Personalized Prediction of Postconcussive Working Memory Decline: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Neuropsychological Evaluation
2.2. MRI Data Acquisition and Experimental Design
2.3. Data Analysis
2.3.1. fMRI Preprocessing
2.3.2. WM Task Activation and Deactivation Map
2.4. Statistical Analyses
2.5. Regions-of-Interest Selection and Percentage Signal Change Calculation
2.6. Postconcussive WM Changes at Predetermined Time Periods during 1-Year Follow-Up
2.7. Individualized Prediction of Postconcussive WM Impairments by Using Biomarkers Measured at Baseline
3. Results
3.1. Demographics
3.2. Postconcussive WM Changes during the 1-Year Follow-Up Period
3.2.1. N-Back WM Task
3.2.2. Neuropsychological Assessment
- Thirty-eight percent (9/24) of patients exhibited no recovery in the WMI at 3 months after a mTBI.
- Seventy-five percent (18/24) of patients exhibited a decline in the WMI from 3 to 6 months after a mTBI.
- Thirty-eight percent (9/24) of patients exhibited no recovery in the WMI from 6 months to 1 year after a mTBI.
- Forty-six percent (11/24) of patients exhibited a worsened WMI at 1-year follow-up compared to the baseline.

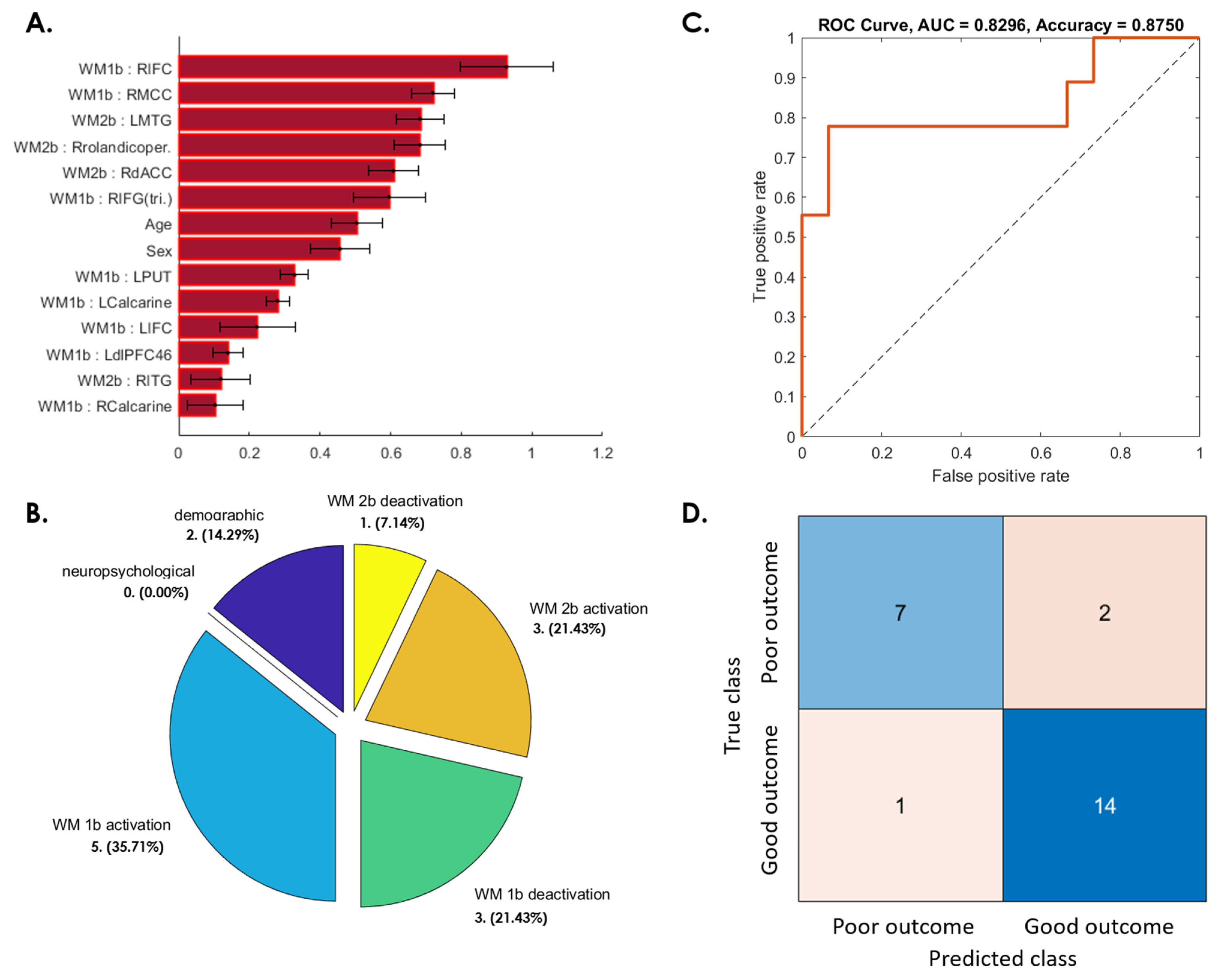
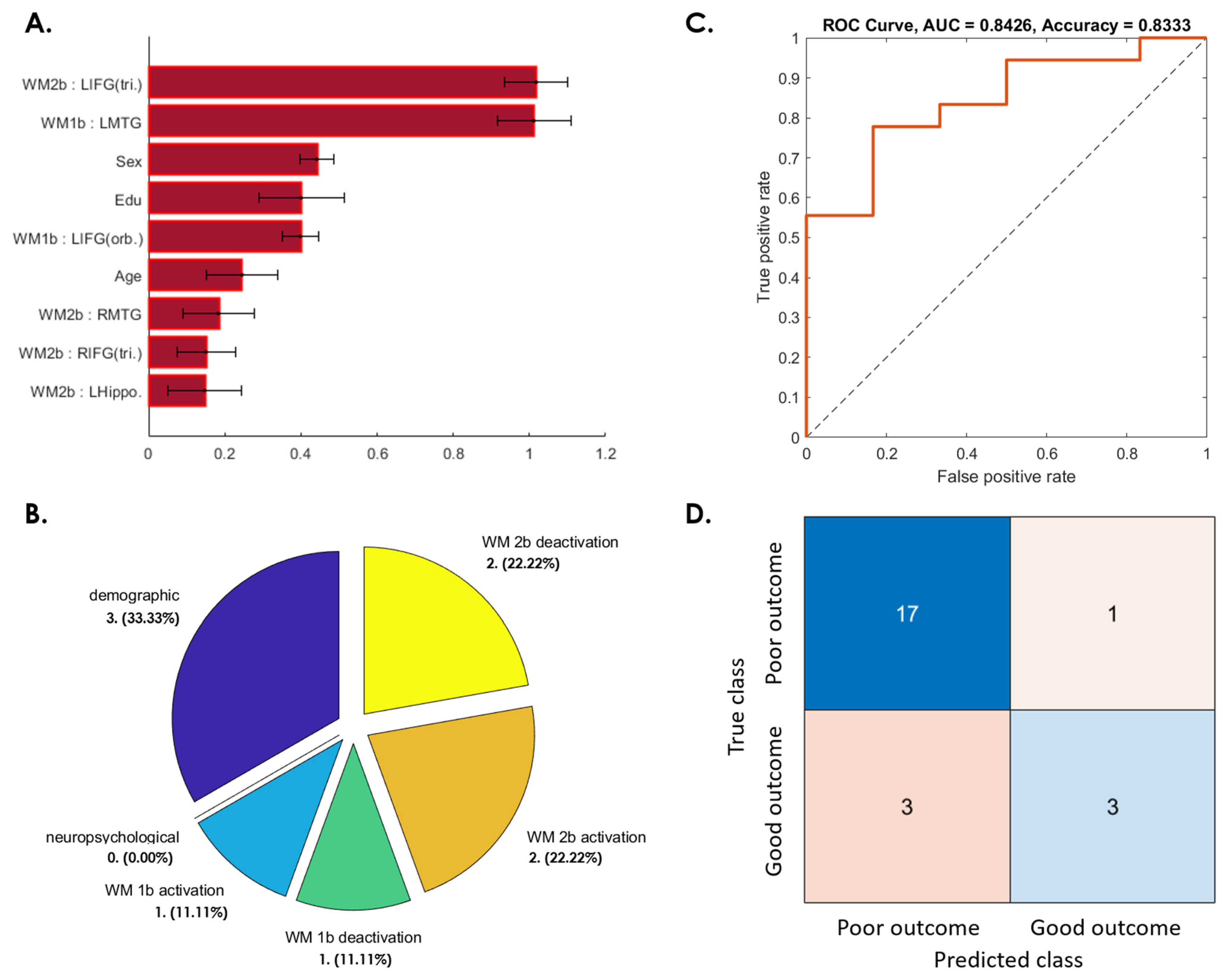
3.3. Prediction of Postconcussive WMI Decline Based on Baseline Studies
3.3.1. WMI Not Recovered at 3 Months after mTBI
3.3.2. WMI Decline from 3 to 6 Months after Initial Recovery
3.3.3. WMI Not Recovered from 6 Months to 1 Year after mTBI
3.3.4. Patients Whose WMI at 1-Year Follow-Up Was Worse Than at Baseline
4. Discussion
4.1. Validate Machine Learning Algorithms in a Limited Data Size
4.2. Neuropsychological Assessments Are Not Predictive of Postconcussion Cognitive Decline
4.3. Age and Sex Effects in Postconcussive Working Memory Impairment
4.4. The Role of WM Task-Induced Deactivation Regions in Reflecting Postconcussive Cognitive Decline
4.5. Scientific Merit and Clinical Implications
4.6. Limitations
4.6.1. Small Data Size and Dropouts in Longitudinal Data
4.6.2. The Handedness and Brain Lateralization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanderploeg, R.D.; Belanger, H.G.; Curtiss, G. Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Arch. Phys. Med. Rehabil. 2009, 90, 1084–1093. [Google Scholar] [CrossRef]
- Rohling, M.L.; Binder, L.M.; Demakis, G.J.; Larrabee, G.J.; Ploetz, D.M.; Rohling-Langhinrichsen, J. A meta-analysis of neuropsychological outcome after mild traumatic brain injury: Re-analyses and reconsiderations of Binder et al. (1997), Frencham et al. (2005), and Pertab et al. (2009). Clin. Neuropsychol. 2011, 25, 608–623. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, D.H.; Riley, D.O.; Nowinski, C.J.; Mckee, A.C.; Stern, R.A.; Cantu, R.C. Long-term consequences: Effects on normal development profile after concussion. Phys. Med. Rehabil. Clin. N. Am. 2011, 22, 683–700. [Google Scholar] [CrossRef]
- Hall, R.C.; Hall, R.C.; Chapman, M.J. Definition, diagnosis, and forensic implications of postconcussional syndrome. Psychosomatics 2005, 46, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, S.; Paradis-Giroux, A.A.; Pépin, M.; Mckerral, M. Impact of divided attention during verbal learning in young adults following mild traumatic brain injury. Brain Inj. 2009, 23, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, D.; Saling, M. Neuropsychological deficits following concussion. Brain Inj. 1996, 10, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Mangels, J.A.; Craik, F.I.M.; Levine, B.; Schwartz, M.L.; Stuss, D.T. Effects of divided attention on episodic memory in chronic traumatic brain injury: A function of severity and strategy. Neuropsychologia 2002, 40, 2369–2385. [Google Scholar] [CrossRef]
- Rutherford, W.; Merrett, J.; McDonald, J. Symptoms at one year following concussion from minor head injuries. J. Inj. 1979, 10, 225–230. [Google Scholar] [CrossRef]
- Sterr, A.; Herron, K.A.; Hayward, C.; Montaldi, D. Are mild head injuries as mild as we think? Neurobehavioral concomitants of chronic post-concussion syndrome. J. BMC Neurol. 2006, 6, 1–10. [Google Scholar] [CrossRef]
- McInnes, K.; Friesen, C.L.; MacKenzie, D.E.; Westwood, D.A.; Boe, S.G. Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS ONE 2017, 12, e0174847. [Google Scholar] [CrossRef]
- Rabinowitz, A.R.; Levin, H.S. Cognitive sequelae of traumatic brain injury. Psychiatr. Clin. N. Am. 2014, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.W.; Flashman, L.A.; McDonald, B.C.; Saykin, A.J. Mechanisms of working memory dysfunction after mild and moderate TBI: Evidence from functional MRI and neurogenetics. J. Neurotrauma 2006, 23, 1450–1467. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Wu, C.-H.; Liao, Y.-P.; Hsu, H.-L.; Tseng, Y.-C.; Liu, H.-L.; Chiu, W.-T. Working memory in patients with mild traumatic brain injury: Functional MR imaging analysis. Radiology 2012, 264, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Wortzel, H.S.; Arciniegas, D.B. Treatment of post-traumatic cognitive impairments. Curr. Treat. Options Neurol. 2012, 14, 493–508. [Google Scholar] [CrossRef]
- Cicerone, K.D.; Langenbahn, D.M.; Braden, C.; Malec, J.F.; Kalmar, K.; Fraas, M.; Felicetti, T.; Laatsch, L.; Harley, J.P.; Bergquist, T.; et al. Evidence-based cognitive rehabilitation: Updated review of the literature from 2003 through 2008. Arch. Phys. Med. Rehabil. 2011, 92, 519–530. [Google Scholar] [CrossRef]
- Laatsch, L.; Thulborn, K.R.; Krisky, C.M.; Shobat, D.M.; Sweeney, J.A. Investigating the neurobiological basis of cognitive rehabilitation therapy with fMRI. Brain Inj. 2004, 18, 957–974. [Google Scholar] [CrossRef]
- Strangman, G.; O’Neil-Pirozzi, T.M.; Burke, D.; Cristina, D.; Goldstein, R.; Rauch, S.L.; Savage, C.R.; Glenn, M.B. Functional neuroimaging and cognitive rehabilitation for people with traumatic brain injury. Am. J. Phys. Med. Rehabil. 2005, 84, 62–75. [Google Scholar] [CrossRef]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef]
- Witt, S.T.; Warntjes, M.; Engström, M. Increased fMRI sensitivity at equal data burden using averaged shifted echo acquisition. J. Front. Neurosci. 2016, 10, 544. [Google Scholar] [CrossRef]
- Luo, W.L.; Nichols, T.E. Diagnosis and exploration of massively univariate neuroimaging models. Neuroimage 2003, 19, 1014–1032. [Google Scholar] [CrossRef]
- Bengio, Y.; Grandvalet, Y. No unbiased estimator of the variance of k-fold cross-validation. J. Mach. Learn. Res. 2004, 5, 1089–1105. [Google Scholar]
- Carroll, E.L.; Outtrim, J.G.; Forsyth, F.; Manktelow, A.E.; Hutchinson, P.J.A.; Tenovou, O.; Posti, J.P.; Wilson, L.; Sahakian, B.J.; Menon, D.K.; et al. Mild traumatic brain injury recovery: A growth curve modelling analysis over 2 years. J. Neurol. 2020, 267, 3223–3234. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.; Jolles, J.; Twijnstra, A. Neuropsychological deficits in patients with persistent symptoms six months after mild head injury. Neurosurgery 1992, 30, 692–696. [Google Scholar] [PubMed]
- Cnossen, M.C.; van der Naalt, J.; Spikman, J.M.; Nieboer, D.; Yue, J.K.; Winkler, E.A.; Manley, G.T.; von Steinbuechel, N.; Polinder, S.; Steyerberg, E.W.; et al. Prediction of persistent post-concussion symptoms after mild traumatic brain injury. J. Neurotrauma 2018, 35, 2691–2698. [Google Scholar] [CrossRef]
- Langer, L.K.; Alavinia, S.M.; Lawrence, D.W.; Munce, S.E.P.; Kam, A.; Tam, A.; Ruttan, L.; Comper, P.; Bayley, M.T. Prediction of risk of prolonged post-concussion symptoms: Derivation and validation of the TRICORDRR (Toronto Rehabilitation Institute Concussion Outcome Determination and Rehab Recommendations) score. PLoS Med. 2021, 18, e1003652. [Google Scholar] [CrossRef]
- Hellstrøm, T.; Kaufmann, T.; Andelic, N.; Soberg, H.L.; Sigurdardottir, S.; Helseth, E.; Andreassen, O.A.; Westlye, L.T. Predicting outcome 12 months after mild traumatic brain injury in patients admitted to a neurosurgery service. Front. Neurol. 2017, 8, 125. [Google Scholar] [CrossRef]
- Tenovuo, O.; Diaz-Arrastia, R.; Goldstein, L.E.; Sharp, D.J.; van der Naalt, J.; Zasler, N.D. Assessing the Severity of Traumatic Brain Injury—Time for a Change? J. Clin. Med. 2021, 10, 148. [Google Scholar] [CrossRef]
- Stenberg, J.; Haberg, A.K.; Follestad, T.; Olsen, A.; Icerson, G.L.; Terry, D.P.; Karlsen, R.H.; Saksvik, S.B.; Karaliute, M.; Ek, J.A.N.; et al. Cognitive reserve moderates cognitive outcome after mild traumatic brain injury. Arch. Phys. Med. Rehabil. 2020, 101, 72–80. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Blyth, B.; Mookerjee, S.; He, H.; McDermott, M.P. Sex differences in outcome after mild traumatic brain injury. J. Neurotrauma 2010, 27, 527–539. [Google Scholar] [CrossRef]
- Hsu, H.L.; Chen, D.Y.-T.; Tseng, Y.-C.; Kuo, Y.-S.; Huang, Y.-L.; Chiu, W.-T.; Yan, F.-X.; Wang, W.-S.; Chen, C.-J. Sex Differences in Working Memory after Mild Traumatic Brain Injury: A Functional MR Imaging Study. Radiology 2015, 276, 828–835. [Google Scholar] [CrossRef]
- Chen, D.Y.; Hsu, H.-L.; Kuo, Y.-S.; Wu, C.W.; Chiu, W.-T.; Yan, F.-X.; Wang, W.-S.; Chen, C.-J.; Tseng, Y.-C. Effect of Age on Working Memory Performance and Cerebral Activation after Mild Traumatic Brain Injury: A Functional MR Imaging Study. Radiology 2016, 278, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Levin, H.S.; Temkin, N.R.; Barber, J.; Nelson, L.D.; Robertson, C.; Brennan, J.; Stein, M.B.; Yue, J.K.; Giacino, J.T.; McCrea, M.A.; et al. Association of sex and age with mild traumatic brain injury–related symptoms: A TRACK-TBI study. JAMA Netw. Open 2021, 4, e213046. [Google Scholar] [CrossRef] [PubMed]
- Anticevic, A.; Repovs, G.; Barch, D.M. Working memory encoding and maintenance deficits in schizophrenia: Neural evidence for activation and deactivation abnormalities. Schizophr. Bull. 2013, 39, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, X.; Gu, H.; Yang, Y. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. J. Neurosci. 2013, 33, 18566–18573. [Google Scholar] [CrossRef]
- Singh, K.D.; Fawcett, I.P. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage 2008, 41, 100–112. [Google Scholar] [CrossRef]
- Anticevic, A.; Repovs, G.; Shulman, G.L.; Barch, D.M. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage 2010, 49, 2638–2648. [Google Scholar] [CrossRef]
- Wylie, G.R.; Freeman, K.; Thomas, A.; Shpaner, M.; OKeefe, M.; Watts, R.; Naylor, M.R. Cognitive improvement after mild traumatic brain injury measured with functional neuroimaging during the acute period. PLoS ONE 2015, 10, e0126110. [Google Scholar] [CrossRef]
- Levin, H.; Troyanskaya, M.; Petrie, J.; Wilde, E.A.; Hunter, J.V.; Abildskov, T.J.; Scheibel, R.S. Methylphenidate treatment of cognitive dysfunction in adults after mild to moderate traumatic brain injury: Rationale, efficacy, and neural mechanisms. Front. Neurol. 2019, 10, 925. [Google Scholar] [CrossRef]
- Manktelow, A.E.; Menon, D.K.; Sahakian, B.J.; Stamatakis, E.A. Working memory after traumatic brain injury: The neural basis of improved performance with methylphenidate. Front. Behav. Neurosci. 2017, 11, 58. [Google Scholar] [CrossRef]
- Willems, R.M.; Francks, C. Your left-handed brain. Front. Young Minds 2014, 2. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Chen, Y.-L.; Kuo, D.-P.; Li, Y.-T.; Chiang, Y.-H.; Chang, J.-J.; Tseng, S.-H.; Chen, C.-Y. Personalized Prediction of Postconcussive Working Memory Decline: A Feasibility Study. J. Pers. Med. 2022, 12, 196. https://doi.org/10.3390/jpm12020196
Chen Y-C, Chen Y-L, Kuo D-P, Li Y-T, Chiang Y-H, Chang J-J, Tseng S-H, Chen C-Y. Personalized Prediction of Postconcussive Working Memory Decline: A Feasibility Study. Journal of Personalized Medicine. 2022; 12(2):196. https://doi.org/10.3390/jpm12020196
Chicago/Turabian StyleChen, Yung-Chieh, Yung-Li Chen, Duen-Pang Kuo, Yi-Tien Li, Yung-Hsiao Chiang, Jyh-Jong Chang, Sung-Hui Tseng, and Cheng-Yu Chen. 2022. "Personalized Prediction of Postconcussive Working Memory Decline: A Feasibility Study" Journal of Personalized Medicine 12, no. 2: 196. https://doi.org/10.3390/jpm12020196
APA StyleChen, Y.-C., Chen, Y.-L., Kuo, D.-P., Li, Y.-T., Chiang, Y.-H., Chang, J.-J., Tseng, S.-H., & Chen, C.-Y. (2022). Personalized Prediction of Postconcussive Working Memory Decline: A Feasibility Study. Journal of Personalized Medicine, 12(2), 196. https://doi.org/10.3390/jpm12020196





