The Influence of Body Mass Index on Glucocorticoid Insensitivity in Chronic Rhinosinusitis with Nasal Polyps
Abstract
:1. Introduction
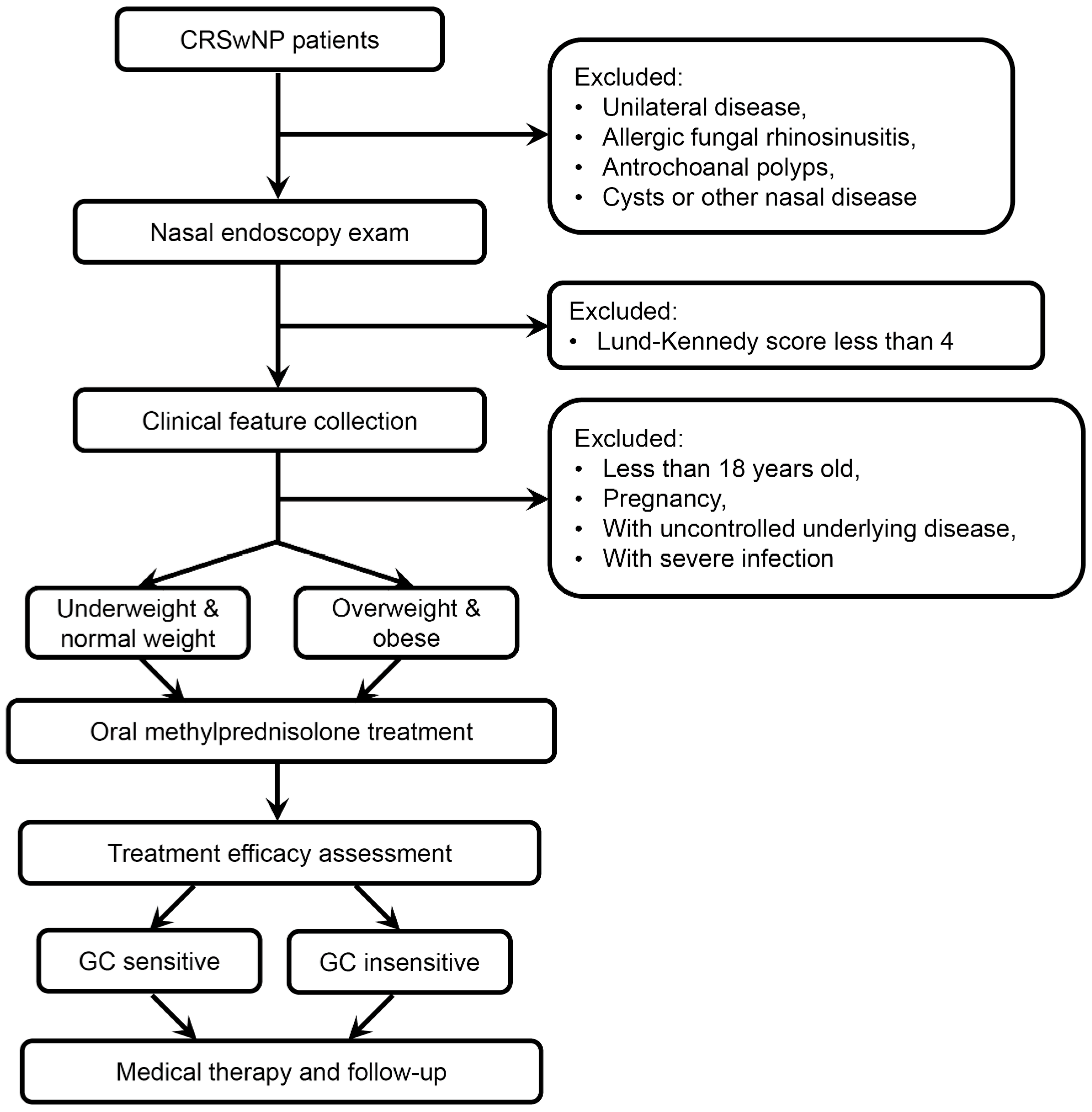
2. Materials and Methods
2.1. Subjects
2.2. Clinical Feature Collection
2.3. Clinical Phenotype Evaluation in CRSwNP Tissue
2.4. Assessment and Follow-Up of GC Treatment Efficacy
2.5. Statistical Analysis
3. Results
3.1. Being Overweight & Obese Was a Risk Factor for GC Insensitivity in CRSwNP
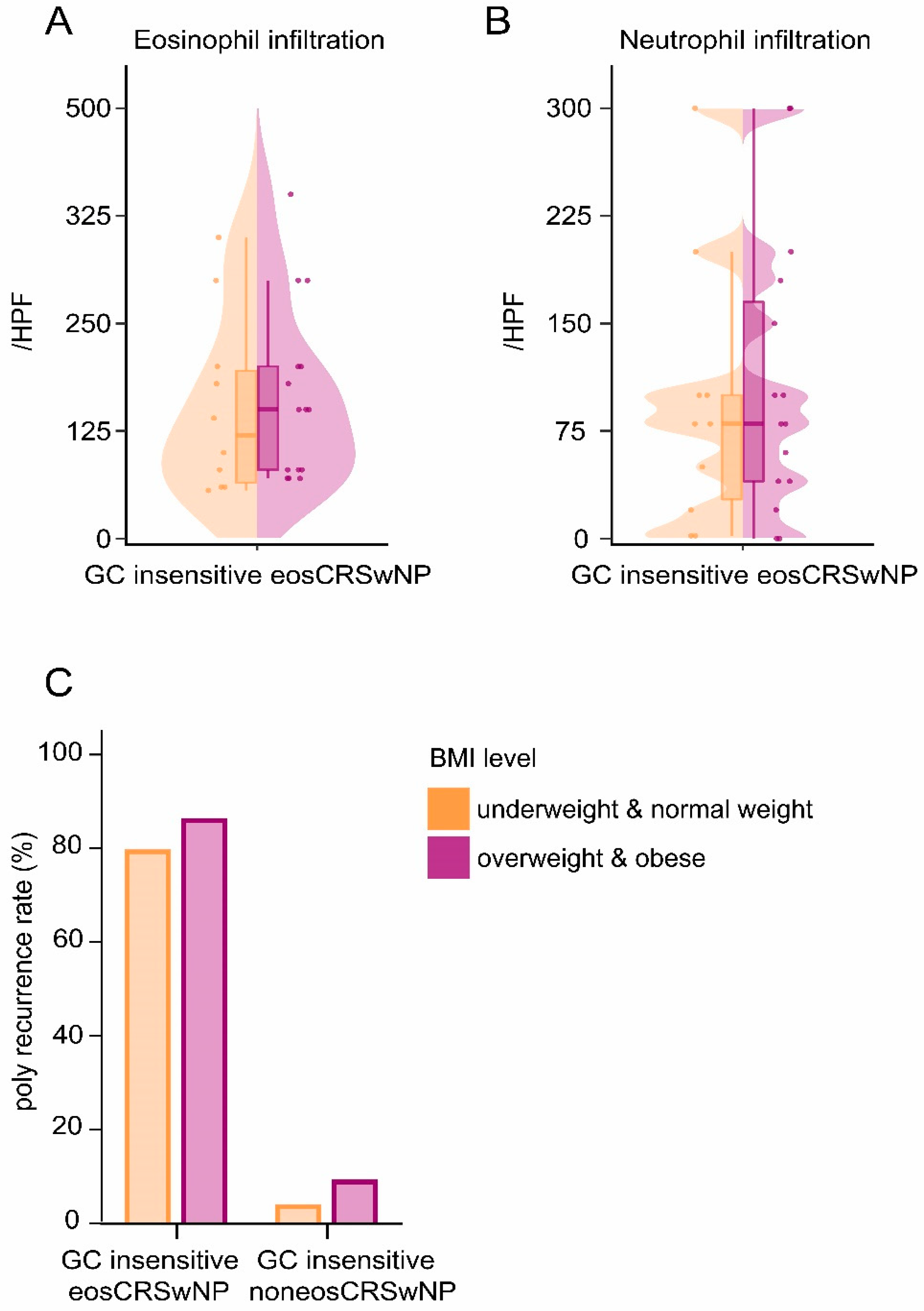
3.2. Being Overweight & Obese Contributed to GC Insensitivity in eosCRSwNP Patients without Affecting Polyp Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bachert, C.; Marple, B.; Schlosser, R.J.; Hopkins, C.; Schleimer, R.P.; Lambrecht, B.N.; Bröker, B.M.; Laidlaw, T.; Song, W.J. Adult chronic rhinosinusitis. Nat. Rev. Dis. Primers 2020, 6, 86. [Google Scholar] [CrossRef]
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Soler, Z.; Welch, K.C.; Wise, S.K.; et al. International consensus statement on allergy and rhinology: Rhinosinusitis 2021. Int. Forum Allergy Rhinol. 2021, 11, 213–739. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Zhang, N.; Bachert, C.; Zhang, L. Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int. Forum Allergy Rhinol. 2018, 8, 1218–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Liu, W.; Zhang, L.; Bai, J.; Fan, Y.; Xia, W.; Luo, Q.; Zheng, J.; Wang, H.; Li, Z.; et al. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J. Allergy Clin. Immunol. 2012, 129, 1522–1528.e5. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.L.; Milara, J.; Lluch, J.; De Diego, A.; Sanz, C.; Cortijo, J. Phosphodiesterase-4 inhibition improves corticosteroid insensitivity in pulmonary endothelial cells under oxidative stress. Allergy 2013, 68, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Contoli, M.; Adcock, I.M.; Bellettato, C.; Padovani, A.; Casolari, P.; Stanciu, L.A.; Barnes, P.J.; Johnston, S.L.; Ito, K.; et al. Rhinovirus infection causes steroid resistance in airway epithelium through nuclear factor kappaB and c-Jun N-terminal kinase activation. J. Allergy Clin. Immunol. 2013, 132, 1075–1085.e6. [Google Scholar] [CrossRef]
- Wang, S.B.; Chen, S.M.; Zhu, K.S.; Zhou, B.; Chen, L.; Zou, X.Y. Increased lipopolysaccharide content is positively correlated with glucocorticoid receptor-beta expression in chronic rhinosinusitis with nasal polyps. Immun. Inflamm. Dis. 2020, 8, 605–614. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Carpaij, O.A.; van den Berge, M. The asthma-obesity relationship: Underlying mechanisms and treatment implications. Curr. Opin. Pulm. Med. 2018, 24, 42–49. [Google Scholar] [CrossRef]
- Sutherland, E.R.; Goleva, E.; Strand, M.; Beuther, D.A.; Leung, D.Y. Body mass and glucocorticoid response in asthma. Am. J. Respir. Crit. Care Med. 2008, 178, 682–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fokkens, W.; Lund, V.; Mullol, J. EP3OS 2007: European position paper on rhinosinusitis and nasal polyps 2007. A summary for otorhinolaryngologists. Rhinology 2007, 45, 97–101. [Google Scholar] [PubMed]
- Psaltis, A.J.; Li, G.; Vaezeafshar, R.; Cho, K.S.; Hwang, P.H. Modification of the Lund-Kennedy endoscopic scoring system improves its reliability and correlation with patient-reported outcome measures. Laryngoscope 2014, 124, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry; Report of a WHO Expert Committee; World Health Organization: Geneva, Switzerland, 1995; Volume 854, pp. 1–452. [Google Scholar]
- Bateman, E.D.; Hurd, S.S.; Barnes, P.J.; Bousquet, J.; Drazen, J.M.; FitzGerald, J.M.; Gibson, P.; Ohta, K.; O’Byrne, P.; Pedersen, S.E.; et al. Global strategy for asthma management and prevention: GINA executive summary. Eur. Respir. J. 2008, 31, 143–178. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008, 63 (Suppl. 86), 8–160. [Google Scholar] [CrossRef]
- Lund, V.J.; Mackay, I.S. Staging in rhinosinusitus. Rhinology 1993, 31, 183–184. [Google Scholar]
- Lou, H.; Meng, Y.; Piao, Y.; Wang, C.; Zhang, L.; Bachert, C. Predictive significance of tissue eosinophilia for nasal polyp recurrence in the Chinese population. Am. J. Rhinol. Allergy 2015, 29, 350–356. [Google Scholar] [CrossRef]
- Wu, D.; Yan, B.; Wang, Y.; Zhang, L.; Wang, C. Predictive Significance of Charcot-Leyden Crystal Protein in Nasal Secretions in Recurrent Chronic Rhinosinusitis with Nasal Polyps. Int. Arch. Allergy Immunol. 2021, 182, 65–75. [Google Scholar] [CrossRef]
- Lou, H.; Meng, Y.; Piao, Y.; Zhang, N.; Bachert, C.; Wang, C.; Zhang, L. Cellular phenotyping of chronic rhinosinusitis with nasal polyps. Rhinology 2016, 54, 150–159. [Google Scholar] [CrossRef]
- Wang, E.; Wechsler, M.E.; Tran, T.N.; Heaney, L.G.; Jones, R.C.; Menzies-Gow, A.N.; Busby, J.; Jackson, D.J.; Pfeffer, P.E.; Rhee, C.K.; et al. Characterization of Severe Asthma Worldwide: Data From the International Severe Asthma Registry. Chest 2020, 157, 790–804. [Google Scholar] [CrossRef]
- Mukadam, S.; Zacharias, J.; Henao, M.P.; Kraschnewski, J.; Ishmael, F. Differential effects of obesity on eosinophilic vs. non-eosinophilic asthma subtypes. J. Asthma Off. J. Assoc. Care Asthma 2018, 55, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Gibeon, D.; Batuwita, K.; Osmond, M.; Heaney, L.G.; Brightling, C.E.; Niven, R.; Mansur, A.; Chaudhuri, R.; Bucknall, C.E.; Rowe, A.; et al. Obesity-associated severe asthma represents a distinct clinical phenotype: Analysis of the British Thoracic Society Difficult Asthma Registry Patient cohort according to BMI. Chest 2013, 143, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Wang, Y.; Zhao, Y.; Xu, Y.; Wang, T.; Du, K.; Bao, S.; Wang, X.; Zhang, L. Identification of multiple isoforms of glucocorticoid receptor in nasal polyps of patients with chronic rhinosinusitis. J. Otolaryngol. Head Neck Surg. 2022, 51, 25. [Google Scholar] [CrossRef] [PubMed]
- Elgazar-Carmon, V.; Rudich, A.; Hadad, N.; Levy, R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 2008, 49, 1894–1903. [Google Scholar] [CrossRef] [Green Version]
- Panettieri, R.A., Jr. The Role of Neutrophils in Asthma. Immunol. Allergy Clin. N. Am. 2018, 38, 629–638. [Google Scholar] [CrossRef]
- Tian, B.P.; Xia, L.X.; Bao, Z.Q.; Zhang, H.; Xu, Z.W.; Mao, Y.Y.; Cao, C.; Che, L.Q.; Liu, J.K.; Li, W.; et al. Bcl-2 inhibitors reduce steroid-insensitive airway inflammation. J. Allergy Clin. Immunol. 2017, 140, 418–430. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.S.; Roh, Y.H.; Fahad, W.A.; Noh, H.E.; Ha, J.G.; Yoon, J.H.; Kim, C.H.; Cho, H.J. Association between obesity and chronic rhinosinusitis with nasal polyps: A national population-based study. BMJ Open 2021, 11, e047230. [Google Scholar] [CrossRef]

| Characteristics | GC-Insensitive | GC-Sensitive | p-Value |
|---|---|---|---|
| Number | 247 | 452 | |
| Age (mean ± SD) | 46.1 ± 13.3 | 46.1 ± 11.9 | 0.894 |
| Gender (%) | |||
| Females | 52 (21.1) | 169 (37.4) | <0.001 |
| Males | 195 (78.9) | 283 (62.6) | |
| BMI levels (%) | |||
| Underweight & normal weight | 128 (51.8) | 282 (62.4) | 0.007 |
| Overweight & obese | 119 (48.2) | 170 (37.6) | |
| BMI (mean ± SD) | 25.1 ± 3.6 | 24.3 ± 3.5 | 0.002 |
| Smoke (%) | 72 (29.1) | 114 (25.2) | 0.261 |
| VAS score (mean ± SD) | |||
| Nasal blockage | 6.3 ± 2.0 | 6.2 ± 2.2 | 0.570 |
| Nasal discharge | 5.3 ± 1.9 | 5.3 ± 2.0 | 0.629 |
| Facial pain | 1.8 ± 2.3 | 2.2 ± 2.4 | 0.068 |
| Smell loss | 4.2 ± 3.4 | 5.1 ± 3.3 | <0.001 |
| Asthma (%) | 32 (13.0) | 160 (35.4) | <0.001 |
| Allergy (%) | 32 (13.0) | 80 (17.7) | 0.102 |
| Polyp Recurrence (%) | 36 (14.6) | 361 (79.9) | <0.001 |
| CT and endoscopic scores (mean ± SD) | |||
| Lund–Kennedy score | 6.9 ± 2.0 | 6.9 ± 1.9 | 0.695 |
| Lund–Mackay score | 17.6 ± 5.0 | 18.9 ± 4.4 | 0.002 |
| CRSwNP | Number | GC Insensitive (%) | GC Sensitive (%) | p Value | |
|---|---|---|---|---|---|
| eosCRSwNP | Underweight & normal weight | 237 | 10 (4.2) | 227 (95.8) | 0.027 |
| Overweight & obese | 152 | 15 (9.9) | 137 (90.1) | ||
| noneosCRSwNP | Underweight & normal weight | 173 | 118 (68.2) a | 55 (31.8) a | 0.135 |
| Overweight & obese | 137 | 104 (75.9) b | 33 (24.1) b | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Shen, S.; Liu, Y.; Wang, Z.; Wang, Q.; Li, Y.; Wang, C.; Lan, F.; Zhang, L. The Influence of Body Mass Index on Glucocorticoid Insensitivity in Chronic Rhinosinusitis with Nasal Polyps. J. Pers. Med. 2022, 12, 1935. https://doi.org/10.3390/jpm12111935
Zhang Y, Shen S, Liu Y, Wang Z, Wang Q, Li Y, Wang C, Lan F, Zhang L. The Influence of Body Mass Index on Glucocorticoid Insensitivity in Chronic Rhinosinusitis with Nasal Polyps. Journal of Personalized Medicine. 2022; 12(11):1935. https://doi.org/10.3390/jpm12111935
Chicago/Turabian StyleZhang, Yuling, Shen Shen, Yating Liu, Zaichuan Wang, Qiqi Wang, Yan Li, Chengshuo Wang, Feng Lan, and Luo Zhang. 2022. "The Influence of Body Mass Index on Glucocorticoid Insensitivity in Chronic Rhinosinusitis with Nasal Polyps" Journal of Personalized Medicine 12, no. 11: 1935. https://doi.org/10.3390/jpm12111935
APA StyleZhang, Y., Shen, S., Liu, Y., Wang, Z., Wang, Q., Li, Y., Wang, C., Lan, F., & Zhang, L. (2022). The Influence of Body Mass Index on Glucocorticoid Insensitivity in Chronic Rhinosinusitis with Nasal Polyps. Journal of Personalized Medicine, 12(11), 1935. https://doi.org/10.3390/jpm12111935







