Liu, Y.; Ramirez-Schrempp, D.; et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: Final report of a phase 2, open-label, multicentre, dose-escalation study. Lancet Child Adolesc. Health 2021, 5, 491–500. [Google Scholar] [CrossRef]
- Zipser, C.M.; Margetis, K.; Pedro, K.M.; Curt, A.; Fehlings, M.; Sadler, I.; Tetreault, L.; Davies, B.M.; Committee, A.O.S.R.D.S.; Members of the Diagnostic Criteria Working, G. Increasing awareness of degenerative cervical myelopathy: A preventative cause of non-traumatic spinal cord injury. Spinal Cord 2021, 59, 1216–1218. [Google Scholar] [CrossRef]
- Scalabrino, G. Subacute combined degeneration one century later. The neurotrophic action of cobalamin (vitamin B12) revisited. J. Neuropathol. Exp. Neurol. 2001, 60, 109–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pant, S.S.; Asbury, A.K.; Richardson, E.P., Jr. The myelopathy of pernicious anemia. A neuropathological reappraisal. Acta Neurol. Scand. 1968, 44, 7–36. [Google Scholar]
- Stabler, S.P. Clinical practice. Vitamin B12 deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef]
- Pugliese, R.S.; Slagle, E.J.; Oettinger, G.R.; Neuburger, K.J.; Ambrose, T.M. Subacute combined degeneration of the spinal cord in a patient abusing nitrous oxide and self-medicating with cyanocobalamin. Am. J. Health Syst. Pharm. 2015, 72, 952–957. [Google Scholar] [CrossRef]
- Lan, S.Y.; Kuo, C.Y.; Chou, C.C.; Kong, S.S.; Hung, P.C.; Tsai, H.Y.; Chen, Y.C.; Lin, J.J.; Chou, I.J.; Lin, K.L.; et al. Recreational nitrous oxide abuse related subacute combined degeneration of the spinal cord in adolescents-A case series and literature review. Brain Dev. 2019, 41, 428–435. [Google Scholar] [CrossRef]
- Salehpour, F.; Mirzaei, F.; Kazemzadeh, M.; Alavi, S.A.N. Spontaneous Epidural Hematoma of Cervical Spine. Int. J. Spine Surg. 2018, 12, 26–29. [Google Scholar]
- Lee, Y.J.; Terbrugge, K.G.; Saliou, G.; Krings, T. Clinical features and outcomes of spinal cord arteriovenous malformations: Comparison between nidus and fistulous types. Stroke 2014, 45, 2606–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheshire, W.P.; Santos, C.C.; Massey, E.W.; Howard, J.F., Jr. Spinal cord infarction: Etiology and outcome. Neurology 1996, 47, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Neri, V.C.; Xavier, M.F.; Barros, P.O.; Melo Bento, C.; Marignier, R.; Papais Alvarenga, R. Case Report: Acute Transverse Myelitis after Zika Virus Infection. Am. J. Trop. Med. Hyg. 2018, 99, 1419–1421. [Google Scholar] [CrossRef] [Green Version]
- Chow, C.C.N.; Magnussen, J.; Ip, J.; Su, Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. 2020, 13, e236720. [Google Scholar] [CrossRef] [PubMed]
- Beh, S.C.; Greenberg, B.M.; Frohman, T.; Frohman, E.M. Transverse myelitis. Neurol. Clin. 2013, 31, 79–138. [Google Scholar] [CrossRef] [PubMed]
- Roman, G.C.; Gracia, F.; Torres, A.; Palacios, A.; Gracia, K.; Harris, D. Acute Transverse Myelitis (ATM):Clinical Review of 43 Patients With COVID-19-Associated ATM and 3 Post-Vaccination ATM Serious Adverse Events With the ChAdOx1 nCoV-19 Vaccine (AZD1222). Front. Immunol. 2021, 12, 653786. [Google Scholar] [CrossRef] [PubMed]
- de Seze, J.; Lanctin, C.; Lebrun, C.; Malikova, I.; Papeix, C.; Wiertlewski, S.; Pelletier, J.; Gout, O.; Clerc, C.; Moreau, C.; et al. Idiopathic acute transverse myelitis: Application of the recent diagnostic criteria. Neurology 2005, 65, 1950–1953. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.F. Nosology of idiopathic transverse myelitis syndromes. Acta Neurol. Scand. 2007, 115, 371–376. [Google Scholar] [CrossRef]
- Krishnan, C.; Kaplin, A.I.; Pardo, C.A.; Kerr, D.A.; Keswani, S.C. Demyelinating disorders: Update on transverse myelitis. Curr. Neurol. Neurosci. Rep. 2006, 6, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1. [Google Scholar] [PubMed]
- Wattjes, M.P.; Ciccarelli, O.; Reich, D.S.; Banwell, B.; de Stefano, N.; Enzinger, C.; Fazekas, F.; Filippi, M.; Frederiksen, J.; Gasperini, C.; et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021, 20, 653–670. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Waters, P.J.; McKeon, A.; Leite, M.I.; Rajasekharan, S.; Lennon, V.A.; Villalobos, A.; Palace, J.; Mandrekar, J.N.; Vincent, A.; Bar-Or, A.; et al. Serologic diagnosis of NMO: A multicenter comparison of aquaporin-4-IgG assays. Neurology 2012, 78, 665–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; de Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, D.; Gillis, C.C.; Shih, P.; O’Toole, J.E.; Fessler, R.G. Intramedullary Spinal Cord Tumors: Part I-Epidemiology, Pathophysiology, and Diagnosis. Glob. Spine J. 2015, 5, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Celano, E.; Salehani, A.; Malcolm, J.G.; Reinertsen, E.; Hadjipanayis, C.G. Spinal cord ependymoma: A review of the literature and case series of ten patients. J. Neurooncol. 2016, 128, 377–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mechtler, L.L.; Nandigam, K. Spinal cord tumors: New views and future directions. Neurol. Clin. 2013, 31, 241–268. [Google Scholar] [CrossRef] [PubMed]
- Ciftdemir, M.; Kaya, M.; Selcuk, E.; Yalniz, E. Tumors of the spine. World J. Orthop. 2016, 7, 109–116. [Google Scholar] [CrossRef]
- Muller-Jensen, L.; Ploner, C.J.; Kroneberg, D.; Schmidt, W.U. Clinical Presentation and Causes of Non-traumatic Spinal Cord Injury: An Observational Study in Emergency Patients. Front. Neurol. 2021, 12, 701927. [Google Scholar] [CrossRef] [PubMed]
- Shah, L.M.; Salzman, K.L. Imaging of spinal metastatic disease. Int. J. Surg. Oncol. 2011, 2011, 769753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanishvili, Z.; Fourney, D.R. Incorporating the Spine Instability Neoplastic Score into a Treatment Strategy for Spinal Metastasis: LMNOP. Glob. Spine J. 2014, 4, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennington, Z.; Ahmed, A.K.; Cottrill, E.; Westbroek, E.M.; Goodwin, M.L.; Sciubba, D.M. Intra- and interobserver reliability of the Spinal Instability Neoplastic Score system for instability in spine metastases: A systematic review and meta-analysis. Ann. Transl. Med. 2019, 7, 218. [Google Scholar] [CrossRef]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef] [Green Version]
- Thomson, C. Spinal cord compression secondary to epidural abscess: The importance of prompt diagnosis and management. BMJ Case Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Krishnamohan, P.; Berger, J.R. Spinal epidural abscess. Curr. Infect. Dis. Rep. 2014, 16, 436. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, R.; Lv, X.; Lai, Q.; Xie, B.; Jiang, X.; Dai, M.; Zhang, B. Challenges in diagnosis of spinal epidural abscess: A case report. Medicine 2019, 98, e14196. [Google Scholar] [CrossRef]
- Tan, S.V.; Guiloff, R.J.; Scaravilli, F. AIDS-associated vacuolar myelopathy. A morphometric study. Brain 1995, 118, 1247–1261. [Google Scholar] [CrossRef]
- Gessain, A.; Mahieux, R. Tropical spastic paraparesis and HTLV-1 associated myelopathy: Clinical, epidemiological, virological and therapeutic aspects. Rev. Neurol. 2012, 168, 257–269. [Google Scholar] [CrossRef]
- Mozhgani, S.H.; Piran, M.; Zarei-Ghobadi, M.; Jafari, M.; Jazayeri, S.M.; Mokhtari-Azad, T.; Teymoori-Rad, M.; Valizadeh, N.; Farajifard, H.; Mirzaie, M.; et al. An insight to HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) pathogenesis; evidence from high-throughput data integration and meta-analysis. Retrovirology 2019, 16, 46. [Google Scholar] [CrossRef] [Green Version]
- Wiechers, D.O.; Hubbell, S.L. Late changes in the motor unit after acute poliomyelitis. Muscle Nerve 1981, 4, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Post-Polio Syndrome: Identifying Best Practices in Diagnosis and Care. Available online: https://www.polioplace.org/sites/default/files/files/MOD-%20Identifying.pdf (accessed on 27 June 2022).
- Lo, J.K.; Robinson, L.R. Postpolio syndrome and the late effects of poliomyelitis. Part 1. pathogenesis, biomechanical considerations, diagnosis, and investigations. Muscle Nerve 2018, 58, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.R. Neurosyphilis and the spinal cord: Then and now. J. Nerv. Ment. Dis. 2011, 199, 912–913. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Koralnik, I.J.; Marra, C.M. Neurosyphilis. Semin. Neurol. 2019, 39, 448–455. [Google Scholar] [CrossRef]
- Ghanem, K.G.; Moore, R.D.; Rompalo, A.M.; Erbelding, E.J.; Zenilman, J.M.; Gebo, K.A. Neurosyphilis in a clinical cohort of HIV-1-infected patients. AIDS 2008, 22, 1145–1151. [Google Scholar] [CrossRef] [Green Version]
- Saberi, S.; Stauffer, J.E.; Schulte, D.J.; Ravits, J. Neuropathology of Amyotrophic Lateral Sclerosis and Its Variants. Neurol. Clin. 2015, 33, 855–876. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.H.; Longo, D.L.; Al-Chalabi, A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [Green Version]
- Rezania, K.; Roos, R.P. Spinal cord: Motor neuron diseases. Neurol. Clin. 2013, 31, 219–239. [Google Scholar] [CrossRef]
- Turner, M.R.; Barohn, R.J.; Corcia, P.; Fink, J.K.; Harms, M.B.; Kiernan, M.C.; Ravits, J.; Silani, V.; Simmons, Z.; Statland, J.; et al. Delegates of the 2nd International, P.L.S.C.; Mitsumoto, H. Primary lateral sclerosis: Consensus diagnostic criteria. J. Neurol. Neurosurg. Psychiatry 2020, 91, 373–377. [Google Scholar] [CrossRef] [Green Version]
- New, P.W.; Guilcher, S.J.T.; Jaglal, S.B.; Biering-Sørensen, F.; Noonan, V.K.; Ho, C. Trends, Challenges, and Opportunities Regarding Research in Non-traumatic Spinal Cord Dysfunction. Top. Spinal Cord Inj. Rehabil. 2017, 23, 313–323. [Google Scholar] [CrossRef]
- Ginsberg, L. Myelopathy: Chameleons and mimics. Pract. Neurol. 2017, 17, 6–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, J. The bare essentials: Functional symptoms in neurology. Pract. Neurol. 2009, 9, 179–189. [Google Scholar] [CrossRef]
- Ditunno, J.F., Jr.; Young, W.; Donovan, W.H.; Creasey, G. The international standards booklet for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Paraplegia 1994, 32, 70–80. [Google Scholar] [PubMed]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupp, R.; Biering-Sørensen, F.; Burns, S.P.; Graves, D.E.; Guest, J.; Jones, L.; Read, M.S.; Rodriguez, G.M.; Schuld, C.; Tansey-Md, K.E.; et al. International Standards for Neurological Classification of Spinal Cord Injury: Revised 2019. Top. Spinal Cord Inj. Rehabil. 2021, 27, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lena, E.; Baroncini, I.; Pavese, C.; Musumeci, G.; Volini, S.; Masciullo, M.; Aiachini, B.; Fizzotti, G.; Puci, M.V.; Scivoletto, G. Reliability and validity of the international standards for neurological classification of spinal cord injury in patients with non-traumatic spinal cord lesions. Spinal Cord 2022, 60, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wecht, J.M.; Krassioukov, A.V.; Alexander, M.; Handrakis, J.P.; McKenna, S.L.; Kennelly, M.; Trbovich, M.; Biering-Sorensen, F.; Burns, S.; Elliott, S.L.; et al. International Standards to document Autonomic Function following SCI (ISAFSCI): Second Edition. Top. Spinal Cord Inj. Rehabil. 2021, 27, 23–49. [Google Scholar] [CrossRef]
- McKinley, W.; Hills, A.; Sima, A. Posterior cord syndrome: Demographics and rehabilitation outcomes. J. Spinal Cord Med. 2021, 44, 241–246. [Google Scholar] [CrossRef]
- Celani, M.G.; Spizzichino, L.; Ricci, S.; Zampolini, M.; Franceschini, M. Retrospective Study Group on, S.C.I., Spinal cord injury in Italy: A multicenter retrospective study. Arch. Phys. Med. Rehabil. 2001, 82, 589–596. [Google Scholar] [CrossRef]
- Pope, D.H.; Mowforth, O.D.; Davies, B.M.; Kotter, M.R.N. Diagnostic Delays Lead to Greater Disability in Degenerative Cervical Myelopathy and Represent a Health Inequality. Spine 2020, 45, 368–377. [Google Scholar] [CrossRef]
- Cosar, S.N.; Yemisci, O.U.; Oztop, P.; Cetin, N.; Sarifakioglu, B.; Yalbuzdag, S.A.; Ustaomer, K.; Karatas, M. Demographic characteristics after traumatic and non-traumatic spinal cord injury: A retrospective comparison study. Spinal Cord 2010, 48, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Osterthun, R. Outcomes after Spinal Cord Injury. Doctoral Dissertation, Rijksuniversiteit Groningen, Groningen, The Netherlands, 2018. [Google Scholar]
- Gedde, M.H.; Lilleberg, H.S.; Assmus, J.; Gilhus, N.E.; Rekand, T. Traumatic vs. non-traumatic spinal cord injury: A comparison of primary rehabilitation outcomes and complications during hospitalization. J. Spinal Cord Med. 2019, 42, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Catz, A.; Goldin, D.; Fishel, B.; Ronen, J.; Bluvshtein, V.; Gelernter, I. Recovery of neurologic function following nontraumatic spinal cord lesions in Israel. Spine 2004, 29, 2278–2282. [Google Scholar] [CrossRef] [PubMed]
- Buzzell, A.; Chamberlain, J.D.; Gmunder, H.P.; Hug, K.; Jordan, X.; Schubert, M.; Brinkhof, M.W.G.; Swi, S.C.I.s.g. Survival after non-traumatic spinal cord injury: Evidence from a population-based rehabilitation cohort in Switzerland. Spinal Cord 2019, 57, 267–275. [Google Scholar] [CrossRef] [PubMed]
| Total | T-SCI | NT-SCI | p-Value | Total | T-SCI | NT-SCI | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Sex | (0.06) | Clinical presentation | (0.002) | ||||||
| Male | 75 (67%) | 22 (81%) | 53 (63%) | Tetraplegia | 32 (29%) | 14 (52%) | 18 (21%) | ||
| Female | 37 (33%) | 5 (19%) | 32 (37%) | Paraplegia | 80 (71%) | 13 (48%) | 67 (79%) | ||
| Total | T-SCI | NT-SCI | p-Value | Total | T-SCI | NT-SCI | p-Value | ||
| Age | (0.0005) | Level of injury | (0.005) | ||||||
| Range Mean ± SD | 22–87 60 ± 14.8 | 22–77 52 ± 17.7 | 25–87 63 ± 12.6 | Cervical Cervical and thoracic | 30.5% 3.5% | 44.5% 15.0% | 26.0% - | ||
| Thoracic | 57.0% | 33.0% | 65.0% | ||||||
| Lumbar-sacral | 9.0% | 7.5% | 9.0% | ||||||
| Total | T-SCI | NT-SCI | p-Value | ||||||
| Completeness of injury | (0.001) | ||||||||
| Complete | 8 (7%) | 7 (21%) | 2 (2%) | ||||||
| Incomplete | 104 (93%) | 29 (79%) | 83 (98%) |
| Extramedullary | ||
|---|---|---|
| Intramedullary | Intradural | Extradural |
| Ependymoma | Meningioma | Metastasis |
| Astrocytoma | Malignant nerve sheet tumors | Vertebral primary & secondary tumors |
| Metastasis | Schwannomas | Chordoma |
| Arterial-venous malformation | Neurofibromas | Sarcomas |
| Hemangioblastoma | Lipoma | Lymphomas |
| Leptomeningeal carcinomatosis | Plasmacytomas | |
| Extramedullary | Clinical Presentation | Intramedullary |
|---|---|---|
| Common | Local pain—vertebral pain | Rare |
| Common | Radicular pain | Rare |
| Less common | Funicular pain | Common |
| Yes, early | Upper motor neuron signs | Yes, late |
| Uncommon; if present segmental | Lower motor neuron signs | Prominent and diffuse |
| Ascending; sacral involvement | Paresthesia progression | Descending; sacral spearing, dissociate loss |
| Cauda equina (late) | Sphincter abnormalities | Conus lesions (early) |
| Element | Score |
|---|---|
| Location | |
| Junctional (occiput-C2, C7-T2, T11-L1, L5-S1) | 3 |
| Mobile spine (C3-C6, L2-L4) | 2 |
| Semirigid spine (T3-T10) | 1 |
| Rigid spine (S2-S5) | 0 |
| Pain with recumbency and/or movement of spine | |
| Yes | 3 |
| Occasional, but not mechanical | 1 |
| No | 0 |
| Bone lesion | |
| Lytic | 2 |
| Mixed (lytic and blastic) | 1 |
| Blastic | 0 |
| Radiographic spinal alignment | |
| Subluxation or translation present | 4 |
| De novo deformity (kyphosis or scoliosis) | 2 |
| Normal alignment | 0 |
| Vertebral body collapse | |
| >50% | 3 |
| <50% | 2 |
| No collapse, with >50% of body involved | 1 |
| None | 0 |
| Involvement of posterolateral spine elements (face, pedicle or costovertebral joint fracture or replacement with tumor) | |
| Bilateral | 3 |
| Unilateral | 1 |
| None of the above | 0 |
| Total score | |
| Stable | 0–6 |
| Indeterminate | 7–12 |
| Unstable | 13–18 |
| Author (Country) | Years | Numbers (n) SCI Group | Age (Years) SCI Group | Admission FIM SCI Group | Discharge FIM SCI Group | Length of Stay (Days) SCI Group | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TSCI | NTSCI | TSCI | NTSCI | TSCI | NTSCI | TSCI | NTSCI | TSCI | NTSCI | ||
| Mckinley et al. (USA) [79] | 1992–99 | 86 | 86 | NR | NR | 36.7 | 37.0 | 68.0 | 55.8 | 41.35 | 22.38 |
| Grazia Celani et al. (Italy) [80] | 1989–99 | 642 | 217 | 34.3 ± 15.5 | 48.2 ± 18.1 | NR | NR | NR | NR | 143.1 ± 89.1 | 91.7 ± 78.9 |
| Ostherthun et al. (Netherlands & Belgium) [83] | 2002–07 | 389 | 530 | 43.4 ± 16.7 | 57.2 ± 14.5 | NR | NR | NR | NR | 227.6 ± 105.2 | 142.7 ± 110.5 |
| New et al. (Australia) [4] | 2002–06 | 1361 | 2241 | 46.0 | 67.0 | 38 | 53 | 74 | 76 | 44 | 21 |
| New (Australia) [6] | 1995–97 | NA | 70 | NA | 69 | NA | 37.5 ± 11.4 | NA | 52.0 ± 18.5 | NA | 55.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinares, D.M.; Gater, D.R.; Daniel, S.; Pontee, N.L. Nontraumatic Spinal Cord Injury: Epidemiology, Etiology and Management. J. Pers. Med. 2022, 12, 1872. https://doi.org/10.3390/jpm12111872
Molinares DM, Gater DR, Daniel S, Pontee NL. Nontraumatic Spinal Cord Injury: Epidemiology, Etiology and Management. Journal of Personalized Medicine. 2022; 12(11):1872. https://doi.org/10.3390/jpm12111872
Chicago/Turabian StyleMolinares, Diana M., David R. Gater, Scott Daniel, and Nicole L. Pontee. 2022. "Nontraumatic Spinal Cord Injury: Epidemiology, Etiology and Management" Journal of Personalized Medicine 12, no. 11: 1872. https://doi.org/10.3390/jpm12111872
APA StyleMolinares, D. M., Gater, D. R., Daniel, S., & Pontee, N. L. (2022). Nontraumatic Spinal Cord Injury: Epidemiology, Etiology and Management. Journal of Personalized Medicine, 12(11), 1872. https://doi.org/10.3390/jpm12111872







