Presence of Helicobacter Species in Gastric Mucosa of Human Patients and Outcome of Helicobacter Eradication Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Samples
2.2. Histological Analysis of Helicobacter Species in Human Gastric Samples
2.3. Immunohistochemical Detection of Helicobacter Species in Human Gastric Samples
2.4. DNA Extraction and Helicobacter Species Identification through PCR Analysis
3. Results
3.1. Patients Data
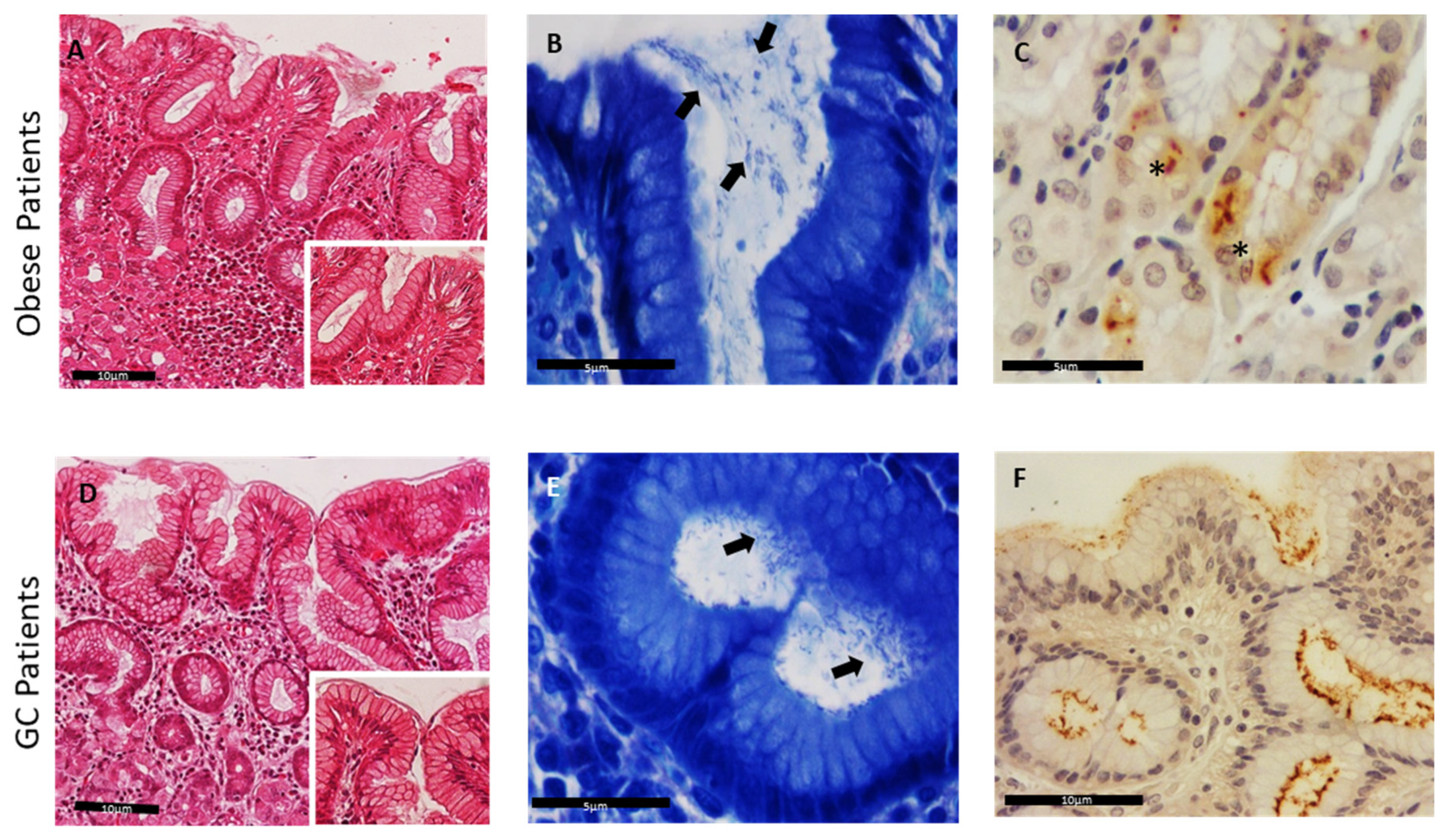
3.2. Histological and Immunohistochemical Detection of Helicobacter Species in Human Gastric Samples
3.3. Detection of Helicobacter Species
3.4. Association between Helicobacter spp. Presence and Animal Contact
3.5. Helicobacter spp. Presence in Patients with Previous History of Eradication Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bahadori, A.; De Witte, C.; Agin, M.; De Bruyckere, S.; Smet, A.; Tümgör, G.; Gökmen, T.G.; Haesebrouck, F.; Köksal, F. Presence of gastricHelicobacterspecies in children suffering from gastric disorders in Southern Turkey. Helicobacter 2018, 23, e12511. [Google Scholar] [CrossRef] [PubMed]
- Doulberis, M.; Srivastava, S.; Polyzos, S.A.; Kountouras, J.; Papaefthymiou, A.; Klukowska-Rötzler, J.; Blank, A.; Exadaktylos, A.K.; Srivastava, D.S. Active Helicobacter pylori Infection is Independently Associated with Nonalcoholic Steatohepatitis in Morbidly Obese Patients. J. Clin. Med. 2020, 9, 933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Amsterdam, K.; van Vliet, A.; Kusters, J.G.; Van Der Ende, A. Of microbe and man: Determinants of Helicobacter pylori-related diseases. FEMS Microbiol. Rev. 2006, 30, 131–156. [Google Scholar] [CrossRef] [Green Version]
- Blaser, N.; Backert, S.; Pachathundikandi, S.K. Immune Cell Signaling by Helicobacter pylori: Impact on Gastric Pathology. Helicobacter Pylori Hum. Dis. 2019, 1149, 77–106. [Google Scholar] [CrossRef]
- Blaecher, C.; Smet, A.; Flahou, B.; Pasmans, F.; Ducatelle, R.; Taylor, D.; Weller, C.; Bjarnason, I.; Charlett, A.; Lawson, A.J.; et al. Significantly higher frequency of Helicobacter suis in patients with idiopathic parkinsonism than in control patients. Aliment. Pharmacol. Ther. 2013, 38, 1347–1353. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.F.; Guerra, M.R.; De Alvarenga, A.V.R.; Souza, D.Z.D.O.; Costa, R.A.V.E.S.; Cupolilo, S.M.N. Helicobacter pylori infection and gastric cancer precursor lesions: Prevalence and associated factors in a reference laboratory in southeastern brazil. Arq. Gastroenterol. 2019, 56, 419–424. [Google Scholar] [CrossRef]
- Cai, X.; Carlson, J.; Stoicov, C.; Li, H.; Wang, T.C.; Houghton, J. Helicobacter felis eradication restores normal architecture and ihnibits gastric cancer progression in C57BL/6 mice. Gastroenterology 2005, 128, 1937–1952. [Google Scholar] [CrossRef]
- Yang-Ou, Y.-B.; Hu, Y.; Zhu, Y.; Lu, N.-H. The effect of antioxidants on Helicobacter pylori eradication: A systematic review with meta-analysis. Helicobacter 2018, 23, e12535. [Google Scholar] [CrossRef]
- De Briyne, N.; Atkinson, J.; Borriello, S.P.; Pokludová, L. Antibiotics used most commonly to treat animals in Europe. Vet. Rec. 2014, 175, 325. [Google Scholar] [CrossRef] [Green Version]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial Activity of Curcumin against Helicobacter pylori Isolates from India and during Infections in Mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bento-Miranda, M.; Figueiredo, C. Helicobacter heilmannii sensu lato: An overview of the infection in humans. World J. Gastroenterol. 2014, 20, 17779–17787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlamont, H.; Smet, A.; De Bruykere, S.; Boyen, F.; Ducatelle, R.; Haesebrouck, F.; De Witte, C. Antimicrobial susceptibility pattern of Helicobacter suis isolates from pigs and macaques. Vet. Microbiol. 2019, 239, 108459. [Google Scholar] [CrossRef] [Green Version]
- De Witte, C.; Taminiau, B.; Flahou, B.; Hautekiet, V.; Daube, G.; Ducatelle, R.; Haesebrouck, F. In-feed bambermycin medication induces anti-inflammatory effects and prevents parietal cell loss without influencing Helicobacter suis colonization in the stomach of mice. Vet. Res. 2018, 49, 35. [Google Scholar] [CrossRef] [Green Version]
- Matos, R.; De Witte, C.; Smet, A.; Berlamont, H.; De Bruyckere, S.; Amorim, I.; Gärtner, F.; Haesebrouck, F. Antimicrobial Susceptibility Pattern of Helicobacter heilmannii and Helicobacter ailurogastricus Isolates. Microorganisms 2020, 8, 957. [Google Scholar] [CrossRef]
- Boyanova, L.; Koumanova, R.; Lazarova, E.; Jelev, C. Helicobacter pylori and Helicobacter heilmannii in children. A Bulgarian study. Diagn. Microbiol. Infect. Dis. 2003, 46, 249–252. [Google Scholar] [CrossRef]
- Haesebrouck, F.; Pasmans, F.; Flahou, B.; Chiers, K.; Baele, M.; Meyns, T.; Decostere, A.; Ducatelle, R. Gastric Helicobacters in Domestic Animals and Nonhuman Primates and Their Significance for Human Health. Clin. Microbiol. Rev. 2009, 22, 202–223. [Google Scholar] [CrossRef] [Green Version]
- Sykora, J.; Hejda, V.; Varvarovská, J.; Stozicky, F.; Gottrand, F.; Siala, K. Helicobacter heilmannii Related Gastric Ulcer in Childhood. J. Pediatric Gastroenterol. Nutr. 2003, 36, 410–413. [Google Scholar] [CrossRef]
- Yakoob, J.; Abbas, Z.; Khan, R.; Naz, S.; Ahmad, Z.; Islam, M.; Awan, S.; Jafri, F.; Jafri, W. Prevalence of non Helicobacter pylori species in patients presenting with dyspepsia. BMC Gastroenterol. 2012, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Joosten, M.; Lindén, S.; Rossi, M.; Tay, A.C.Y.; Skoog, E.; Padra, M.; Peters, F.; Perkins, T.; Vandamme, P.; Van Nieuwerburgh, F.; et al. Divergence between the Highly Virulent Zoonotic Pathogen Helicobacter heilmannii and Its Closest Relative, the Low-Virulence “Helicobacter ailurogastricus” sp. nov. Infect. Immun. 2016, 84, 293–306. [Google Scholar] [CrossRef] [Green Version]
- Morgner, A.; Lehn, N.; Andersen, L.P.; Thiede, C.; Bennedsen, M.; Trebesius, K.; Neubauer, B.; Neubauer, A.; Stolte, M.; Bayerdörffer, E. Helicobacter heilmannii–Associated Primary Gastric Low-Grade MALT Lymphoma: Complete Remission After Curing the Infection. Gastroenterology 2000, 118, 821–828. [Google Scholar] [CrossRef]
- De Cooman, L.; Flahou, B.; Houf, K.; Smet, A.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F. Survival of Helicobacter suis bacteria in retail pig meat. Int. J. Food Microbiol. 2013, 166, 164–167. [Google Scholar] [CrossRef] [PubMed]
- De Cooman, L.; Houf, K.; Smet, A.; Flahou, B.; Ducatelle, R.; De Bruyne, E.; Pasmans, F.; Haesebrouck, F. Presence of Helicobacter suis on pork carcasses. Int. J. Food Microbiol. 2014, 187, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Joosten, M.; Blaecher, C.; Flahou, B.; Ducatelle, R.; Haesebrouck, F.; Smet, A. Diversity in bacterium-host interactions within the species Helicobacter heilmannii sensu stricto. Vet. Res. 2013, 44, 65. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; He, L.; Haesebrouck, F.; Gong, Y.; Flahou, B.; Cao, Q.; Zhang, J. Prevalence of Coinfection with Gastric Non-Helicobacter pylori Helicobacter(NHPH) Species in Helicobacter pylori-infected Patients Suffering from Gastric Disease in Beijing, China. Helicobacter 2014, 20, 284–290. [Google Scholar] [CrossRef]
- Borges, S.S.; Ramos, A.F.P.L.; Filho, A.V.D.M.; Braga, C.A.D.S.B.; Carneiro, L.C.; Barbosa, M.S. [Article partial retraction] prevalence of helicobacter pylori infection in dyspeptic patients and its association with clinical risk factors for developing gastric adenocarcinoma. Arq. Gastroenterol. 2019, 56, 66–70. [Google Scholar] [CrossRef]
- Prachasilpchai, W.; Nuanualsuwan, S.; Chatsuwan, T.; Techangamsuwan, S.; Wangnaitham, S.; Sailasuta, A. Diagnosis of Helicobacter spp. infection in canine stomach. J. Vet. Sci. 2007, 8, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Amorim, I.; Smet, A.; Alves, O.; Teixeira, S.; Saraiva, A.L.; Taulescu, M.; Reis, C.; Haesebrouck, F.; Gärtner, F. Presence and significance of Helicobacter spp. in the gastric mucosa of Portuguese dogs. Gut Pathog. 2015, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.K.; Han, J.H.; Joo, H.S. Identification of Novel Helicobacter Species in Pig Stomachs by PCR and Partial Sequencing. J. Clin. Microbiol. 2001, 39, 3311–3315. [Google Scholar] [CrossRef] [Green Version]
- Farshad, S.; Alborzi, A.; Hosseini, S.A.M.; Oboodi, B.; Rasouli, M.; Japoni, A.; Nasiri, J. Identification of Helicobacter pylori DNA in Iranian patients with gallstones. Epidemiol. Infect. 2004, 132, 1185–1189. [Google Scholar] [CrossRef]
- Farshad, S.; Alborzi, A.; Malekhosseini, S.A.; Gramizadeh, B.; Oboodi, B.; Rasouli, M.; Japoni, A.; Kalani, M.; Pourabbas, B. Detection of Helicobacter DNA in Bile Samples of Patients with Biliary Diseases Living in South of Iran. IJMS 2006, 31, 186–190. [Google Scholar]
- Baele, M.; Decostere, A.; Vandamme, P.; Ceelen, L.; Hellemans, A.; Mast, J.; Chiers, K.; Ducatelle, R.; Haesebrouck, F. Isolation and characterization of Helicobacter suis sp. nov. from pig stomachs. Int. J. Syst. Evol. Microbiol. 2008, 58, 1350–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praet, J.; Cnockaert, M.; Meeus, I.; Smagghe, G.; Vandamme, P. Gilliamella intestini sp. nov., Gilliamella bombicola sp. nov., Gilliamella bombi sp. nov. and Gilliamella mensalis sp. nov.: Four novel Gilliamella species isolated from the bumblebee gut. Syst. Appl. Microbiol. 2017, 40, 199–204. [Google Scholar] [CrossRef]
- Dubois, A.B.T. Helicobacter pylori is invasive and it may be a facultative intracellular organism. Cell Microbiol. 2007, 9, 1108–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scanziani, E.S.K.; Monestiroli, S.; Soldati, S.; Strauss-Ayali, D.; Del Piero, F. Histological and immunohistochemical detection of different Helicobacter species in the gastric mucosa of cats. J. Vet. Diagn. Investig. 2001, 13, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiinberg, B.S.A.; Dietz, H.H.; Egelund, T.; Greiter-Wilke, A.; McDonough, S.P.; Olsen, J.; Priestnall, S.; Chang, Y.F.; Simpson, K.W. Quantitative Analysis of Inflammatory and Immune Responses in Dogs with Gastritis and Their Relationship to Helicobacter spp. Infection. J. Vet. Intern. Med. 2005, 19, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-H.; Kim, H.-D.; Lee, Y.-S.; Hwang, C.-Y. Determination of the Prevalence of Helicobacter heilmannii-Like Organisms Type 2 (HHLO-2) Infection in Humans and Dogs Using Non-Invasive Genus/Species-Specific PCR in Korea. J. Vet. Med. Sci. 2014, 76, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, F.; Chaves, P. Pathologic Risk Factors of Adenocarcinoma of the Gastric Cardia and Gastroesophageal Junction. Surg. Oncol. Clin. N. Am. 2006, 15, 697–714. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chiang, T.-H.; Chou, C.-K.; Tu, Y.-K.; Liao, W.-C.; Wu, M.-S.; Graham, D.Y. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016, 150, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Dicksved, J.; Lindberg, M.; Rosenquist, M.; Enroth, H.; Jansson, J.; Engstrand, L. Molecular characterization of the stomach microbiota in patients with gastric cancer and in controls. J. Med. Microbiol. 2009, 58, 509–516. [Google Scholar] [CrossRef]
- Noto, J.M.; Peek, R.M., Jr. The gastric microbiome, its interaction with Helicobacter pylori, and its potential role in the progression to stomach cancer. PLoS Pathog. 2017, 13, e1006573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- Ghotaslou, R.; Leylabadlo, H.E.; Asl, Y.M. Prevalence of antibiotic resistance in Helicobacter pylori: A recent literature review. World J. Methodol. 2015, 5, 164–174. [Google Scholar] [CrossRef] [PubMed]



| Gastric Samples | Detection Methods Positive (Percentage and Number) | ||||
|---|---|---|---|---|---|
| MG | IHC | Genus-Specific PCR + Sequencing | Species-Specific PCR + Sequencing | ||
| H.pylori | H. felis | ||||
| Total (n = 80) | 53.8% (43/80) | 66.3% (53/80) | 46.3% (37/80) | 21.3% (17/80) | 2.5% (2/80) |
| Gastric cancer patients (n = 17) | 41.2% (7/17) | 52.9% (9/17) | 11.8% (2/17) | 11.8% (2/17) | - |
| Obese patients (n = 63) | 57.1% (36/63) | 69.8% (44/63) | 55.5% (35/63) | 23.8% (15/63) | 3.2% (2/63) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, R.; Taillieu, E.; De Bruyckere, S.; De Witte, C.; Rêma, A.; Santos-Sousa, H.; Nogueiro, J.; Reis, C.A.; Carneiro, F.; Haesebrouck, F.; et al. Presence of Helicobacter Species in Gastric Mucosa of Human Patients and Outcome of Helicobacter Eradication Treatment. J. Pers. Med. 2022, 12, 181. https://doi.org/10.3390/jpm12020181
Matos R, Taillieu E, De Bruyckere S, De Witte C, Rêma A, Santos-Sousa H, Nogueiro J, Reis CA, Carneiro F, Haesebrouck F, et al. Presence of Helicobacter Species in Gastric Mucosa of Human Patients and Outcome of Helicobacter Eradication Treatment. Journal of Personalized Medicine. 2022; 12(2):181. https://doi.org/10.3390/jpm12020181
Chicago/Turabian StyleMatos, Rita, Emily Taillieu, Sofie De Bruyckere, Chloë De Witte, Alexandra Rêma, Hugo Santos-Sousa, Jorge Nogueiro, Celso A. Reis, Fátima Carneiro, Freddy Haesebrouck, and et al. 2022. "Presence of Helicobacter Species in Gastric Mucosa of Human Patients and Outcome of Helicobacter Eradication Treatment" Journal of Personalized Medicine 12, no. 2: 181. https://doi.org/10.3390/jpm12020181






