Intra- and Early Postoperative Evaluation of Malperfused Areas in an Irradiated Random Pattern Skin Flap Model Using Indocyanine Green Angiography and Near-Infrared Reflectance-Based Imaging and Infrared Thermography
Abstract
1. Introduction
2. Materials and Methods
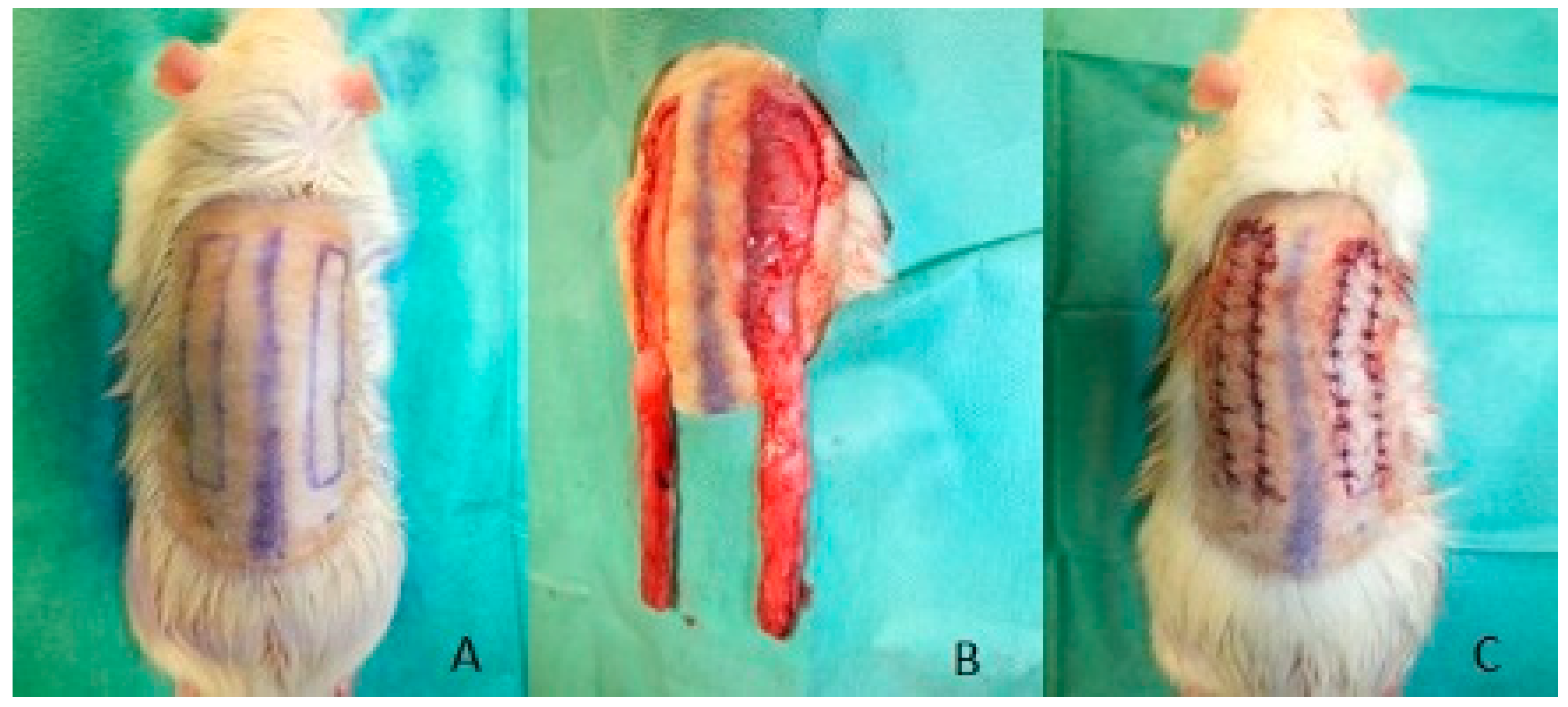
2.1. Surgical Procedure
2.2. Irradiation Procedure
2.3. Groups
2.4. Imaging
2.5. Blood Samples
2.6. Statistical Analysis
3. Results
3.1. Flap Size
3.2. Necrotic Areas of Flaps
3.3. Nonperfused Areas of the Flaps in the Indocyanine Green Angiography
3.4. Staining of Double-Strand Breaks
3.5. Near-Infrared Reflectance-Based Imaging and Infrared Thermography
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fourman, M.S.; Gersch, R.P.; Phillips, B.T.; Nasser, A.; Rivara, A.; Verma, R.; Dagum, A.B.; Rosengart, T.K.; Bui, D.T. Comparison of laser doppler and laser-assisted indocyanine green angiography prediction of flap survival in a novel modification of the mcfarlane flap. Ann. Plast. Surg. 2015, 75, 102–107. [Google Scholar] [CrossRef]
- Wei, J.W.; Dong, Z.G.; Ni, J.D.; Liu, L.H.; Luo, S.H.; Luo, Z.B.; Zheng, L.; He, A.Y. Influence of flap factors on partial necrosis of reverse sural artery flap: A study of 179 consecutive flaps. J. Trauma Acute Care Surg. 2012, 72, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Moyer, H.R.; Losken, A. Predicting mastectomy skin flap necrosis with indocyanine green angiography: The gray area defined. Plast. Reconstr. Surg. 2012, 129, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Jaschke, W.; Schmuth, M.; Trianni, A.; Bartal, G. Radiation-induced skin injuries to patients: What the interventional radiologist needs to know. Cardiovasc. Intervent. Radiol. 2017, 40, 1131–1140. [Google Scholar] [CrossRef]
- Thanik, V.D.; Chang, C.C.; Zoumalan, R.A.; Lerman, O.Z.; Allen, R.J., Jr.; Nguyen, P.D.; Warren, S.M.; Coleman, S.R.; Hazen, A. A novel mouse model of cutaneous radiation injury. Plast. Reconstr. Surg. 2011, 127, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Yoshimoto, H.; Hirano, A.; Akita, S. Wound healing and angiogenesis through combined use of a vascularized tissue flap and adipose-derived stem cells in a rat hindlimb irradiated ischemia model. Plast. Reconstr. Surg. 2016, 137, 1486–1497. [Google Scholar] [CrossRef]
- Borrelli, M.R.; Shen, A.H.; Lee, G.K.; Momeni, A.; Longaker, M.T.; Wan, D.C. Radiation-induced skin fibrosis: Pathogenesis, current treatment options, and emerging therapeutics. Ann. Plast. Surg. 2019, 83, S59–S64. [Google Scholar] [CrossRef]
- Yarnold, J.; Brotons, M.C. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 2010, 97, 149–161. [Google Scholar] [CrossRef]
- Kimura, H.; Wu, N.Z.; Dodge, R.; Spencer, D.P.; Klitzman, B.M.; McIntyre, T.M.; Dewhirst, M.W. Inhibition of radiation-induced up-regulation of leukocyte adhesion to endothelial cells with the platelet-activating factor inhibitor, BN52021. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 627–633. [Google Scholar] [CrossRef]
- Phulpin, B.; Gangloff, P.; Tran, N.; Bravetti, P.; Merlin, J.L.; Dolivet, G. Rehabilitation of irradiated head and neck tissues by autologous fat transplantation. Plast. Reconstr. Surg. 2009, 123, 1187–1197. [Google Scholar] [CrossRef]
- Lin, K.Y.; Patterson, J.W.; Simmons, J.; Long, M.D.; Schultz, R.O.; Amiss, L.R.; Molloy, J.A.; Kelly, M.D. Effects of external beam irradiation on the TRAM flap: An experimental model. Plast. Reconstr. Surg. 2001, 107, 1190–1197, discussion 1198–1200. [Google Scholar] [CrossRef]
- Özyazgan, İ.; Baykan, H. The effect of TENS on random pattern flap survival in nicotinized rats. Ann. Plast. Surg. 2015, 74, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, M.; Jiang, Z.; Liu, Y.; Lu, L.; Gong, X. Visualized identification of the maximal surgical delay effect in a rat flap model. Wound Repair Regen. 2019, 27, 39–48. [Google Scholar] [CrossRef]
- Giunta, R.E.; Holzbach, T.; Taskov, C.; Holm, P.S.; Brill, T.; Busch, R.; Gansbacher, B.; Biemer, E. Prediction of flap necrosis with laser induced indocyanine green fluorescence in a rat model. Br. J. Plast. Surg. 2005, 58, 695–701. [Google Scholar] [CrossRef]
- Chin, M.S.; Chappell, A.G.; Giatsidis, G.; Perry, D.J.; Lujan-Hernandez, J.; Haddad, A.; Matsumine, H.; Orgill, D.P.; Lalikos, J.F. Hyperspectral imaging provides early prediction of random axial flap necrosis in a preclinical model. Plast. Reconstr. Surg. 2017, 139, 1285e–1290e. [Google Scholar] [CrossRef] [PubMed]
- Müller-Seubert, W.; Roth, S.; Hauck, T.; Arkudas, A.; Horch, R.E.; Ludolph, I. Novel imaging methods reveal positive impact of topical negative pressure application on tissue perfusion in an in vivo skin model. Int. Wound J. 2021, 18, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Schmauss, D.; Beier, J.P.; Eisenhardt, S.U.; Horch, R.E.; Momeni, A.; Rab, M.; Rieck, B.; Rieger, U.; Schaefer, D.J.; Schmidt, V.J.; et al. The “safe” flap—Preoperative perforator-mapping and intraoperative perfusion assessment to reduce flap-associated morbidity—Consensus statement of the German speaking working group for microsurgery of the peripheral nerves and vessels. Handchir. Mikrochir. Plast. Chir. 2019, 51, 410–417. [Google Scholar] [PubMed]
- Landsman, A.S.; Barnhart, D.; Sowa, M. Near-infrared spectroscopy imaging for assessing skin and wound oxygen perfusion. Clin. Podiatr. Med. Surg. 2018, 35, 343–355. [Google Scholar] [CrossRef]
- Kuefner, M.A.; Grudzenski, S.; Schwab, S.A.; Wiederseiner, M.; Heckmann, M.; Bautz, W.; Lobrich, M.; Uder, M. DNA double-strand breaks and their repair in blood lymphocytes of patients undergoing angiographic procedures. Investig. Radiol. 2009, 44, 440–446. [Google Scholar] [CrossRef]
- Müller-Seubert, W.; Horch, R.E.; Schmidt, V.F.; Ludolph, I.; Schmitz, M.; Arkudas, A. Retrospective analysis of free temporoparietal fascial flap for defect reconstruction of the hand and the distal upper extremity. Arch. Orthop. Trauma Surg. 2021, 141, 165–171. [Google Scholar] [CrossRef]
- Horch, R.E.; Ludolph, I.; Arkudas, A. Reconstruction of oncological defects of the perianal region. Chirurg 2021, 92, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Horch, R.E.; Ludolph, I.; Cai, A.; Weber, K.; Grützmann, R.; Arkudas, A. Interdisciplinary surgical approaches in vaginal and perineal reconstruction of advanced rectal and anal female cancer patients. Front. Oncol. 2020, 10, 719. [Google Scholar] [CrossRef]
- Schellerer, V.S.; Bartholomé, L.; Langheinrich, M.C.; Grützmann, R.; Horch, R.E.; Merkel, S.; Weber, K. Donor site morbidity of patients receiving vertical rectus abdominis myocutaneous flap for perineal, vaginal or inguinal reconstruction. World J. Surg. 2021, 45, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, B.B.; Bagavathiappan, S.; Jayakumar, T.; Philip, J. Medical applications of infrared thermography: A review. Infrared Phys. Technol. 2012, 55, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, J.F. The pathophysiology of skin flap circulation. The delay phenomenon. Plast. Reconstr. Surg. 1974, 54, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.C.; Taylor, G.I. The delay phenomenon: The story unfolds. Plast. Reconstr. Surg. 1999, 104, 2079–2091. [Google Scholar] [CrossRef]
- Heitland, A.S.; Markowicz, M.; Koellensperger, E.; Schoth, F.; Feller, A.M.; Pallua, N. Duplex ultrasound imaging in free transverse rectus abdominis muscle, deep inferior epigastric artery perforator, and superior gluteal artery perforator flaps: Early and long-term comparison of perfusion changes in free flaps following breast reconstruction. Ann. Plast. Surg. 2005, 55, 117–121. [Google Scholar]
- McBride, W.H.; Schaue, D. Radiation-induced tissue damage and response. J. Pathol. 2020, 250, 647–655. [Google Scholar] [CrossRef]
- Martin, M.; Lefaix, J.; Delanian, S. TGF-beta1 and radiation fibrosis: A master switch and a specific therapeutic target? Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 277–290. [Google Scholar] [CrossRef]
- Chin, M.S.; Freniere, B.B.; Lo, Y.C.; Saleeby, J.H.; Baker, S.P.; Strom, H.M.; Ignotz, R.A.; Lalikos, J.F.; Fitzgerald, T.J. Hyperspectral imaging for early detection of oxygenation and perfusion changes in irradiated skin. J. Biomed. Opt. 2012, 17, 026010. [Google Scholar] [CrossRef]
- Virolainen, P.; Aitasalo, K. Effect of postoperative irradiation on free skin flaps: An experimental study in rats. Scand J. Plast. Reconstr. Surg. Hand Surg. 2002, 36, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Sumi, Y.; Ueda, M.; Oka, T.; Torii, S. Effects of irradiation of skin flaps. J. Oral Maxillofac. Surg. 1984, 42, 447–452. [Google Scholar] [CrossRef]
- Thanik, V.D.; Hang, C.C.; Lerman, O.Z.; Greives, M.R.; Le, H.; Warren, S.M.; Schneider, R.J.; Formenti, S.C.; Saadeh, P.B.; Levine, J.P. Cutaneous low-dose radiation increases tissue vascularity through upregulation of angiogenic and vasculogenic pathways. J. Vasc. Res. 2010, 47, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Heissig, B.; Rafii, S.; Akiyama, H.; Ohki, Y.; Sato, Y.; Rafael, T.; Zhu, Z.; Hicklin, D.J.; Okumura, K.; Ogawa, H.; et al. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J. Exp. Med. 2005, 202, 739–750. [Google Scholar] [CrossRef]
- Ministro, A.; de Oliveira, P.; Nunes, R.J.; Dos Santos Rocha, A.; Correia, A.; Carvalho, T.; Rino, J.; Faísca, P.; Becker, J.D.; Goyri-O’Neill, J.; et al. Low-dose ionizing radiation induces therapeutic neovascularization in a pre-clinical model of hindlimb ischemia. Cardiovasc. Res. 2017, 113, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Kermani, P.; Leclerc, G.; Martel, R.; Fareh, J. Effect of ionizing radiation on thymidine uptake, differentiation, and VEGFR2 receptor expression in endothelial cells: The role of VEGF(165). Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 213–220. [Google Scholar] [CrossRef]
- Luginbuhl, A.; Modest, M.; Yan, K.; Curry, J.; Heffelfinger, R. Novel irradiated axial rotational flap model in the rodent. JAMA Facial Plast. Surg. 2013, 15, 344–348. [Google Scholar] [CrossRef][Green Version]
- Geis, S.; Schreml, S.; Lamby, P.; Obed, A.; Jung, E.M.; Nerlich, M.; Babilas, P.; Szeimies, R.M.; Prantl, L. Postoperative assessment of free skin flap viability by transcutaneous pO2 measurement using dynamic phosphorescence imaging. Clin. Hemorheol. Microcirc. 2009, 43, 11–18. [Google Scholar] [CrossRef]
- Pan, X.; Chen, G.; Wu, P.; Han, C.; Ho, J.K. Skin perfusion pressure as a predictor of ischemic wound healing potential. Biomed. Rep. 2018, 8, 330–334. [Google Scholar] [CrossRef]









| Group. | Irradiation Regimen |
|---|---|
| Group 1 | None=control |
| Group 2 | Single stage postoperative irradiation 20 Gy 1 day after flap harvest |
| Group 3 | Fractional postoperative irradiation 12 Gy each day 1, 2 and 3 after flap harvest |
| Group 4 | Single stage preoperative irradiation 20 Gy 28 days before flap harvest |
| Group 5 | Fractional preoperative irradiation 12 Gy each day 28, 27 and 26 before flap harvest |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller-Seubert, W.; Ostermaier, P.; Horch, R.E.; Distel, L.; Frey, B.; Cai, A.; Arkudas, A. Intra- and Early Postoperative Evaluation of Malperfused Areas in an Irradiated Random Pattern Skin Flap Model Using Indocyanine Green Angiography and Near-Infrared Reflectance-Based Imaging and Infrared Thermography. J. Pers. Med. 2022, 12, 237. https://doi.org/10.3390/jpm12020237
Müller-Seubert W, Ostermaier P, Horch RE, Distel L, Frey B, Cai A, Arkudas A. Intra- and Early Postoperative Evaluation of Malperfused Areas in an Irradiated Random Pattern Skin Flap Model Using Indocyanine Green Angiography and Near-Infrared Reflectance-Based Imaging and Infrared Thermography. Journal of Personalized Medicine. 2022; 12(2):237. https://doi.org/10.3390/jpm12020237
Chicago/Turabian StyleMüller-Seubert, Wibke, Patrick Ostermaier, Raymund E. Horch, Luitpold Distel, Benjamin Frey, Aijia Cai, and Andreas Arkudas. 2022. "Intra- and Early Postoperative Evaluation of Malperfused Areas in an Irradiated Random Pattern Skin Flap Model Using Indocyanine Green Angiography and Near-Infrared Reflectance-Based Imaging and Infrared Thermography" Journal of Personalized Medicine 12, no. 2: 237. https://doi.org/10.3390/jpm12020237
APA StyleMüller-Seubert, W., Ostermaier, P., Horch, R. E., Distel, L., Frey, B., Cai, A., & Arkudas, A. (2022). Intra- and Early Postoperative Evaluation of Malperfused Areas in an Irradiated Random Pattern Skin Flap Model Using Indocyanine Green Angiography and Near-Infrared Reflectance-Based Imaging and Infrared Thermography. Journal of Personalized Medicine, 12(2), 237. https://doi.org/10.3390/jpm12020237











