The Association of uPA, uPAR, and suPAR System with Inflammation and Joint Damage in Rheumatoid Arthritis: suPAR as a Biomarker in the Light of a Personalized Medicine Perspective
Abstract
1. Introduction
2. Materials and Methods
3. Results
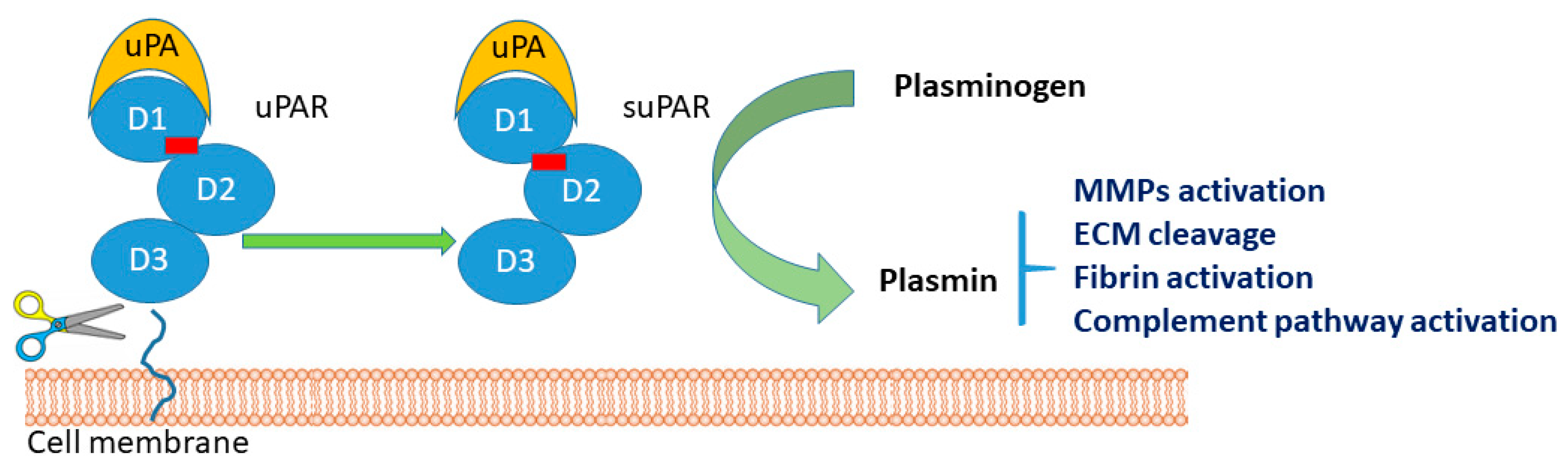
3.1. Molecular Biochemical Aspects of suPAR
3.2. suPAR as a Novel Biomarker for Systemic Chronic Inflammation (SCI) and COVID-19
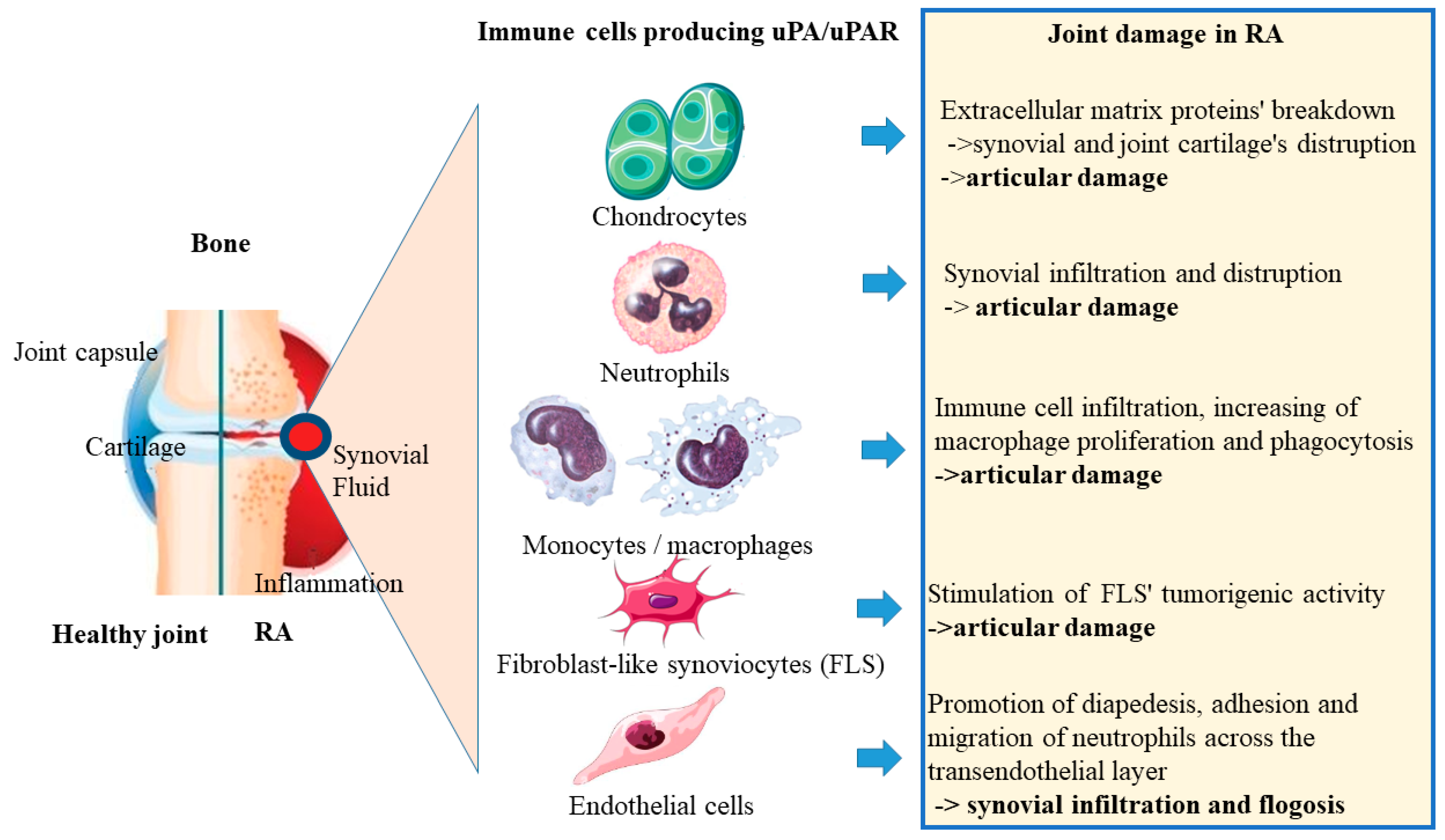
3.3. uPA/uPAR Secretion by Immune Cells in Rheumatoid Arthritis
3.3.1. uPA/uPAR System in Chondrocytes
3.3.2. uPA/uPAR System in Neutrophils
3.3.3. uPA/uPAR System in Monocytes/Macrophages
3.3.4. uPA/uPAR System in Fibroblast-Like Synoviocytes (FLS)
3.3.5. uPA/uPAR System in Endothelial Cells
3.4. Signaling Pathways Mediated through uPA/uPAR during RA Progression
3.5. suPAR as a New Potential Biomarker in Rheumatoid Arthritis
4. Perspectives and Research Agenda
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef] [PubMed]
- Trouw, L.A.; Pickering, M.C.; Blom, A.M. The complement system as a potential therapeutic target in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 538–547. [Google Scholar] [CrossRef]
- Cohen, S.; Kay, J. Biosimilars: Implications for rheumatoid arthritis therapy. Curr. Opin. Rheumatol. 2017, 29, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Szondy, Z.; Pallai, A. Transmembrane TNF-alpha reverse signaling leading to TGF-beta production is selectively activated by TNF targeting molecules: Therapeutic implications. Pharmacol. Res. 2017, 115, 124–132. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Lee, Y.A.; Yang, H.I.; Yoo, M.C.; Kim, K.S. Increased levels of thymosin beta4 in synovial fluid of patients with rheumatoid arthritis: Association of thymosin beta4 with other factors that are involved in inflammation and bone erosion in joints. Int. J. Rheum. Dis. 2011, 14, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.W.; Marshall, C.J. Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 2010, 11, 23–36. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, Y.-A.; Choi, H.M.; Yoo, M.C.; Yang, H.I. Implication of MMP-9 and urokinase plasminogen activator (uPA) in the activation of pro-matrix metalloproteinase (MMP)-13. Rheumatol. Int. 2012, 32, 3069–3075. [Google Scholar] [CrossRef]
- Pavon, M.A.; Arroyo-Solera, I.; Céspedes, M.V.; Casanova, I.; León, X.; Mangues, R. uPA/uPAR and SERPINE1 in head and neck cancer: Role in tumor resistance, metastasis, prognosis and therapy. Oncotarget 2016, 7, 57351–57366. [Google Scholar] [CrossRef]
- Del Rosso, M.; Margheri, F.; Serrati, S.; Chilla, A.; Laurenzana, A.; Fibbi, G. The urokinase receptor system, a key regulator at the intersection between inflammation, immunity, and coagulation. Curr. Pharm. Des. 2011, 17, 1924–1943. [Google Scholar] [CrossRef]
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.; Josefsson, E.; Jin, T. Fibrinolysis is down-regulated in mouse collagen-induced arthritis, but its normalization does not alleviate the course of disease. Inflamm. Res. 2011, 60, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Ronday, H.K.; Smits, H.H.; Van Muijen, G.N.; Pruszczynski, M.S.; Dolhain, R.J.; Van Langelaan, E.J.; Verheijen, J.H. Difference in expression of the plasminogen activation system in synovial tissue of patients with rheumatoid arthritis and osteoarthritis. Br. J. Rheumatol. 1996, 35, 416–423. [Google Scholar] [CrossRef][Green Version]
- Busso, N.; Péclat, V.; So, A.; Sappino, A.-P. Plasminogen activation in synovial tissues: Differences between normal, osteoarthritis, and rheumatoid arthritis joints. Ann. Rheum. Dis. 1997, 56, 550–557. [Google Scholar] [CrossRef]
- Almholt, K.; Hebsgaard, J.B.; Nansen, A.; Andersson, C.; Pass, J.; Rønø, B.; Thygesen, P.; Pelzer, H.; Loftager, M.; Lund, I.K.; et al. Antibody-Mediated Neutralization of uPA Proteolytic Function Reduces Disease Progression in Mouse Arthritis Models. J. Immunol. 2018, 200, 957–965. [Google Scholar] [CrossRef]
- Kanno, Y.; Ishisaki, A.; Kawashita, E.; Kuretake, H.; Ikeda, K.; Matsuo, O. uPA Attenuated LPS-induced Inflammatory Osteoclastogenesis through the Plasmin/PAR-1/Ca(2+)/CaMKK/AMPK Axis. Int. J. Biol. Sci. 2016, 12, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.C.; Al-Rashed, F.; Calay, D.; Birdsey, G.M.; Bauer, A.; Mylroie, H.; Morley, B.J.; Randi, A.M.; Haskard, D.O.; Boyle, J.J.; et al. Methotrexate-mediated activation of an AMPK-CREB-dependent pathway: A novel mechanism for vascular protection in chronic systemic inflammation. Ann. Rheum. Dis. 2016, 75, 439–448. [Google Scholar] [CrossRef]
- Kanno, Y.; Ishisaki, A.; Miyashita, M.; Matsuo, O. The blocking of uPAR suppresses lipopolysaccharide-induced inflammatory osteoclastogenesis and the resultant bone loss through attenuation of integrin beta3/Akt pathway. Immun. Inflamm. Dis. 2016, 4, 338–349. [Google Scholar] [CrossRef]
- Rasmussen, L.J.H.; Petersen, J.E.V.; Eugen-Olsen, J. Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a Biomarker of Systemic Chronic Inflammation. Front. Immunol. 2021, 12, 780641. [Google Scholar] [CrossRef] [PubMed]
- Danø, K.; Brünner, N.; Ellis, V.; Ploug, M.; Pyke, C. The urokinase receptor. Protein structure and role in plasminogen activation and cancer invasion. Fibrinolysis 1994, 8, 189–203. [Google Scholar] [CrossRef]
- Behrendt, N.S.R. The urokinase receptor. Fibrinolysis Proteol. 1998, 12, 191–204. [Google Scholar] [CrossRef]
- Plesner, T.; Behrendt, N.; Ploug, M. Structure, function and expression on blood and bone marrow cells of the urokinase-type plasminogen activator receptor, uPAR. Stem Cells 1997, 15, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Plesner, T.; Ralfkiaer, E.; Wittrup, M.; Johnsen, H.; Pyke, C.; Pedersen, T.L.; Dano, K. Expression of the receptor for urokinase-type plasminogen activator in normal and neoplastic blood cells and hematopoietic tissue. Am. J. Clin. Pathol. 1994, 102, 835–841. [Google Scholar] [CrossRef]
- Koshelnick, Y.; Ehart, M.; Stockinger, H.; Binder, B.R. Mechanisms of signaling through urokinase receptor and the cellular response. Thromb. Haemost. 1999, 82, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.W.; Pedersen, A.N.; Nielsen, H.J.; Hamers, M.J.; Hoyer-Hansen, G.; Ronne, E.; Brunner, N. ELISA determination of soluble urokinase receptor in blood from healthy donors and cancer patients. Clin. Chem. 1997, 43, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Rønne, E.; Pappot, H.; Grøndahl-Hansen, J.; Høyer-Hansen, G.; Plesner, T.; Hansen, N.E.; Danø, K. The receptor for urokinase plasminogen activator is present in plasma from healthy donors and elevated in patients with paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 1995, 89, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.J.H. Clinical Prognostication with the Inflammatory Biomarker suPAR. Ph.D. Thesis, University of Copenhagen, København, Denmark, 2018. [Google Scholar]
- Altintas, I.; Eugen-Olsen, J.; Seppälä, S.; Tingleff, J.; Stauning, M.A.; El Caidi, N.O.; Elmajdoubi, S.; Gamst-Jensen, H.; Lindstrøm, M.B.; Rasmussen, L.J.H.; et al. suPAR Cut-Offs for Risk Stratification in Patients with Symptoms of COVID-19. Biomark. Insights 2021, 16, 11772719211034685. [Google Scholar] [CrossRef] [PubMed]
- Infantino, M.; Morena, L.; Di Pietro, M.A.; Romanin, B.; Cimolato, B.; Rocca, B.A.L.; Tunnera, S.; Modi, G.; Tilli, M.; Grossi, V.; et al. Soluble urokinase Plasminogen Activator Receptor (suPAR) levels are predictive of COVID-19 severity: An Italian experience. Clin. Immunol. 2022, 242, 109091. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; Poulakou, G.; Milionis, H.; Metallidis, S.; Adamis, G.; Tsiakos, K.; Fragkou, A.; Rapti, A.; Damoulari, C.; Fantoni, M.; et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat. Med. 2021, 27, 1752–1760. [Google Scholar] [CrossRef]
- Bedaiwi, M.K.; Almaghlouth, I.; Omair, M.A. Effectiveness and adverse effects of anakinra in treatment of rheumatoid arthritis: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7833–7839. [Google Scholar]
- Enocsson, H.; Idoff, C.; Gustafsson, A.; Govender, M.; Hopkins, F.; Larsson, M.; Nilsdotter-Augustinsson, A.; Sjöwall, J. Soluble Urokinase Plasminogen Activator Receptor (suPAR) Independently Predicts Severity and Length of Hospitalisation in Patients With COVID-19. Front. Med. 2021, 8, 791716. [Google Scholar] [CrossRef] [PubMed]
- Guiducci, S.; Del Rosso, A.; Cinelli, M.; Margheri, F.; D’Alessio, S.; Fibbi, G.; Cerinic, M.M.; Del Rosso, M. Rheumatoid synovial fibroblasts constitutively express the fibrinolytic pattern of invasive tumor-like cells. Clin. Exp. Rheumatol. 2005, 23, 364–372. [Google Scholar] [PubMed]
- Shakibaei, M.; Schulze-Tanzil, G.; Mobasheri, A.; Beichler, T.; Dressler, J.; Schwab, W. Expression of the VEGF receptor-3 in osteoarthritic chondrocytes: Stimulation by interleukin-1 beta and association with beta 1-integrins. Histochem. Cell Biol. 2003, 120, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.D.; De Nardo, C.M.; Braine, E.L.; Turner, A.L.; Vlahos, R.; Way, K.J.; Beckman, S.K.; Lenzo, J.C.; Hamilton, J.A. Urokinase-type plasminogen activator and arthritis progression: Role in systemic disease with immune complex involvement. Arthritis Res. Ther. 2010, 12, R37. [Google Scholar] [CrossRef]
- Slot, O.; Brünner, N.; Locht, H.; Oxholm, P.; Stephens, R.W. Soluble urokinase plasminogen activator receptor in plasma of patients with inflammatory rheumatic disorders: Increased concentrations in rheumatoid arthritis. Ann. Rheum. Dis. 1999, 58, 488–492. [Google Scholar] [CrossRef]
- Huh, Y.H.; Lee, G.; Song, W.-H.; Koh, J.-T.; Ryu, J.-H. Crosstalk between FLS and chondrocytes is regulated by HIF-2alpha-mediated cytokines in arthritis. Exp. Mol. Med. 2015, 47, e197. [Google Scholar] [CrossRef]
- Hu, J.; Zhai, C.; Hu, J.; Li, Z.; Fei, H.; Wang, Z.; Fan, W. MiR-23a inhibited IL-17-mediated proinflammatory mediators expression via targeting IKKalpha in articular chondrocytes. Int. Immunopharmacol. 2017, 43, 1–6. [Google Scholar] [CrossRef]
- Withrow, J.; Murphy, C.; Liu, Y.; Hunter, M.; Fulzele, S.; Hamrick, M.W. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2016, 18, 286. [Google Scholar] [CrossRef]
- Yeh, C.C.; Chang, S.F.; Huang, T.Y.; Chang, H.I.; Kuo, H.C.; Wu, Y.C.; Chen, C.N. Shear stress modulates macrophage-induced urokinase plasminogen activator expression in human chondrocytes. Arthritis Res. Ther. 2013, 15, R53. [Google Scholar] [CrossRef]
- Busso, N.; Hamilton, J.A. Extravascular coagulation and the plasminogen activator/plasmin system in rheumatoid arthritis. Arthritis Rheum. 2002, 46, 2268–2279. [Google Scholar] [CrossRef]
- Milner, J.M.; Patel, A.; Rowan, A.D. Emerging roles of serine proteinases in tissue turnover in arthritis. Arthritis Rheum. 2008, 58, 3644–3656. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Tang, Y.; Liang, X.; Zheng, M.; Yang, J.; Zhou, H.; Li, L.; Qin, T. Role of hypoxia-inducible factor-1 alpha in the regulation of plasminogen activator activity in rat knee joint chondrocytes. Osteoarthr. Cartil. 2009, 17, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kronbichler, A.; Park, D.D.-Y.; Park, Y.M.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.S.; Shim, S.; Lyu, I.S.; et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Morell, M.; Varela, N.; Maranon, C. Myeloid Populations in Systemic Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2017, 53, 198–218. [Google Scholar] [CrossRef] [PubMed]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Corsiero, E.; Pratesi, F.; Prediletto, E.; Bombardieri, M.; Migliorini, P. NETosis as Source of Autoantigens in Rheumatoid Arthritis. Front. Immunol. 2016, 7, 485. [Google Scholar] [CrossRef]
- Skopelja-Gardner, S.; Jones, J.D.; Rigby, W.F.C. “NETtling” the host: Breaking of tolerance in chronic inflammation and chronic infection. J. Autoimmun. 2018, 88, 1–10. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ikata, T.; Mishiro, T.; Nakano, S.; Ikebe, M.; Yasuoka, S. Cathepsins B and L in synovial fluids from patients with rheumatoid arthritis and the effect of cathepsin B on the activation of pro-urokinase. J. Med. Investig. 2000, 47, 61–75. [Google Scholar]
- Jin, T.; Tarkowski, A.; Carmeliet, P.; Bokarewa, M. Urokinase, a constitutive component of the inflamed synovial fluid, induces arthritis. Arthritis Res. Ther. 2003, 5, R9–R17. [Google Scholar] [CrossRef]
- Cuda, C.M.; Pope, R.M.; Perlman, H. The inflammatory role of phagocyte apoptotic pathways in rheumatic diseases. Nat. Rev. Rheumatol. 2016, 12, 543–558. [Google Scholar] [CrossRef]
- McInnes, I.B.; Buckley, C.D.; Isaacs, J.D. Cytokines in rheumatoid arthritis-shaping the immunological landscape. Nat. Rev. Rheumatol. 2016, 12, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Goldman, N.; Valiuskyte, K.; Londregan, J.; Swider, A.; Somerville, J.; Riggs, J.E. Macrophage regulation of B cell proliferation. Cell Immunol. 2017, 314, 54–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, B.; Tang, Y.; Sun, X.; Ouyang, X.; Li, H.; Wei, J.; Zhang, Y.; Li, X. Increased IL-6 expression on THP-1 by IL-34 stimulation up-regulated rheumatoid arthritis Th17 cells. Clin. Rheumatol. 2018, 37, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Fennen, M.; Pap, T.; Dankbar, B. Smad-dependent mechanisms of inflammatory bone destruction. Arthritis Res. Ther. 2016, 18, 279. [Google Scholar] [CrossRef] [PubMed]
- Gigliotti, C.L.; Boggio, E.; Clemente, N.; Shivakumar, Y.; Toth, E.; Sblattero, D.; D’Amelio, P.; Isaia, G.C.; Dianzani, C.; Yagi, J.; et al. ICOS-Ligand Triggering Impairs Osteoclast Differentiation and Function In Vitro and In Vivo. J. Immunol. 2016, 197, 3905–3916. [Google Scholar] [CrossRef]
- Szekanecz, Z.; Haines, G.K.; Koch, A.E. Differential expression of the urokinase receptor (CD87) in arthritic and normal synovial tissues. J. Clin. Pathol. 1997, 50, 314–319. [Google Scholar] [CrossRef]
- Yang, Y.H.; Carmeliet, P.; Hamilton, J.A. Tissue-type plasminogen activator deficiency exacerbates arthritis. J. Immunol. 2001, 167, 1047–1052. [Google Scholar] [CrossRef]
- Kofoed, K.; Andersen, O.; Kronborg, G.; Tvede, M.; Petersen, J.; Eugen-Olsen, J.; Larsen, K. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: A prospective study. Crit Care 2007, 11, R38. [Google Scholar]
- Pliyev, B.K.; Menshikov, M.Y. Release of the soluble urokinase-type plasminogen activator receptor (suPAR) by activated neutrophils in rheumatoid arthritis. Inflammation 2010, 33, 1–9. [Google Scholar] [CrossRef]
- Koga, T.; Okada, A.; Kawashiri, S.; Kita, J.; Suzuki, T.; Nakashima, Y.; Kawakami, A. Soluble urokinase plasminogen activator receptor as a useful biomarker to predict the response to adalimumab in patients with rheumatoid arthritis in a Japanese population. Clin. Exp. Rheumatol. 2011, 29, 811–815. [Google Scholar]
- Fleetwood, A.J.; Achuthan, A.; Schultz, H.; Nansen, A.; Almholt, K.; Usher, P.; Hamilton, J.A. Urokinase plasminogen activator is a central regulator of macrophage three-dimensional invasion, matrix degradation, and adhesion. J. Immunol. 2014, 192, 3540–3547. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Xu, L.; Sun, X.; Wang, Y.; Xuan, W.; Zhang, Q.; Zhang, M. Adiponectin aggravates bone erosion by promoting osteopontin production in synovial tissue of rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Asif Amin, M.; Fox, D.A.; Ruth, J.H. Synovial cellular and molecular markers in rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, S.; Meier, F.M.; Neumann, E.; Muller-Ladner, U. Role of synovial fibroblasts in rheumatoid arthritis. Curr. Pharm. Des. 2015, 21, 130–141. [Google Scholar] [CrossRef]
- Orr, C.; Vieira-Sousa, E.; Boyle, D.L.; Buch, M.; Buckley, C.D.; Cañete, J.D.; Catrina, A.I.; Choy, E.H.S.; Emery, P.; Fearon, U.; et al. Synovial tissue research: A state-of-the-art review. Nat. Rev. Rheumatol. 2017, 13, 463–475. [Google Scholar] [CrossRef]
- McNaughton, E.F.; Eustace, A.D.; King, S.; Sessions, R.B.; Kay, A.; Farris, M.; Broadbridge, R.; Kehoe, O.; Kungl, A.J.; Middleton, J. Novel Anti-Inflammatory Peptides Based on Chemokine-Glycosaminoglycan Interactions Reduce Leukocyte Migration and Disease Severity in a Model of Rheumatoid Arthritis. J. Immunol. 2018, 200, 3201–3217. [Google Scholar] [CrossRef]
- Nonaka, T.; Kikuchi, H.; Ikeda, T.; Okamoto, Y.; Hamanishi, C.; Tanaka, S. Hyaluronic acid inhibits the expression of u-PA, PAI-1, and u-PAR in human synovial fibroblasts of osteoarthritis and rheumatoid arthritis. J. Rheumatol. 2000, 27, 997–1004. [Google Scholar]
- Nonaka, T.; Kikuchi, H.; Sohen, S.; Fukuda, K.; Hamanishi, C.; Tanaka, S. Comparison of the inhibitory effects of two types (90 kDa and 190 kDa) of hyaluronic acid on the expression of fibrinolytic factors in human synovial fibroblasts. Mod. Rheumatol. 2002, 12, 160–166. [Google Scholar] [CrossRef]
- Baran, M.; Mollers, L.N.; Andersson, S.; Jonsson, I.M.; Ekwall, A.K.; Bjersing, J.; Bokarewa, M. Survivin is an essential mediator of arthritis interacting with urokinase signalling. J. Cell Mol. Med. 2009, 13, 3797–3808. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.F.; Xue, Y.Q.; Fang, L.K.; Guo, X.H.; Guo, X.; Zheng, S.G. uPAR promotes tumor-like biologic behaviors of fibroblast-like synoviocytes through PI3K/Akt signaling pathway in patients with rheumatoid arthritis. Cell Mol. Immunol. 2018, 15, 171–181. [Google Scholar] [CrossRef]
- Kobori, T.; Hamasaki, S.; Kitaura, A.; Yamazaki, Y.; Nishinaka, T.; Niwa, A.; Takahashi, H. Interleukin-18 Amplifies Macrophage Polarization and Morphological Alteration, Leading to Excessive Angiogenesis. Front. Immunol. 2018, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, R.; Schuett, J.; Schuett, H.; Koch, A.K.; Luchtefeld, M.; Grote, K.; Schieffer, B. Targeting Tumor Necrosis Factor-alpha with Adalimumab: Effects on Endothelial Activation and Monocyte Adhesion. PLoS ONE 2016, 11, e0160145. [Google Scholar] [CrossRef] [PubMed]
- Pliyev, B.K.; Antonova, O.A.; Menshikov, M. Participation of the urokinase-type plasminogen activator receptor (uPAR) in neutrophil transendothelial migration. Mol. Immunol. 2011, 48, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, S.W.; Lee, S.Y.; Hong, K.W.; Bae, S.S.; Kim, K.; Kim, C.D. SIRT1/Adenosine Monophosphate-Activated Protein Kinase alpha Signaling Enhances Macrophage Polarization to an Anti-inflammatory Phenotype in Rheumatoid Arthritis. Front. Immunol. 2017, 8, 1135. [Google Scholar] [CrossRef]
- Conde, J.; Scotece, M.; Lopez, V.; Gomez, R.; Lago, F.; Pino, J.; Gualillo, O. Adiponectin and leptin induce VCAM-1 expression in human and murine chondrocytes. PLoS ONE 2012, 7, e52533. [Google Scholar] [CrossRef]
- Malemud, C.J. The PI3K/Akt/PTEN/mTOR pathway: A fruitful target for inducing cell death in rheumatoid arthritis? Future Med. Chem. 2015, 7, 1137–1147. [Google Scholar] [CrossRef]
- Charbonneau, M.; Lavoie, R.R.; Lauzier, A.; Harper, K.; McDonald, P.P.; Dubois, C.M. Platelet-Derived Growth Factor Receptor Activation Promotes the Prodestructive Invadosome-Forming Phenotype of Synoviocytes from Patients with Rheumatoid Arthritis. J. Immunol. 2016, 196, 3264–3275. [Google Scholar] [CrossRef]
- Kalbasi Anaraki, P.; Patecki, M.; Tkachuk, S.; Kiyan, Y.; Haller, H.; Dumler, I. Urokinase receptor mediates osteoclastogenesis via M-CSF release from osteoblasts and the c-Fms/PI3K/Akt/NF-kappaB pathway in osteoclasts. J. Bone Miner. Res. 2015, 30, 379–388. [Google Scholar] [CrossRef]
- Toldi, G.; Bekő, G.; Kádár, G.; Mácsai, E.; Kovács, L.; Vásárhelyi, B.; Balog, A. Soluble urokinase plasminogen activator receptor (suPAR) in the assessment of inflammatory activity of rheumatoid arthritis patients in remission. Clin. Chem. Lab. Med. 2013, 51, 327–332. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewe, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; van der Heijde, D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef]
- Enocsson, H.; Lukic, T.; Ziegelasch, M.; Kastbom, A. Serum levels of the soluble urokinase plasminogen activator receptor (suPAR) correlates with disease activity in early rheumatoid arthritis and reflects joint damage over time. Transl. Res. 2021, 232, 142–149. [Google Scholar] [CrossRef] [PubMed]
| Cell Type | Pathways Activated by uPA/uPAR | Effect on RA |
|---|---|---|
| Chondrocytes | MMP1, MMP3 and MMP13, TNF, IL-1β, and retinoids secretion | Extracellular matrix proteins’ breakdown -> synovial and joint cartilage’s distruption -> articular damage |
| Neutrophils | Stimulation various of inflammatory processes (releasing of cytotoxic degradative enzymes, cytokines, chemokynes, NETs formation) | Synovial infiltration and distruption -> articular damage |
| Monocytes/macrophages | Improvment of fibrin clot formation, cytokine release | Immune cell infiltration, increasing of macrophage proliferation and phagocytosis -> articular damage |
| Fibroblast-like synoviocytes (FLS) | Promotion of fibrinolytic activity, stimulation of β1/PI3K/Akt integrin, tyrosine and mitogen kinase’s signaling pathways | Stimulation of FLS’ tumorigenic activity -> articular damage |
| Endothelial cells | Proteolytic activity | Promotion of diapedesis, adhesion and migration of neutrophils across the transendothelial layer -> synovial infiltration and flogosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benucci, M.; Damiani, A.; Russo, E.; Guiducci, S.; Li Gobbi, F.; Fusi, P.; Grossi, V.; Amedei, A.; Manfredi, M.; Infantino, M. The Association of uPA, uPAR, and suPAR System with Inflammation and Joint Damage in Rheumatoid Arthritis: suPAR as a Biomarker in the Light of a Personalized Medicine Perspective. J. Pers. Med. 2022, 12, 1984. https://doi.org/10.3390/jpm12121984
Benucci M, Damiani A, Russo E, Guiducci S, Li Gobbi F, Fusi P, Grossi V, Amedei A, Manfredi M, Infantino M. The Association of uPA, uPAR, and suPAR System with Inflammation and Joint Damage in Rheumatoid Arthritis: suPAR as a Biomarker in the Light of a Personalized Medicine Perspective. Journal of Personalized Medicine. 2022; 12(12):1984. https://doi.org/10.3390/jpm12121984
Chicago/Turabian StyleBenucci, Maurizio, Arianna Damiani, Edda Russo, Serena Guiducci, Francesca Li Gobbi, Paola Fusi, Valentina Grossi, Amedeo Amedei, Mariangela Manfredi, and Maria Infantino. 2022. "The Association of uPA, uPAR, and suPAR System with Inflammation and Joint Damage in Rheumatoid Arthritis: suPAR as a Biomarker in the Light of a Personalized Medicine Perspective" Journal of Personalized Medicine 12, no. 12: 1984. https://doi.org/10.3390/jpm12121984
APA StyleBenucci, M., Damiani, A., Russo, E., Guiducci, S., Li Gobbi, F., Fusi, P., Grossi, V., Amedei, A., Manfredi, M., & Infantino, M. (2022). The Association of uPA, uPAR, and suPAR System with Inflammation and Joint Damage in Rheumatoid Arthritis: suPAR as a Biomarker in the Light of a Personalized Medicine Perspective. Journal of Personalized Medicine, 12(12), 1984. https://doi.org/10.3390/jpm12121984








