Clinical Response and Hospital Costs of Therapeutic Drug Monitoring for Vancomycin in Elderly Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Performance of Vancomycin TDM with Pharmacokinetic Consultation
2.2. Study Design and Patients
2.3. Identificaition of Microorganisms and Quantification of Vancomycin Concentration
2.4. Cost-Effectiveness Variables
2.5. Statistical Analysis
3. Results
3.1. Patients
3.2. Baseline Demographic and Clinical Characteristics
3.3. Univariate Cost-Effectiveness Analysis
3.4. Multivariate Cost-Effectiveness Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bond, C.A.; Raehl, C.L. Clinical and economic outcomes of pharmacist-managed aminoglycoside or vancomycin therapy. Am. J. Health Syst. Pharm. 2005, 62, 1596–1605. [Google Scholar] [CrossRef]
- Ali, A.S.; Abdel-Rhaman, M.S.; Osman, O.H. Basic Principles of Therapeutic Drug Monitoring. J. Appl. Biopharm. Pharmacokinet. 2013, 1, 87–95. [Google Scholar] [CrossRef]
- Kang, J.-S.; Lee, M.-H. Overview of therapeutic drug monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef]
- Russell, L. The Role of Cost-effectiveness Analysis in Health and Medicine. J. Am. Med. Assoc. 1996, 276, 1172. [Google Scholar] [CrossRef]
- Haji, E.O.; Mann, K.; Dragicevic, A.; Müller, M.J.; Boland, K.; Rao, M.-L.; Fric, M.; Laux, G.; Hiemke, C. Potential cost-effectiveness of therapeutic drug monitoring for depressed patients treated with citalopram. Ther. Drug Monit. 2013, 35, 396–401. [Google Scholar] [CrossRef]
- Touw, D.J.; Neef, C.; Thomson, A.H.; Vinks, A.A. Cost-effectiveness of therapeutic drug monitoring: A systematic review. Ther. Drug Monit. 2005, 27, 10–17. [Google Scholar] [CrossRef]
- Eisenberg, J.M.; Koffer, H.; Glick, H.A.; Connell, M.L.; Loss, L.E.; Talbot, G.H.; Shusterman, N.H.; Strom, B.L. What is the cost of nephrotoxicity associated with aminoglycosides? Ann. Intern. Med. 1987, 107, 900–909. [Google Scholar] [CrossRef]
- Streetman, D.S.; Nafziger, A.N.; Destache, C.J.; Bertino, J.S. Individualized Pharmacokinetic Monitoring Results in Less Aminoglycoside-Associated Nephrotoxicity and Fewer Associated Costs. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2001, 21, 443–451. [Google Scholar] [CrossRef]
- Darko, W.; Medicis, J.J.; Smith, A.; Guharoy, R.; Lehmann, D.E. Mississippi mud no more: Cost-effectiveness of pharmacokinetic dosage adjustment of vancomycin to prevent nephrotoxicity. Pharmacotherapy 2003, 23, 643–650. [Google Scholar] [CrossRef]
- Fernandez de Gatta, M.D.; Calvo, M.V.; Hernandez, J.M.; Caballero, D.; San Miguel, J.F.; Dominguez-Gil, A. Cost-effectiveness analysis of serum vancomycin concentration monitoring in patients with hematologic malignancies. Clin. Pharmacol. Ther. 1996, 60, 332–340. [Google Scholar] [CrossRef]
- Rybak, M.J.; Albrecht, L.M.; Boike, S.C.; Chandrasekar, P.H. Nephrotoxicity of vancomycin, alone and with an aminoglycoside. J. Antimicrob. Chemother. 1990, 25, 679–687. [Google Scholar] [CrossRef]
- Ye, Z.K.; Tang, H.L.; Zhai, S.D. Benefits of therapeutic drug monitoring of vancomycin: A systematic review and meta-analysis. PLoS ONE 2013, 8, e77169. [Google Scholar] [CrossRef]
- Farber, B.F.; Moellering, R.C., Jr. Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother 1983, 23, 138–141. [Google Scholar] [CrossRef] [Green Version]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R., Jr.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef]
- Guay, D.R.P.; Vance-Bryan, K.; Gilliland, S.; Rodvold, K.; Rotschafer, J. Comparison of vancomycin pharmacokinetics in hospitalized elderly and young patients using a Bayesian forecaster. J. Clin. Pharmacol. 1993, 33, 918–922. [Google Scholar] [CrossRef]
- Matsumoto, K.; Takesue, Y.; Ohmagari, N.; Mochizuki, T.; Mikamo, H.; Seki, M.; Takakura, S.; Tokimatsu, I.; Takahashi, Y.; Kasahara, K.; et al. Practice guidelines for therapeutic drug monitoring of vancomycin: A consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J. Infect. Chemother. 2013, 19, 365–380. [Google Scholar] [CrossRef] [Green Version]
- Hidayat, L.K.; Hsu, D.I.; Quist, R.; Shriner, K.A.; Wong-Beringer, A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: Efficacy and toxicity. Arch. Intern. Med. 2006, 166, 2138–2144. [Google Scholar] [CrossRef] [Green Version]
- Jeffres, M.N.; Isakow, W.; Doherty, J.A.; Micek, S.T.; Kollef, M.H. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin. Ther. 2007, 29, 1107–1115. [Google Scholar] [CrossRef]
- Lodise, T.P.; Lomaestro, B.; Graves, J.; Drusano, G.L. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob. Agents Chemother. 2008, 52, 1330–1336. [Google Scholar] [CrossRef] [Green Version]
- Han, H.K.; An, H.; Shin, K.H.; Shin, D.; Lee, S.H.; Kim, J.H.; Cho, S.H.; Kang, H.R.; Jang, I.J.; Yu, K.S.; et al. Trough concentration over 12.1 mg/L is a major risk factor of vancomycin-related nephrotoxicity in patients with therapeutic drug monitoring. Ther. Drug Monit. 2014, 36, 606–611. [Google Scholar] [CrossRef]
- Naughton, C.A. Drug-induced nephrotoxicity. Am. Fam. Physician 2008, 78, 743–750. [Google Scholar]
- Pazhayattil, G.S.; Shirali, A.C. Drug-induced impairment of renal function. Int. J. Nephrol. Renov. Dis. 2014, 7, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Welty, T.E.; Copa, A.K. Impact of vancomycin therapeutic drug monitoring on patient care. Ann. Pharm. 1994, 28, 1335–1339. [Google Scholar] [CrossRef]
- Suryadevara, M.; Steidl, K.E.; Probst, L.A.; Shaw, J. Inappropriate vancomycin therapeutic drug monitoring in hospitalized pediatric patients increases pediatric trauma and hospital costs. J. Pediatr. Pharmacol. Ther. 2012, 17, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Loh, J.M.; Tran, A.L.; Ji, L.; Groshen, S.; Daneshmand, S.; Schuckman, A.; Quinn, D.I.; Dorff, T.B. Baseline Glomerular Filtration Rate and Cisplatin- Induced Renal Toxicity in Urothelial Cancer Patients. Clin. Genitourin Cancer 2018, 16, 90–98.e1. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef] [Green Version]
- Rhee, K.Y.; Gardiner, D.F.; Charles, M. Decreasing in vitro susceptibility of clinical Staphylococcus aureus isolates to vancomycin at the New York Hospital: Quantitative testing redux. Clin Infect. Dis. 2005, 40, 1705–1706. [Google Scholar] [CrossRef]
- Wang, G.; Hindler, J.F.; Ward, K.W.; Bruckner, D.A. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J. Clin. Microbiol. 2006, 44, 3883–3886. [Google Scholar] [CrossRef] [Green Version]
- Steinkraus, G.; White, R.; Friedrich, L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–2005. J. Antimicrob. Chemother. 2007, 60, 788–794. [Google Scholar] [CrossRef] [Green Version]
- Diaz, R.; Afreixo, V.; Ramalheira, E.; Rodrigues, C.; Gago, B. Evaluation of vancomycin MIC creep in methicillin-resistant Staphylococcus aureus infections-a systematic review and meta-analysis. Clin. Microbiol. Infect. 2018, 24, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Sader, H.S.; Flamm, R.K.; Castanheira, M.; Smart, J.I.; Mendes, R.E. In Vitro Activity of Telavancin Against Clinically Important Gram-Positive Pathogens from 69 U.S. Medical Centers (2015): Potency Analysis by U.S. Census Divisions. Microb. Drug Resist. 2017, 23, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Diekema, D.J.; Pfaller, M.A.; Shortridge, D.; Zervos, M.; Jones, R.N. Twenty-Year Trends in Antimicrobial Susceptibilities Among Staphylococcus aureus From the SENTRY Antimicrobial Surveillance Program. Open Forum. Infect. Dis. 2019, 6, S47–S53. [Google Scholar] [CrossRef]
- Kim, J.S.; Gong, S.Y.; Kim, J.W.; Rheem, I.; Kim, G.Y. Antimicrobial Susceptibility Patterns of Microorganisms Isolated from Blood Culture during the Last 8 Years: 2010∼2017. Korean J. Clin Lab. Sci. 2019, 51, 155–163. [Google Scholar] [CrossRef]
- Oh, H.; Heo, S.T.; Kim, M.; Kim, Y.R.; Yoo, J.R. Antimicrobial Susceptibility Trends of Streptococcus pneumoniae by Age Groups Over Recent 10 Years in a Single Hospital in South Korea. Yonsei Med. J. 2021, 62, 306–314. [Google Scholar] [CrossRef]
- Hsu, D.I.; Hidayat, L.K.; Quist, R.; Hindler, J.; Karlsson, A.; Yusof, A.; Wong-Beringer, A. Comparison of method-specific vancomycin minimum inhibitory concentration values and their predictability for treatment outcome of meticillin-resistant Staphylococcus aureus (MRSA) infections. Int. J. Antimicrob Agents 2008, 32, 378–385. [Google Scholar] [CrossRef]
- van Hal, S.J.; Lodise, T.P.; Paterson, D.L. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: A systematic review and meta-analysis. Clin. Infect. Dis. 2012, 54, 755–771. [Google Scholar] [CrossRef] [Green Version]
- Peleg, A.Y.; Monga, D.; Pillai, S.; Mylonakis, E.; Moellering, R.C., Jr.; Eliopoulos, G.M. Reduced susceptibility to vancomycin influences pathogenicity in Staphylococcus aureus infection. J. Infect. Dis. 2009, 199, 532–536. [Google Scholar] [CrossRef] [Green Version]
- Yoo, R.N.; Kim, S.H.; Lee, J. Impact of Initial Vancomycin Trough Concentration on Clinical and Microbiological Outcomes of Methicillin-Resistant Staphylococcus aureus Bacteremia in Children. J. Korean Med. Sci. 2017, 32, 22–28. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, H.S.; Hwang, N.Y.; Kim, K.; Park, H.D.; Lee, S.Y. Individualized Vancomycin Dosing with Therapeutic Drug Monitoring and Pharmacokinetic Consultation Service: A Large-Scale Retrospective Observational Study. Drug Des. Devel. Ther. 2021, 15, 423–440. [Google Scholar] [CrossRef]
- Gajdacs, M. The Continuing Threat of Methicillin-Resistant Staphylococcus aureus. Antibiotics 2019, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Osorio, C.; Garzon, L.; Jaimes, D.; Silva, E.; Bustos, R.H. Impact on Antibiotic Resistance, Therapeutic Success, and Control of Side Effects in Therapeutic Drug Monitoring (TDM) of Daptomycin: A Scoping Review. Antibiotics 2021, 10, 263. [Google Scholar] [CrossRef]
- Totoli, E.G.; Garg, S.; Salgado, H.R. Daptomycin: Physicochemical, Analytical, and Pharmacological Properties. Ther. Drug Monit. 2015, 37, 699–710. [Google Scholar] [CrossRef]
- Galar, A.; Munoz, P.; Valerio, M.; Cercenado, E.; Garcia-Gonzalez, X.; Burillo, A.; Sanchez-Somolinos, M.; Juarez, M.; Verde, E.; Bouza, E. Current use of daptomycin and systematic therapeutic drug monitoring: Clinical experience in a tertiary care institution. Int. J. Antimicrob. Agents 2019, 53, 40–48. [Google Scholar] [CrossRef]
- Rao, G.G.; Konicki, R.; Cattaneo, D.; Alffenaar, J.W.; Marriott, D.J.E.; Neely, M.; Committee, I.A.S. Therapeutic Drug Monitoring Can Improve Linezolid Dosing Regimens in Current Clinical Practice: A Review of Linezolid Pharmacokinetics and Pharmacodynamics. Ther. Drug Monit. 2020, 42, 83–92. [Google Scholar] [CrossRef]
- Pea, F.; Cojutti, P.G.; Baraldo, M. A 10-Year Experience of Therapeutic Drug Monitoring (TDM) of Linezolid in a Hospital-wide Population of Patients Receiving Conventional Dosing: Is there Enough Evidence for Suggesting TDM in the Majority of Patients? Basic Clin. Pharm. Toxicol. 2017, 121, 303–308. [Google Scholar] [CrossRef]
- Falcone, M.; Russo, A.; Cassetta, M.I.; Lappa, A.; Tritapepe, L.; d’Ettorre, G.; Fallani, S.; Novelli, A.; Venditti, M. Variability of pharmacokinetic parameters in patients receiving different dosages of daptomycin: Is therapeutic drug monitoring necessary? J. Infect. Chemother 2013, 19, 732–739. [Google Scholar] [CrossRef]
- Urban, E.; Stone, G.G. Impact of EUCAST ceftaroline breakpoint change on the susceptibility of methicillin-resistant Staphylococcus aureus isolates collected from patients with complicated skin and soft-tissue infections. Clin. Microbiol. Infect. 2019, 25, 1429.e1–1429.e4. [Google Scholar] [CrossRef] [Green Version]
- Livermore, D.M.; Mushtaq, S.; Warner, M.; James, D.; Kearns, A.; Woodford, N. Pathogens of skin and skin-structure infections in the UK and their susceptibility to antibiotics, including ceftaroline. J. Antimicrob. Chemother. 2015, 70, 2844–2853. [Google Scholar] [CrossRef] [Green Version]
- Duplessis, C.; Crum-Cianflone, N.F. Ceftaroline: A New Cephalosporin with Activity against Methicillin-Resistant Staphylococcus aureus (MRSA). Clin Med. Rev. Ther. 2011, 3, a2466. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.; Mouton, J.W.; Pea, F. Pharmacokinetics and Dosing of Ceftobiprole Medocaril for the Treatment of Hospital- and Community-Acquired Pneumonia in Different Patient Populations. Clin. Pharm. 2016, 55, 1507–1520. [Google Scholar] [CrossRef] [Green Version]
- Kiang, T.K.; Wilby, K.J.; Ensom, M.H. A critical review on the clinical pharmacokinetics, pharmacodynamics, and clinical trials of ceftaroline. Clin. Pharm. 2015, 54, 915–931. [Google Scholar] [CrossRef]
- Cies, J.J.; Moore, W.S., II; Enache, A.; Chopra, A. Ceftaroline for Suspected or Confirmed Invasive Methicillin-Resistant Staphylococcus aureus: A Pharmacokinetic Case Series. Pediatr. Crit. Care Med. 2018, 19, e292–e299. [Google Scholar] [CrossRef]
- Llopis, B.; Bleibtreu, A.; Schlemmer, D.; Robidou, P.; Paccoud, O.; Tissot, N.; Noe, G.; Junot, H.; Luyt, C.E.; Funck-Brentano, C.; et al. Simple and accurate quantitative analysis of cefiderocol and ceftobiprole in human plasma using liquid chromatography-isotope dilution tandem mass spectrometry: Interest for their therapeutic drug monitoring and pharmacokinetic studies. Clin Chem. Lab. Med. 2021, 59, 1800–1810. [Google Scholar] [CrossRef]
- Lima, B.; Bodeau, S.; Quinton, M.C.; Leven, C.; Lemaire-Hurtel, A.S.; Bennis, Y. Validation and Application of an HPLC-DAD Method for Routine Therapeutic Drug Monitoring of Ceftobiprole. Antimicrob. Agents Chemother 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.K.; Li, C.; Zhai, S.D. Guidelines for therapeutic drug monitoring of vancomycin: A systematic review. PLoS ONE 2014, 9, e99044. [Google Scholar] [CrossRef]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef] [Green Version]

| Characteristic | Non-ICU (n = 1258) | ICU (n = 686) | Total (n = 1944) | p-Value 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-TDM | n | TDM | n | Non-TDM | n | TDM | n | Non-TDM | n | TDM | n | Non-ICU | ICU | Total | |
| Males/Females/%Females | 441/265/37.5 | 706 | 338/214/38.8 | 552 | 240/148/38.1 | 388 | 210/88/29.5 | 298 | 681/413/37.8 | 1094 | 548/302/35.5 | 850 | 0.655 | 0.0186 | 0.3136 |
| Age, years | 72.3 ± 5.6 | 706 | 73.6 ± 6.4 | 552 | 73.1 ± 5.9 | 388 | 73.8 ± 6.0 | 298 | 72.6 ± 5.7 | 1094 | 73.7 ± 6.2 | 850 | 0.0006 | 0.0978 | 0.0002 |
| Body weight, kg | 58.1 ± 10.9 | 704 | 57.3 ± 11.2 | 552 | 60.3 ± 11.2 | 388 | 57.5 ± 10.5 | 298 | 58.9 ± 11.1 | 1092 | 57.3 ± 11.0 | 850 | 0.2961 | 0.001 | 0.006 |
| Serum creatinine, mg/dL | 1.0 ± 0.7 | 652 | 1.1 ± 0.9 | 530 | 1.2 ± 0.8 | 370 | 1.3 ± 0.9 | 298 | 1.0 ± 0.7 | 1022 | 1.2 ± 0.9 | 828 | 0.0063 | 0.1199 | 0.0037 |
| Glomerular filtration rate, mL/min/1.73 m2 | 88.6 ± 39.3 | 652 | 83.7 ± 45.4 | 530 | 75.5 ± 40.4 | 370 | 77.1 ± 51.8 | 298 | 83.9 ± 40.2 | 1022 | 81.3 ± 47.9 | 828 | 0.0036 | 0.2523 | 0.0058 |
| White blood cell count, 103 cells/μL | 9.7 ± 6.1 | 674 | 10.7 ± 8.7 | 537 | 10.7 ± 7.0 | 368 | 13.6 ± 8.4 | 297 | 10.0 ± 6.4 | 1042 | 11.8 ± 8.7 | 834 | 0.05 | <0.0001 | <0.0001 |
| High sensitivity C-reactive protein, mg/dL | 8.0 ± 8.1 | 620 | 10.1 ± 7.6 | 507 | 10.6 ± 10.0 | 335 | 14.9 ± 9.0 | 294 | 8.9 ± 8.9 | 955 | 11.9 ± 8.4 | 801 | <0.0001 | <0.0001 | <0.0001 |
| Use of nephrotoxic comedications, % | 19.8 | 706 | 18.7 | 552 | 18.3 | 388 | 31.2 | 298 | 19.3 | 1094 | 23.1 | 850 | 0.6017 | <0.0001 | 0.0426 |
| No. of nephrotoxic comedications | 1.1 ± 0.4 | 140 | 1.3 ± 0.5 | 103 | 1.3 ± 0.7 | 71 | 1.4 ± 0.7 | 93 | 1.2 ± 0.5 | 211 | 1.3 ± 0.6 | 196 | 0.0053 | 0.6241 | 0.00083 |
| Site of infection (%) | |||||||||||||||
| Blood stream | 8.6 | 706 | 13.6 | 552 | 8.2 | 388 | 13.1 | 298 | 8.5 | 1094 | 13.4 | 850 | 0.005 | 0.0391 | 0.0005 |
| Bone and joint | 13.0 | 706 | 8.3 | 552 | 0.3 | 388 | 2.0 | 298 | 8.5 | 1094 | 6.1 | 850 | 0.0081 | 0.0233 | 0.0473 |
| Central nervous system | 5.9 | 706 | 2.4 | 552 | 5.2 | 388 | 5.0 | 298 | 5.7 | 1094 | 3.3 | 850 | 0.002 | 0.943 | 0.0135 |
| Ear, nose and throat | 2.0 | 706 | 3.8 | 552 | 0.8 | 388 | 1.7 | 298 | 1.6 | 1094 | 3.1 | 850 | 0.0513 | 0.274 | 0.0252 |
| Intra-abdominal | 19.8 | 706 | 25.9 | 552 | 8.2 | 388 | 13.8 | 298 | 15.7 | 1094 | 21.6 | 850 | 0.0104 | 0.0203 | 0.0008 |
| Reproductive organ | 0.8 | 706 | 0.9 | 552 | 0.3 | 388 | 0.7 | 298 | 0.6 | 1094 | 0.8 | 850 | 0.9158 | 0.416 | 0.6347 |
| Respiratory | 21.5 | 706 | 27.0 | 552 | 57.2 | 388 | 53.7 | 298 | 34.2 | 1094 | 36.4 | 850 | 0.0242 | 0.3569 | 0.3209 |
| Skin and soft tissue | 9.5 | 706 | 4.3 | 552 | 1.0 | 388 | 0.0 | 298 | 6.5 | 1094 | 2.8 | 850 | 0.0005 | 0.0788 | 0.0002 |
| Surgical prophylaxis | 15.9 | 706 | 9.6 | 552 | 17.8 | 388 | 9.1 | 298 | 16.5 | 1094 | 9.4 | 850 | 0.0011 | 0.0011 | <0.0001 |
| Urinary tract | 2.8 | 706 | 4.2 | 552 | 1.0 | 388 | 1.0 | 298 | 2.2 | 1094 | 3.1 | 850 | 0.1963 | 0.975 | 0.232 |
| Clinical Outcome | Non-ICU (n = 1258) | ICU (n = 686) | Total (n = 1944) | p-Value 1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Non-TDM (n = 706) | TDM (n = 552) | Non-TDM (n = 388) | TDM (n = 298) | Non-TDM (n = 1094) | TDM (n = 850) | Non-ICU | ICU | Total | |
| Duration of vancomycin treatment, days | 10.0 ± 9.7 | 10.4 ± 7.7 | 8.5 ± 7.8 | 11.4 ± 8.2 | 9.5 ± 9.1 | 10.8 ± 7.9 | <0.0001 | <0.0001 | <0.0001 |
| Vancomycin dosage, mg/day/kg | 33.3 ± 23.1 2 | 30.5 ± 19.0 | 26.8 ± 14.1 | 28.6 ± 29.7 | 31.0 ± 20.6 3 | 29.8 ± 23.3 | 0.0003 | 0.6104 | 0.0029 |
| Length of stay in hospital, days | 16.3 ± 11.6 | 17.2 ± 10.2 | 16.5 ± 10.4 | 17.9 ± 11.4 | 16.3 ± 11.2 | 17.4 ± 10.6 | 0.0038 | 0.1804 | 0.0019 |
| Duration of fever, days | 1.9 ± 3.2 | 2.2 ± 3.4 | 0.5 ± 1.7 | 0.7 ± 2.0 | 1.4 ± 2.9 | 1.7 ± 3.1 | 0.0613 | 0.0087 | 0.0054 |
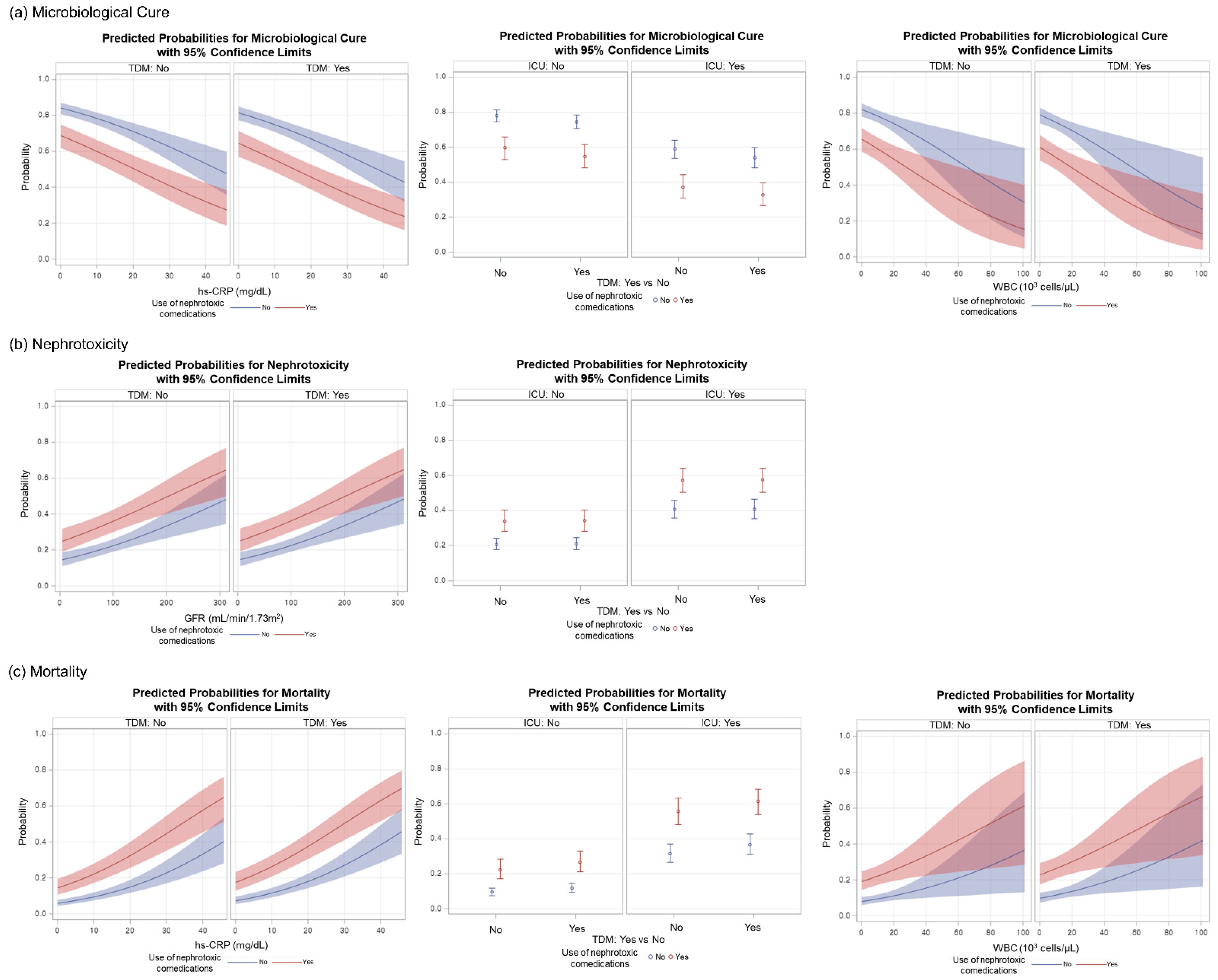
| Microbiological cure, n (%) | 544 (77.1) | 399 (72.3) | 240 (61.9) | 121 (40.6) | 784 (71.7) | 520 (61.2) | 0.0526 | <0.0001 | <0.0001 |
| Nephrotoxicity, n (%) | 143 (20.3) | 136 (24.6) | 164 (42.3) | 134 (45.0) | 307 (28.1) | 270 (31.8) | 0.0633 | 0.4797 | 0.0763 |
| Mortality, n (%) | 75 (10.6) | 81 (14.7) | 124 (32.0) | 153 (51.3) | 199 (18.2) | 234 (27.5) | 0.0305 | <0.0001 | <0.0001 |
| Medical Expenses, USD | Non-ICU (n = 1258) | ICU (n = 686) | Total (n = 1944) | p-Value 1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Non-TDM (n = 706) | TDM (n = 552) | Non-TDM (n = 388) | TDM (n = 298) | Non-TDM (n = 1094) | TDM (n = 850) | Non-ICU | ICU | Total | |
| Hospitalization | 911.2 ± 1027.1 | 1143.2 ± 1370.8 | 1070.8 ± 959.8 | 1403.8 ± 948.0 | 967.8 ± 1006.2 | 1234.6 ± 1244.8 | <0.0001 | <0.0001 | <0.0001 |
| Medications | 84.3 ± 156.0 | 60.0 ± 85.4 | 92.1 ± 113.8 | 68.0 ± 94.2 | 87.1 ± 145.3 | 62.8 ± 88.6 | 0.7014 | 0.0024 | 0.0278 |
| Laboratory tests | 690.4 ± 752.0 | 748.3 ± 643.5 | 1825.1 ± 1538.1 | 2182.8 ± 1461.0 | 1092.8 ± 1223.6 | 1251.2 ± 1218.4 | 0.0005 | <0.0001 | 0.0003 |
| TDM Service | - | 89.1 ± 68.1 | - | 102.0 ± 75.3 | - | 93.6 ± 71.0 | - | - | - |
| Total 2 | 5048.6 ± 5944.1 | 4121.7 ± 3933.9 | 14,464 ± 10,608 | 11,258 ± 10,015 | 8388.1 ± 9107.2 | 6623.6 ± 7532.4 | 0.5394 | <0.0001 | 0.0070 |
| Variable | Non-ICU (n = 1101) | ICU (n = 628) | Total (n = 1729) | ANCOVA | |||||
|---|---|---|---|---|---|---|---|---|---|
| p-Value 1 | |||||||||
| Non-TDM (n = 602) | TDM (n = 499) | Non-TDM (n = 334) | TDM (n = 294) | Non-TDM (n = 936) | TDM (n = 793) | Non-ICU | ICU | Total | |
| Duration of vancomycin treatment, days | 11.0 (10.2–11.8) | 11.4 (10.5–12.3) | 10.5 (9.5–11.5) | 11.8 (10.8–12.8) | 10.8 (10.2–11.4) | 11.7 (11.0–12.3) | 0.4928 | 0.0522 | 0.0439 |
| Vancomycin dosage, mg/day/kg | 31.5 (29.6–33.4) | 29.8 (27.7–31.8) | 27.5 (24.9–30.1) | 28.7 (26.0–31.5) | 29.8 (28.3–31.4) | 29.2 (27.5–30.8) | 0.1650 | 0.4846 | 0.5169 |
| Length of stay in hospital, days | 17.6 (16.6–18.6) | 18.6 (17.5–19.8) | 17.1 (15.8–18.5) | 18.3 (16.9–19.7) | 17.5 (16.7–18.3) | 18.5 (17.7–19.4) | 0.1243 | 0.2097 | 0.0531 |
| Duration of fever, days | 2.4 (2.1–2.7) | 2.6 (2.2–2.9) | 0.7 (0.4–0.9) | 0.8 (0.6–1.1) | 1.5 (1.3–1.8) | 1.7 (1.4–1.9) | 0.3832 | 0.3760 | 0.3897 |
| Medical expenses, USD | |||||||||
| Hospitalization | 1026.3 (914.4–1138.3) | 1214.0 (1092.5–1335.4) | 1320.0 (1204.5–1435.5) | 1456.4 (1336.6–1576.2) | 1155.5 (1072.2–1238.8) | 1342.7 (1254.1–1431.3) | 0.0109 | 0.0832 | 0.0006 |
| Medications | 84.8 (72.4–97.2) | 70.0 (56.6–83.5) | 97.4 (84.3–110.4) | 78.9 (65.4–92.4) | 91.4 (82.1–100.6) | 74.7 (64.8–84.5) | 0.0697 | 0.0383 | 0.0062 |
| Laboratory tests | 803.1 (740.3–865.8) | 862.6 (794.5–930.7) | 2240.2 (2059.2–2421.2) | 2381.8 (2194.1–2569.6) | 1505.3 (1426.8–1583.8) | 1611.5 (1527.9–1695.0) | 0.1493 | 0.2509 | 0.0399 |
| Total 2 | 5583.5 (4649.8–5517.3) | 4623.2 (4152.4–5093.9) | 14,112 (12,865–15,359) | 12,716 (11,422–14,010) | 9502.8 (8963.1–10,043) | 8661.4 (8087.2–9235.6) | 0.1068 | 0.1007 | 0.0178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, S.; Park, J.; Lee, H. Clinical Response and Hospital Costs of Therapeutic Drug Monitoring for Vancomycin in Elderly Patients. J. Pers. Med. 2022, 12, 163. https://doi.org/10.3390/jpm12020163
Kim Y, Kim S, Park J, Lee H. Clinical Response and Hospital Costs of Therapeutic Drug Monitoring for Vancomycin in Elderly Patients. Journal of Personalized Medicine. 2022; 12(2):163. https://doi.org/10.3390/jpm12020163
Chicago/Turabian StyleKim, Yun, Soohyun Kim, Jinsook Park, and Howard Lee. 2022. "Clinical Response and Hospital Costs of Therapeutic Drug Monitoring for Vancomycin in Elderly Patients" Journal of Personalized Medicine 12, no. 2: 163. https://doi.org/10.3390/jpm12020163






