Effect of Exercise Using an Exoskeletal Hip-Assist Robot on Physical Function and Walking Efficiency in Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Exercise
2.3. Assessment Tools and Data Collection
2.4. EX1 Wearable Hip Exoskeleton
2.5. Statistical Analysis
3. Results
3.1. Subjects
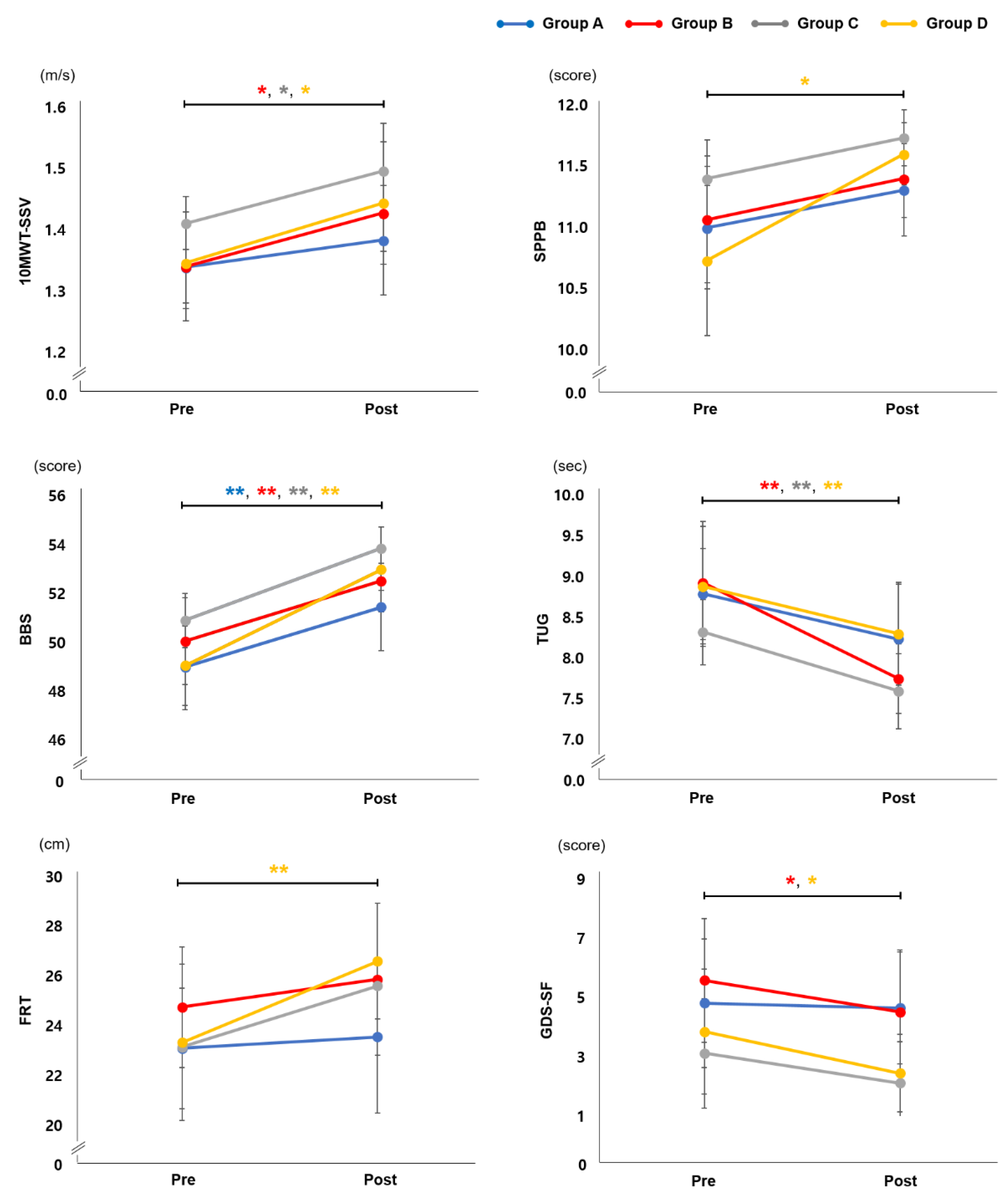
3.2. Effect of EX1 Exercise on Physical Function
3.3. Effect of EX1 Exercise on Muscle Strength
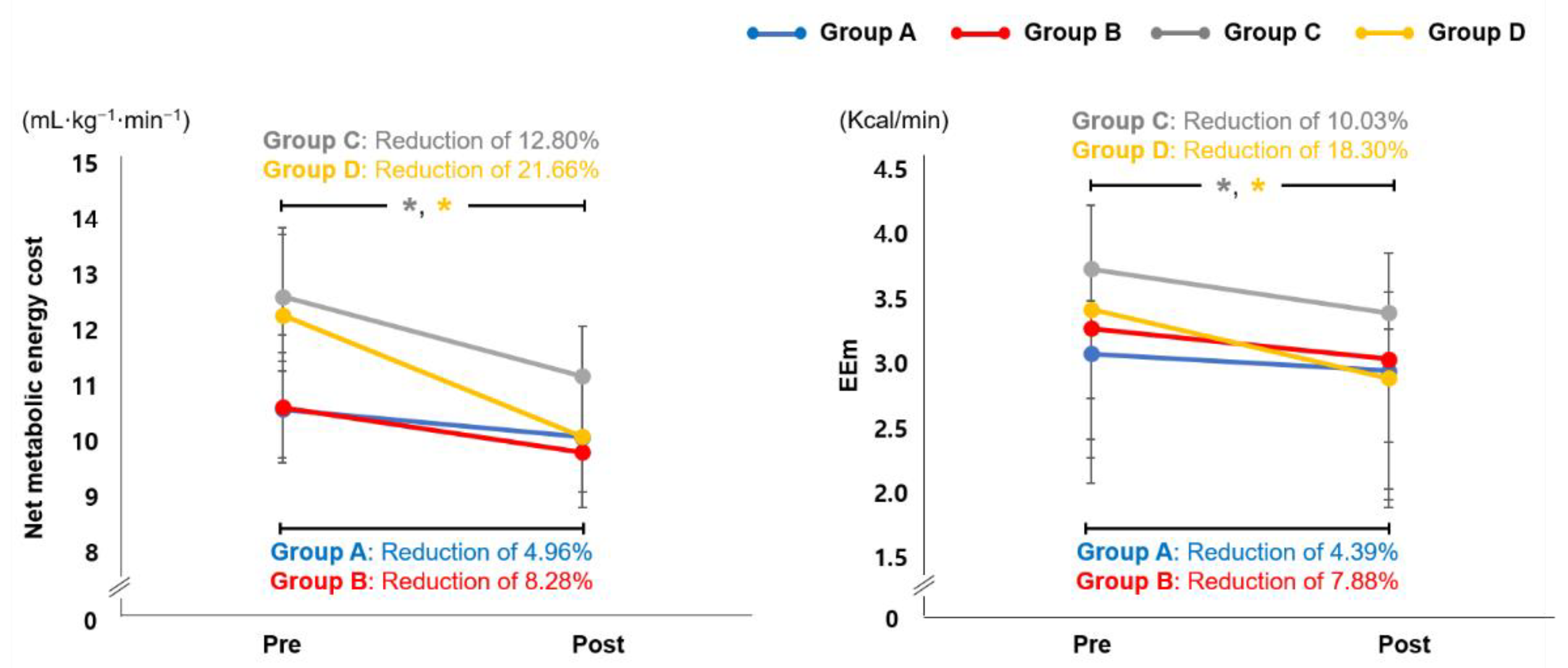
3.4. Effect of EX1 Exercise on Cardiopulmonary Metabolic Energy Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pirker, W.; Katzenschlager, R. Gait disorders in adults and the elderly. Wien. Klin. Wochenschr. 2017, 129, 81–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snijders, A.H.; Van De Warrenburg, B.P.; Giladi, N.; Bloem, B.R. Neurological gait disorders in elderly people: Clinical approach and classification. Lancet Neurol. 2007, 6, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, V.J.; van der Geest, J.N.; Hoogendam, Y.Y.; Hofman, A.; Breteler, M.M.; Ikram, M.A. Gait patterns in a community-dwelling population aged 50 years and older. Gait Posture 2013, 37, 500–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, L.; Hahn, M.E. Comparison of lower extremity joint mechanics between healthy active young and middle age people in walking and running gait. Sci. Rep. 2019, 9, 5568. [Google Scholar] [CrossRef] [Green Version]
- Prince, F.; Corriveau, H.; Hébert, R.; Winter, D.A. Gait in the elderly. Gait Posture 1997, 5, 128–135. [Google Scholar] [CrossRef]
- Salzman, B. Gait and balance disorders in older adults. Am. Fam. Physician 2010, 82, 61–68. [Google Scholar]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Singh, M.A.F.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. Exercise and physical activity for older adults. Med. Sci. Sport. Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Mcleod, J.C.; Stokes, T.; Phillips, S.M. Resistance exercise training as a primary countermeasure to age-related chronic disease. Front. Physiol. 2019, 645. [Google Scholar] [CrossRef] [Green Version]
- Angulo, J.; El Assar, M.; Álvarez-Bustos, A.; Rodríguez-Mañas, L. Physical activity and exercise: Strategies to manage frailty. Redox Biol. 2020, 35, 101513. [Google Scholar] [CrossRef]
- Suzuki, Y. The Effect of Physical Play Experiences on Early Childhood Non-Cognitive Skills Development. J. Educ. Dev. 2020, 4, 54. [Google Scholar] [CrossRef]
- King, A.C. Interventions to promote physical activity by older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, B.; Bergland, A.; Rydwik, E. The importance of physical activity exercise among older people. BioMed Res. Int. 2018, 2018, 7856823. [Google Scholar] [CrossRef] [PubMed]
- Lautenschlager, N.T.; Almeida, O.P.; Flicker, L.; Janca, A. Can physical activity improve the mental health of older adults? Ann. Gen. Hosp. Psychiatry 2004, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, X.; Tian, X.; Zhang, H.; Huang, R.; Li, N.; Chen, P.; Wang, R. Exercise as a prescription for patients with various diseases. J. Sport Health Sci. 2019, 8, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Bean, J.F.; Vora, A.; Frontera, W.R. Benefits of exercise for community-dwelling older adults. Arch. Phys. Med. Rehabil. 2004, 85, 31–42. [Google Scholar] [CrossRef]
- Lee, P.G.; Jackson, E.A.; Richardson, C.R. Exercise prescriptions in older adults. Am. Fam. Physician 2017, 95, 425–432. [Google Scholar]
- Alves, C.R.R.; da Cunha, T.F.; da Paixão, N.A.; Brum, P.C. Aerobic exercise training as therapy for cardiac and cancer cachexia. Life Sci. 2015, 125, 9–14. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Chmelo, E.; Delbono, O.; Carr, J.J.; Lyles, M.F.; Marsh, A.P. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 991–999. [Google Scholar] [CrossRef] [Green Version]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance training for older adults: Position statement from the national strength and conditioning association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Bai, X.; Soh, K.G.; Omar Dev, R.D.; Talib, O.; Xiao, W.; Soh, K.L.; Ong, S.L.; Zhao, C.; Galeru, O.; Casaru, C. Aerobic Exercise Combination Intervention to Improve Physical Performance Among the Elderly: A Systematic Review. Front. Physiol. 2022, 2311. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Smith, G.I.; Sinacore, D.R.; Shah, K.; Mittendorfer, B. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity 2011, 19, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Freiberger, E.; Häberle, L.; Spirduso, W.W.; Rixt Zijlstra, G.A. Long-term effects of three multicomponent exercise interventions on physical performance and fall-related psychological outcomes in community-dwelling older adults: A randomized controlled trial. J. Am. Geriatr. Soc. 2012, 60, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Villelabeitia Jaureguizar, K.; Vicente-Campos, D.; Ruiz Bautista, L.; Hernández de la Peña, C.; Arriaza Gómez, M.J.; Calero Rueda, M.J.; Fernandez Mahillo, I. Effect of high-intensity interval versus continuous exercise training on functional capacity and quality of life in patients with coronary artery disease. J. Cardiopulm. Rehabil. Prev. 2016, 36, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Esain, I.; González-Suarez, Á.M.; Gil, S.M. The effectiveness of a basic exercise intervention to improve strength and balance in women with osteoporosis. Clin. Interv. Aging 2017, 12, 505. [Google Scholar] [CrossRef] [Green Version]
- Kloos, A.D.; Kegelmeyer, D.A.; Ambrogi, K.; Kline, D.; McCormack-Mager, M.; Schroeder, B.; Kostyk, S.K. The step test evaluation of performance on stairs (STEPS): Validation and reliability in a neurological disorder. PLoS ONE 2019, 14, e0213698. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, H.-J.; Lee, S.-H.; Chang, W.H.; Jang, J.; Choi, B.-O.; Ryu, G.-H.; Kim, Y.-H. A wearable hip-assist robot reduces the cardiopulmonary metabolic energy expenditure during stair ascent in elderly adults: A pilot cross-sectional study. BMC Geriatr. 2018, 18, 230. [Google Scholar] [CrossRef]
- Knaggs, J.D.; Larkin, K.A.; Manini, T.M. Metabolic cost of daily activities and effect of mobility impairment in older adults. J. Am. Geriatr. Soc. 2011, 59, 2118–2123. [Google Scholar] [CrossRef] [Green Version]
- Hongu, N.; Shimada, M.; Miyake, R.; Nakajima, Y.; Nakajima, I.; Yoshitake, Y. Promoting stair climbing as an exercise routine among healthy older adults attending a community-based physical activity program. Sports 2019, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, H.-J.; Lee, S.-H.; Lee, J.; Chang, W.H.; Ryu, G.-H.; Kim, Y.-H. Correlation between cardiopulmonary metabolic energy cost and lower-limb muscle activity during inclined treadmill gait in older adults. BMC Geriatr. 2021, 21, 469. [Google Scholar] [CrossRef]
- Kling, R.; Chung, A.; Cox, C.; Kimbro, E.; Grodzielanek, J.; Ayres, S.; Hosseini, S.; Shiraishi, M.; Soangra, R. Surface Inclination Influences Fall Risk and Lower Extremity Joint Moments During Walking. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting; 2020; pp. 1416–1420. [Google Scholar]
- Ehlen, K.A.; Reiser, R.F.; Browning, R.C. Energetics and biomechanics of inclined treadmill walking in obese adults. Med. Sci Sport. Exerc 2011, 43, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, M.; Dickin, D.C.; Popp, J.; Wang, H. The influence of incline walking on joint mechanics. Gait Posture 2014, 39, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zi, B.; Qin, L.; Pan, Q. State-of-the-art research in robotic hip exoskeletons: A general review. J. Orthop. Transl. 2020, 20, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, X.; Du, F.; Zhang, X. Design and control of a powered hip exoskeleton for walking assistance. Int. J. Adv. Robot. Syst. 2015, 12, 18. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Fernández, A.; Lobo-Prat, J.; Font-Llagunes, J.M. Systematic review on wearable lower-limb exoskeletons for gait training in neuromuscular impairments. J. Neuroeng. Rehabil. 2021, 18, 22. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sport. Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lee, S.-H.; Seo, K.; Lee, M.; Chang, W.H.; Choi, B.-O.; Ryu, G.-H.; Kim, Y.-H. Training for walking efficiency with a wearable hip-assist robot in patients with stroke: A pilot randomized controlled trial. Stroke 2019, 50, 3545–3552. [Google Scholar] [CrossRef]
- Dal Jae Im, J.K.; Kim, Y.J.; Cho, S.; Cho, Y.K.; Lim, T.; Lee, H.S.; Kim, H.J.; Kang, Y.J. Utility of a three-dimensional interactive augmented reality program for balance and mobility rehabilitation in the elderly: A feasibility study. Ann. Rehabil. Med. 2015, 39, 462. [Google Scholar]
- Lim, B.; Lee, J.; Jang, J.; Kim, K.; Park, Y.J.; Seo, K.; Shim, Y. Delayed output feedback control for gait assistance with a robotic hip exoskeleton. IEEE Trans. Robot. 2019, 35, 1055–1062. [Google Scholar] [CrossRef]
- Laufer, Y. Effect of age on characteristics of forward and backward gait at preferred and accelerated walking speed. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 627–632. [Google Scholar] [CrossRef]
- Afilalo, J.; Eisenberg, M.J.; Morin, J.-F.; Bergman, H.; Monette, J.; Noiseux, N.; Perrault, L.P.; Alexander, K.P.; Langlois, Y.; Dendukuri, N. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J. Am. Coll. Cardiol. 2010, 56, 1668–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peel, N.M.; Kuys, S.S.; Klein, K. Gait speed as a measure in geriatric assessment in clinical settings: A systematic review. J. Gerontol. Ser. A 2013, 68, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Umakalyani, K.; Kumar, M.S. An observational study of gait speed in elderly participants attending outpatient clinic geriatric department, madras medical college, chennai. J. Evol. Med. Dent. Sci. 2018, 7, 1356–1360. [Google Scholar] [CrossRef]
- Ferrucci, L.; Bandinelli, S.; Benvenuti, E.; Di Iorio, A.; Macchi, C.; Harris, T.B.; Guralnik, J.M. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J. Am. Geriatr. Soc. 2000, 48, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Hardy, S.E.; Perera, S.; Roumani, Y.F.; Chandler, J.M.; Studenski, S.A. Improvement in usual gait speed predicts better survival in older adults. J. Am. Geriatr. Soc. 2007, 55, 1727–1734. [Google Scholar] [CrossRef]
- Rubenstein, L.Z.; Powers, C.M.; MacLean, C.H. Quality indicators for the management and prevention of falls and mobility problems in vulnerable elders. Ann. Intern. Med. 2001, 135, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Thurman, D.J.; Stevens, J.A.; Rao, J.K. Practice parameter: Assessing patients in a neurology practice for risk of falls (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2008, 70, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Hausdorff, J.M.; Nelson, M.E.; Kaliton, D.; Layne, J.E.; Bernstein, M.J.; Nuernberger, A.; Singh, M.A.F. Etiology and modification of gait instability in older adults: A randomized controlled trial of exercise. J. Appl. Physiol. 2001, 90, 2117–2129. [Google Scholar] [CrossRef]
- Howe, T.E.; Rochester, L.; Neil, F.; Skelton, D.A.; Ballinger, C. Exercise for improving balance in older people. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Leroux, A.; Fung, J.; Barbeau, H. Postural adaptation to walking on inclined surfaces: I. Normal strategies. Gait Posture 2002, 15, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, R.A.; Pinto-Zipp, G.; Simpkins, S.; Clark, M. Effects of an inclined walking surface and balance abilities on spatiotemporal gait parameters of older adults. J. Geriatr. Phys. Ther. 2013, 36, 31–38. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.S.; Beatty, K.T.; Dwan, L.N.; Vickers, D.R. Gait dynamics on an inclined walkway. J. Biomech. 2006, 39, 2491–2502. [Google Scholar] [CrossRef] [PubMed]
- Alıpsatıcı, Ç.; Alaca, N.; Canbora, M. Comparison of the Effects of Treadmill Trainings on Walking and Balance Functions by Increasing the Speed and Incline in Chronic Patients with Stroke. Turk. J. Neurol. 2020, 26, 316–321. [Google Scholar] [CrossRef]
- da Silva, R.S.; da Silva, S.T.; de Souza, J.M.; de Figueiredo, M.C.C.; Mendes, T.A.S.; de Sena Nunes, M.C.; de Oliveira, S.K.R.; Cardoso, D.C.R.; da Câmara Silva, R.G.; de Oliveira, D.C. Effects of inclined treadmill training on functional and cardiovascular parameters of stroke patients: Study protocol for a randomized controlled trial. Trials 2019, 20, 252. [Google Scholar] [CrossRef]
- Fukukawa, Y.; Nakashima, C.; Tsuboi, S.; Kozakai, R.; Doyo, W.; Niino, N.; Ando, F.; Shimokata, H. Age differences in the effect of physical activity on depressive symptoms. Psychol. Aging 2004, 19, 346. [Google Scholar] [CrossRef] [Green Version]
- Barbour, K.A.; Edenfield, T.M.; Blumenthal, J.A. Exercise as a treatment for depression and other psychiatric disorders: A review. J. Cardiopulm. Rehabil. Prev. 2007, 27, 359–367. [Google Scholar] [CrossRef]
- Peterson, M.D.; Rhea, M.R.; Sen, A.; Gordon, P.M. Resistance exercise for muscular strength in older adults: A meta-analysis. Ageing Res. Rev. 2010, 9, 226–237. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Kavazis, A.N.; McClung, J.M. Oxidative stress and disuse muscle atrophy. J. Appl. Physiol. 2007, 102, 2389–2397. [Google Scholar] [CrossRef]
- Seene, T.; Kaasik, P. Muscle weakness in the elderly: Role of sarcopenia, dynapenia, and possibilities for rehabilitation. Eur. Rev. Aging Phys. Act. 2012, 9, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Melov, S.; Tarnopolsky, M.A.; Beckman, K.; Felkey, K.; Hubbard, A. Resistance exercise reverses aging in human skeletal muscle. PLoS ONE 2007, 2, e465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumann, M.; Rønnestad, B.R. Concurrent Aerobic and Strength Training; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Brach, J.S.; VanSwearingen, J.M. Interventions to improve walking in older adults. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2013, 2, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabell, A.; Nayak, U. The effect of age on variability in gait. J. Gerontol. 1984, 39, 662–666. [Google Scholar] [CrossRef]
- Jacquelin Perry, M. Gait Analysis: Normal and Pathological Function; SLACK: Thorofare, NJ, USA, 2010. [Google Scholar]
- Polcyn, A.F.; Lipsitz, L.A.; Kerrigan, D.C.; Collins, J.J. Age-related changes in the initiation of gait: Degradation of central mechanisms for momentum generation. Arch. Phys. Med. Rehabil. 1998, 79, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Halsey, L.G.; Watkins, D.A.; Duggan, B.M. The energy expenditure of stair climbing one step and two steps at a time: Estimations from measures of heart rate. PLoS ONE 2012, 7, e51213. [Google Scholar] [CrossRef] [Green Version]
- Myers, J. Exercise and cardiovascular health. Circulation 2003, 107, e2–e5. [Google Scholar] [CrossRef] [Green Version]
- Boreham, C.A.; Wallace, W.F.; Nevill, A. Training effects of accumulated daily stair-climbing exercise in previously sedentary young women. Prev. Med. 2000, 30, 277–281. [Google Scholar] [CrossRef]
- Teh, K.C.; Aziz, A.R. Heart rate, oxygen uptake, and energy cost of ascending and descending the stairs. Med. Sci. Sport. Exerc. 2002, 34, 695–699. [Google Scholar]

| Characteristics | Group A | Group B | Group C | Group D | χ2/F(p) |
|---|---|---|---|---|---|
| Sex (male/female) | 6/7 | 8/7 | 9/6 | 7/8 | 0.742 (0.863) |
| Age, years | 76.38 (4.98) | 75.20 (3.41) | 72.73 (3.10) | 74.67 (5.05) | 1.866 (0.146) |
| Height, cm | 157.62 (9.90) | 163.90 (5.86) | 162.87 (9.89) | 160.53 (6.97) | 1.571 (0.207) |
| Weight, kg | 59.50 (9.92) | 62.80 (8.84) | 62.03 (12.88) | 59.40 (10.81) | 0.382 (0.766) |
| BMI, kg/m2 | 24.01 (3.89) | 23.43 (3.57) | 23.22 (3.43) | 22.93 (2.94) | 0.242 (0.867) |
| MMSE-K | 25.92 (2.06) | 27.27 (1.44) | 27.80 (2.21) | 27.07 (2.02) | 6.214 (0.102) |
| Group A | Group B | Group C | Group D | χ2/F(p) | |
|---|---|---|---|---|---|
| Δ10MWT-SSV, m/s | 0.04 (0.17) | 0.09 (0.13) a | 0.09 (0.12) a | 0.10 (0.15) a | 0.381 (0.767) |
| ΔSPPB | 0.31 (0.63) | 0.33 (0.82) | 0.33 (0.62) | 0.87 (1.19) a | 2.514 (0.473) |
| ΔBBS | 2.46 (2.03) | 2.46 (1.69) | 2.95 (2.47) | 3.93 (2.15) | 4.539 (0.209) |
| ΔTUG, sec | 0.56 (1.05) | 1.18 (0.95) | 0.73 (0.81) | 0.58 (0.61) | 1.631 (0.193) |
| ΔFRT, cm | 0.46 (2.56) | 1.12 (5.47) | 2.45 (6.13) | 3.38 (4.18) | 1.048 (0.379) |
| ΔGDS-SF | 0.16 (1.77) | 1.07 (1.49) | 1.00 (2.18) | 1.40 (2.03) | 4.109 (0.250) |
| Muscle Strength (kg) | Group A | Group B | Group C | Group D | ||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Trunk flexion | 21.62 (6.98) | 22.84 (5.55) | 18.84 (5.08) | 24.19 (6.66) ** | 24.16 (5.08) | 27.77 (7.29) * | 21.94 (6.33) | 24.06 (5.90) |
| Trunk extension | 26.26 (6.58) | 27.47 (9.61) | 24.78 (6.75) | 26.52 (4.54) | 28.20 (6.99) | 30.28 (9.86) | 26.81 (7.40) | 31.92 (5.43) ** |
| Hip flexion | 25.94 (8.35) | 28.63 (9.17) | 28.80 (6.52) | 33.48 (6.71) * | 31.12 (8.16) | 32.92 (7.45) | 28.82 (8.92) | 33.36 (10.12) * |
| Hip extension | 16.64 (7.28) | 19.35 (6.20) | 17.82 (6.46) | 21.70 (6.42) ** | 20.44 (5.54) | 22.99 (6.31) | 18.22 (6.61) | 22.90 (8.15) ** |
| Hip abduction | 18.89 (6.81) | 21.17 (7.01) | 18.15 (4.96) | 24.63 (5.22) ** | 24.11 (6.30) | 25.55 (7.02) | 20.37 (6.56) | 22.90 (8.38) |
| Hip adduction | 34.12 (13.29) | 34.59 (10.09) | 36.65 (11.74) | 38.38 (8.45) | 34.00 (9.44) | 41.44 (9.12) ** | 36.92 (12.34) | 38.90 (12.36) |
| Knee flexion | 30.68 (13.11) | 34.87 (9.82) * | 35.60 (10.66) | 40.10 (9.21) * | 40.08 (9.92) | 41.98 (11.64) | 39.99 (12.19) | 44.59 (13.31) |
| Knee extension | 28.89 (7.62) | 33.77 (4.90) ** | 32.12 (8.29) | 35.38 (8.73) ** | 34.08 (4.45) | 37.38 (5.92) * | 30.70 (5.14) | 35.66 (8.17) ** |
| Ankle DF | 32.87 (10.88) | 34.58 (12.87) | 36.16 (10.52) | 42.29 (9.67) * | 39.70 (9.40) | 44.32 (10.79) | 38.37 (10.60) | 40.06 (11.97) |
| Ankle PF | 38.26 (13.83) | 42.32 (14.81) | 31.39 (10.16) | 43.11 (14.71) §§ | 36.15 (8.48) | 44.30 (15.34) § | 42.48 (12.60) | 52.62 (19.11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-H.; Lee, H.-J.; Kim, K.; Lee, B.-H.; Kim, Y.-H. Effect of Exercise Using an Exoskeletal Hip-Assist Robot on Physical Function and Walking Efficiency in Older Adults. J. Pers. Med. 2022, 12, 2077. https://doi.org/10.3390/jpm12122077
Lee S-H, Lee H-J, Kim K, Lee B-H, Kim Y-H. Effect of Exercise Using an Exoskeletal Hip-Assist Robot on Physical Function and Walking Efficiency in Older Adults. Journal of Personalized Medicine. 2022; 12(12):2077. https://doi.org/10.3390/jpm12122077
Chicago/Turabian StyleLee, Su-Hyun, Hwang-Jae Lee, Kyungrock Kim, Byoung-Hee Lee, and Yun-Hee Kim. 2022. "Effect of Exercise Using an Exoskeletal Hip-Assist Robot on Physical Function and Walking Efficiency in Older Adults" Journal of Personalized Medicine 12, no. 12: 2077. https://doi.org/10.3390/jpm12122077
APA StyleLee, S.-H., Lee, H.-J., Kim, K., Lee, B.-H., & Kim, Y.-H. (2022). Effect of Exercise Using an Exoskeletal Hip-Assist Robot on Physical Function and Walking Efficiency in Older Adults. Journal of Personalized Medicine, 12(12), 2077. https://doi.org/10.3390/jpm12122077







