Effects of Tumor-Rib Distance and Dose-Dependent Rib Volume on Radiation-Induced Rib Fractures in Patients with Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Radiotherapy
2.3. Follow-Up and RIRF Assessment
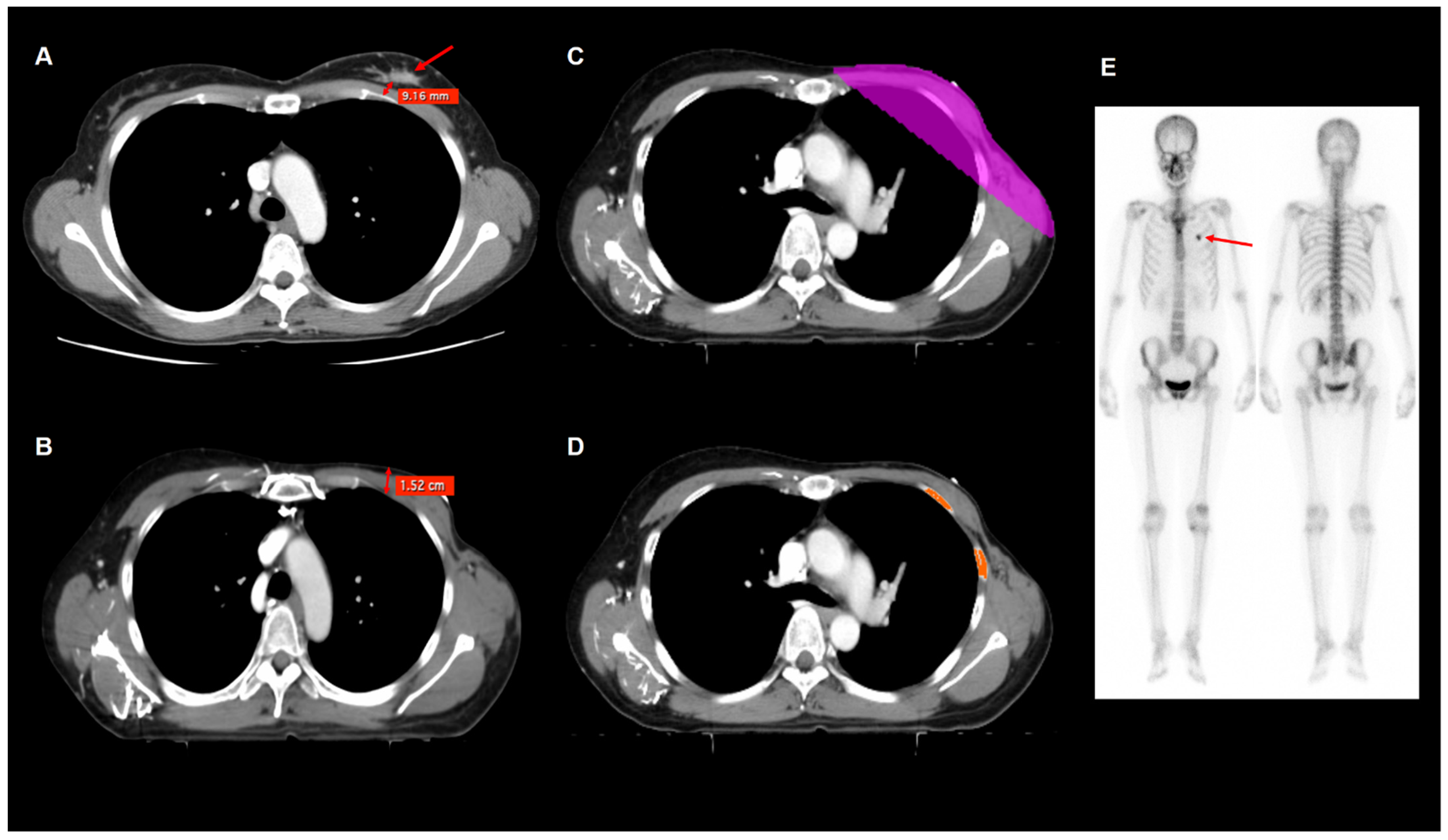
2.4. Imaging Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparisons of Imaging Parameters
3.3. Risk Factors for RIRF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Breast Cancer (Version 5.2020). Available online: https://www2.tri-kobe.org/nccn/guideline/breast/english/breast.pdf (accessed on 14 September 2021).
- Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haviland, J.S.; Owen, J.R.; Dewar, J.A.; Agrawal, R.K.; Barrett, J.; Barrett-Lee, P.J.; Dobbs, H.J.; Hopwood, P.; Lawton, P.A.; Magee, B.J.; et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013, 14, 1086–1094. [Google Scholar] [CrossRef]
- Robinson, P.J.; Bell, R.J.; Zecena Morales, C.S.; Fradkin, P.; Davis, S.R. Minimal-trauma fracture in women with breast cancer surviving for at least 5 years from diagnosis. Osteoporos. Int. 2015, 26, 795–800. [Google Scholar] [CrossRef]
- Pierce, S.M.; Recht, A.; Lingos, T.I.; Abner, A.; Vicini, F.; Silver, B.; Herzog, A.; Harris, J.R. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 915–923. [Google Scholar] [CrossRef]
- Harris, S.R. Differentiating the Causes of Spontaneous Rib Fracture After Breast Cancer. Clin. Breast Cancer 2016, 16, 431–436. [Google Scholar] [CrossRef]
- Overgaard, M. Spontaneous radiation-induced rib fractures in breast cancer patients treated with postmastectomy irradiation. A clinical radiobiological analysis of the influence of fraction size and dose-response relationships on late bone damage. Acta Oncol. 1988, 27, 117–122. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, J.S.; Kim, K.; Shin, K.H. Spontaneous rib fractures after breast cancer treatment based on bone scans: Comparison of conventional versus hypofractionated radiotherapy. Clin. Breast Cancer 2021, 21, e80–e87. [Google Scholar] [CrossRef]
- Kim, H.W.; Won, K.S.; Zeon, S.K.; Kim, J.H. Radiation induced rib fractures on bone scan after breast cancer surgery and radiation therapy. Nucl. Med. Mol. Imaging 2009, 43, 287–293. [Google Scholar]
- Pettersson, N.; Nyman, J.; Johansson, K.A. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy of non-small cell lung cancer: A dose- and volume-response analysis. Radiother. Oncol. 2009, 91, 360–368. [Google Scholar] [CrossRef]
- Park, W.; Huh, S.J.; Yang, J.H.; Nam, S.J.; Kim, J.H.; Choi, J.Y.; Woo, S.Y.; Kim, H.Y.; Noh, J.M.; Ryu, J.I. The implication of hot spots on bone scans within the irradiated field of breast cancer patients treated with mastectomy followed by radiotherapy. Ann. Nucl. Med. 2008, 22, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Asai, K.; Shioyama, Y.; Nakamura, K.; Sasaki, T.; Ohga, S.; Nonoshita, T.; Yoshitake, T.; Ohnishi, K.; Terashima, K.; Matsumoto, K.; et al. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy: Risk factors and dose-volume relationship. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Dalinka, M.K.; Edeiken, J.; Finkelstein, J.B. Complications of radiation therapy: Adult bone. Semin. Roentgenol. 1974, 9, 29–40. [Google Scholar] [CrossRef]
- Welsh, J.; Thomas, J.; Shah, D.; Allen, P.K.; Wei, X.; Mitchell, K.; Gao, S.; Balter, P.; Komaki, R.; Chang, J.Y. Obesity increases the risk of chest wall pain from thoracic stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 91–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentile, M.S.; Usman, A.A.; Neuschler, E.I.; Sathiaseelan, V.; Hayes, J.P.; Small, W., Jr. Contouring guidelines for the axillary lymph nodes for the delivery of radiation therapy in breast cancer: Evaluation of the RTOG breast cancer atlas. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 257–265. [Google Scholar] [CrossRef]
- Schroeder, E.; Valdez, C.; Krauthamer, A.; Khati, N.; Rasmus, J.; Amdur, R.; Brindle, K.; Sarani, B. Average chest wall thickness at two anatomic locations in trauma patients. Injury 2013, 44, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Lin, T.Y.; Ou, J.C.; Ong, J.R.; Ma, H.P. Risk values of weight and body mass index for chest wall thickness in patients requiring needle thoracostomy decompression. Emerg. Med. Int. 2020, 2020, 2070157. [Google Scholar] [CrossRef] [PubMed]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [Green Version]
- Hopewell, J.W. Radiation-therapy effects on bone density. Med. Pediatr. Oncol. 2003, 41, 208–211. [Google Scholar] [CrossRef]
- Pacheco, R.; Stock, H. Effects of radiation on bone. Curr. Osteoporos. Rep. 2013, 11, 299–304. [Google Scholar] [CrossRef]
- Sams, A. The effects of 2000r of X-rays on the acid and alkaline phsphatase of mouse tibiae. Int. J. Radiat. Biol. 1966, 10, 123–140. [Google Scholar]
- Gyorkey, J.; Pollock, F.J. Radiation necrosis of the ossicles. AMA Arch. Otolaryngol. 1960, 71, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, N.E.; Cai, J.; Biedermann, G.B.; Yang, W.; Benedict, S.H.; Sheng, K.; Schefter, T.E.; Kavanagh, B.D.; Larner, J.M. Chest wall volume receiving >30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Leonard, C.E.; Wang, Y.; Asmar, L.; Lei, R.Y.; Howell, K.T.; Henkenberns, P.L.; Johnson, T.K.; Hobart, T.L.; Tole, S.P.; Kercher, J.M.; et al. A prospective Phase III trial evaluating patient self-reported pain and cosmesis in accelerated partial breast irradiation utilizing 3-D versus intensity-modulated radiotherapy. Cancer Med. 2021, 10, 7089–7100. [Google Scholar] [CrossRef]
- Gortman, A.M.; Aherne, N.J.; Amalaseelan, J.; Last, A.; Westhuyzen, J.; Chamberlain, L.; Shakespeare, T.P. Long-term outcomes of patients with conserved breast cancer treated with adjuvant hypofractionated prone breast intensity-modulated radiation therapy. J. Med. Imaging Radiat. Oncol. 2020, 64, 845–851. [Google Scholar] [CrossRef]
- Testolin, A.; Ciccarelli, S.; Vidano, G.; Avitabile, R.; Dusi, F.; Alongi, F. Deep inspiration breath-hold intensity modulated radiation therapy in a large clinical series of 239 left-sided breast cancer patients: A dosimetric analysis of organs at risk doses and clinical feasibility from a single center experience. Br. J. Radiol. 2019, 92, 20190150. [Google Scholar] [CrossRef]
- Pandeli, C.; Smyth, L.M.L.; David, S.; See, A.W. Dose reduction to organs at risk with deep-inspiration breath-hold during right breast radiotherapy: A treatment planning study. Radiat. Oncol. 2019, 14, 223. [Google Scholar] [CrossRef] [Green Version]
- Oechsner, M.; Düsberg, M.; Borm, K.J.; Combs, S.E.; Wilkens, J.J.; Duma, M.N. Deep inspiration breath-hold for left-sided breast irradiation: Analysis of dose-mass histograms and the impact of lung expansion. Radiat. Oncol. 2019, 14, 109. [Google Scholar] [CrossRef]
- Vargo, J.A.; Beriwal, S. RTOG chest wall contouring guidelines for post-mastectomy radiation therapy: Is it evidence-based? Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 266–267. [Google Scholar] [CrossRef]

| Characteristics | Number of Patients (%) | |
|---|---|---|
| Age (years) | 49 (25–83) * | |
| Weight (kg) | 58 (38–90) * | |
| Body mass index (kg/m2) | 23.4 (15.8–35.2) * | |
| Obesity | Underweight/normal | 337 (66.1%) |
| Overweight/obesity | 173 (33.9%) | |
| Menopausal status | Premenopausal | 268 (52.5%) |
| Postmenopausal | 242 (47.5%) | |
| Bone mineral density | Normal | 395 (77.5%) |
| Osteopenia | 82 (16.1%) | |
| Osteoporosis | 33 (6.5%) | |
| Histopathology | Ductal carcinoma in situ | 56 (11.0%) |
| Intraductal carcinoma | 430 (84.3%) | |
| Intralobular carcinoma | 10 (2.0%) | |
| Mucinous carcinoma | 6 (1.2%) | |
| Papillary carcinoma | 4 (0.8%) | |
| Others | 4 (0.8%) | |
| T stage | Tis, T1–T2 | 493 (96.7%) |
| T3–T4 | 17 (3.3%) | |
| N stage | N0 | 375 (73.5%) |
| N1–N3 | 135 (26.5%) | |
| Surgery type | Breast-conserving surgery | 467 (91.6%) |
| Total mastectomy | 43 (8.4%) | |
| Chemotherapy | No | 189 (37.1%) |
| Yes | 321 (62.9%) | |
| Hormone therapy | No | 115 (22.5%) |
| Tamoxifen | 243 (47.6%) | |
| Aromatase inhibitor | 152 (29.8%) | |
| Trastuzumab | No | 444 (87.1%) |
| Yes | 66 (12.9%) | |
| Radiotherapy technique | Three-dimensional conformal radiotherapy | 501 (98.2%) |
| Volumetric modulated arc therapy | 9 (1.8%) | |
| Radiation field | Whole breast | 437 (85.7%) |
| Whole breast/chest wall + regional nodes | 73 (14.3%) | |
| Tumor bed boost | No | 65 (12.7%) |
| Yes | 445 (87.3%) | |
| Tumor-rib distance (cm) | 1.3 (0.0–6.7) * | |
| Post-operative chest wall thickness (cm) | 3.1 (0.6–8.0) * | |
| V20 (cm3) | 35.6 (5.2–159.4) * | |
| V30 (cm3) | 32.1 (4.0–132.5) * | |
| V40 (cm3) | 28.8 (2.7–91.2) * | |
| V45 (cm3) | 25.1 (1.4–64.3) * | |
| V50 (cm3) | 9.8 (0.0–57.4) * | |
| Imaging Parameters | RIRF | Radiation Field | ||||
| No RIRF (n = 418) | RIRF (n = 92) | p-Value | Whole Breast (n = 437) | Whole Breast/Chest Wall + Regional Nodes (n = 73) | p-Value | |
| Tumor-rib distance (cm) | 1.5 ± 0.9 | 1.2 ± 0.6 | <0.001 | 1.5 ± 0.8 | 1.4 ± 0.7 | 0.312 |
| Post-operative chest wall thickness (cm) | 3.3 ± 1.2 | 3.1 ± 1.3 | 0.072 | 3.3 ± 1.2 | 2.8 ± 1.5 | 0.008 |
| V20 (cm3) | 38.3 ± 15.8 | 44.3 ± 24.6 | 0.026 | 36.2 ± 13.3 | 58.5 ± 27.5 | <0.001 |
| V30 (cm3) | 33.1 ± 11.8 | 38.0 ± 19.0 | 0.021 | 31.6 ± 10.1 | 48.5 ± 20.5 | <0.001 |
| V40 (cm3) | 29.5 ± 9.9 | 32.4 ± 13.3 | 0.044 | 28.4 ± 9.0 | 39.5 ± 13.9 | <0.001 |
| V45 (cm3) | 25.5 ± 8.7 | 27.9 ± 11.6 | 0.068 | 24.8 ± 8.0 | 33.0 ± 12.9 | <0.001 |
| V50 (cm3) | 11.0 ± 9.2 | 13.1 ± 10.7 | 0.078 | 10.4 ± 8.2 | 17.5 ± 13.7 | <0.001 |
| Imaging parameters | Radiotherapy technique | Tumor bed boost | ||||
| 3D-CRT (n = 501) | VMAT (n = 9) | p-value | No (n = 65) | Yes (n = 445) | p-value | |
| Tumor-rib distance (cm) | 1.5 ± 0.8 | 1.2 ± 0.3 | 0.298 | 1.5 ± 0.8 | 1.5 ± 0.8 | 0.802 |
| Post-operative chest wall thickness (cm) | 3.3 ± 1.2 | 1.5 ± 1.2 | 0.383 | 2.7 ± 1.5 | 3.4 ± 1.2 | 0.002 |
| V20 (cm3) | 38.8 ± 17.3 | 71.7 ± 17.9 | <0.001 | 45.1 ± 27.9 | 38.5 ± 15.9 | 0.305 |
| V30 (cm3) | 33.7 ± 13.3 | 48.9 ± 15.9 | 0.001 | 38.7 ± 21.3 | 33.3 ± 11.8 | 0.212 |
| V40 (cm3) | 29.9 ± 10.5 | 35.8 ± 15.1 | 0.320 | 32.8 ± 14.8 | 29.6 ± 9.8 | 0.259 |
| V45 (cm3) | 25.9 ± 9.2 | 30.2 ± 15.7 | 0.776 | 27.6 ± 12.3 | 25.7 ± 8.8 | 0.598 |
| V50 (cm3) | 11.3 ± 9.4 | 17.4 ± 14.4 | 0.099 | 13.9 ± 13.0 | 11.0 ± 8.8 | 0.230 |
| Variables | p-Value | Hazard Ratio | 95% Confidence Interval | |
|---|---|---|---|---|
| Age (<60 years vs. ≥60 years) | 0.215 | 1.37 | 0.83–2.25 | |
| Weight (<50 kg vs. ≥50 kg) | 0.123 | 1.68 | 0.87–3.23 | |
| Obesity (underweight/normal vs. overweight/obesity) | 0.461 | 0.85 | 0.54–1.32 | |
| Menopausal status (pre- vs. post-) | 0.264 | 1.26 | 0.84–1.90 | |
| Bone mineral density (normal vs.) | Osteopenia | 0.410 | 1.25 | 0.73–2.14 |
| Osteoporosis | 0.006 | 2.44 | 1.28–4.62 | |
| T stage (Tis, T1–T2 vs. T3–T4) | 0.435 | 1.49 | 0.55–4.06 | |
| N stage (N0 vs. N1–N3) | 0.072 | 1.49 | 0.97–2.29 | |
| Surgery type (breast-conserving surgery vs. total mastectomy) | 0.004 | 2.33 | 1.32–4.11 | |
| Chemotherapy (no vs. yes) | 0.462 | 1.17 | 0.76–1.80 | |
| Hormone therapy (no vs.) | Tamoxifen | 0.218 | 0.72 | 0.42–1.22 |
| Aromatase inhibitor | 0.714 | 1.11 | 0.64–1.90 | |
| Trastuzumab (no vs. yes) | 0.184 | 0.61 | 0.30–1.26 | |
| Radiotherapy technique (3D-CRT vs. VMAT) | 0.0820 | 2.89 | 0.91–9.20 | |
| Radiation field (whole breast vs. whole breast/chest wall + regional nodes) | <0.001 | 2.53 | 1.60–4.01 | |
| Tumor bed boost (no vs. yes) | 0.576 | 0.85 | 0.47–1.52 | |
| Tumor-rib distance (<1.3 cm vs. ≥1.3 cm) | <0.001 | 0.43 | 0.28–0.67 | |
| Post-operative chest wall thickness (<3.6 cm vs. ≥3.6 cm) | 0.033 | 0.61 | 0.38–0.96 | |
| V20 (<45.1 cm3 vs. ≥45.1 cm3) | 0.008 | 2.05 | 1.35–3.12 | |
| V30 (<41.7 cm3 vs. ≥41.7 cm3) | <0.001 | 2.12 | 1.36–3.29 | |
| V40 (<40.8 cm3 vs. ≥40.8 cm3) | <0.001 | 2.95 | 1.84–4.72 | |
| V45 (<28.2 cm3 vs. ≥28.2 cm3) | 0.011 | 1.70 | 1.12–2.56 | |
| V50 (<10.2 cm3 vs. ≥10.2 cm3) | 0.004 | 1.86 | 1.23–2.84 |
| Variables | Model with V20 | Model with V30 | Model with V40 | Model with V45 | Model with V50 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | ||
| Bone mineral density | Osteopenia | 0.396 | 1.26 (0.74–2.17) | 0.406 | 1.26 (0.73–2.16) | 0.365 | 1.29 (0.75–2.21) | 0.244 | 1.39 (0.80–2.44) | 0.396 | 1.26 (0.74–2.17) |
| Osteoporosis | 0.004 | 2.64 (1.37–5.11) | 0.003 | 2.77 (1.43–5.37) | 0.002 | 2.91 (1.50–5.62) | 0.002 | 2.87 (1.47–5.26) | 0.005 | 2.57 (1.33–4.95) | |
| Surgery type | 0.562 | 1.22 (0.62–2.42) | 0.538 | 1.24 (0.63–2.43) | 0.459 | 1.29 (0.66–2.54) | 0.423 | 1.32 (0.67–2.60) | 0.645 | 1.18 (0.59–2.34) | |
| Radiation field | 0.049 | 1.79 (1.00–3.20) | 0.071 | 1.73 (0.95–3.13) | 0.123 | 1.59 (0.88–2.86) | 0.023 | 1.91 (1.09–3.32) | 0.005 | 2.18 (1.26–3.78) | |
| Tumor-rib distance | 0.001 | 0.46 (0.29–0.74) | 0.001 | 0.46 (0.29–0.73) | <0.001 | 0.45 (0.28–0.71) | 0.001 | 0.46 (0.29–0.73) | 0.002 | 0.48 (0.30–0.77) | |
| Post-operative chest wall thickness | 0.374 | 0.80 (0.49–1.31) | 0.305 | 0.78 (0.48–1.26) | 0.478 | 0.84 (0.51–1.37) | 0.457 | 0.83 (0.51–.35) | 0.517 | 0.85 (0.52–1.39) | |
| V20 | 0.029 | 1.68 (1.06–2.69) | - | - | - | - | - | - | - | - | |
| V30 | - | - | 0.035 | 1.73 (1.04–2.89) | - | - | - | - | - | - | |
| V40 | - | - | - | - | <0.001 | 2.51 (1.47–4.29) | - | - | - | - | |
| V45 | - | - | - | - | - | - | 0.028 | 1.65 (1.06–2.58) | - | - | |
| V50 | - | - | - | - | - | - | - | - | 0.039 | 1.58 (1.02–2.45) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.M.; Lee, J.W.; Kim, W.C.; Min, C.K.; Kim, E.S.; Jo, I.Y. Effects of Tumor-Rib Distance and Dose-Dependent Rib Volume on Radiation-Induced Rib Fractures in Patients with Breast Cancer. J. Pers. Med. 2022, 12, 240. https://doi.org/10.3390/jpm12020240
Lee SM, Lee JW, Kim WC, Min CK, Kim ES, Jo IY. Effects of Tumor-Rib Distance and Dose-Dependent Rib Volume on Radiation-Induced Rib Fractures in Patients with Breast Cancer. Journal of Personalized Medicine. 2022; 12(2):240. https://doi.org/10.3390/jpm12020240
Chicago/Turabian StyleLee, Sang Mi, Jeong Won Lee, Woo Chul Kim, Chul Kee Min, Eun Seog Kim, and In Young Jo. 2022. "Effects of Tumor-Rib Distance and Dose-Dependent Rib Volume on Radiation-Induced Rib Fractures in Patients with Breast Cancer" Journal of Personalized Medicine 12, no. 2: 240. https://doi.org/10.3390/jpm12020240
APA StyleLee, S. M., Lee, J. W., Kim, W. C., Min, C. K., Kim, E. S., & Jo, I. Y. (2022). Effects of Tumor-Rib Distance and Dose-Dependent Rib Volume on Radiation-Induced Rib Fractures in Patients with Breast Cancer. Journal of Personalized Medicine, 12(2), 240. https://doi.org/10.3390/jpm12020240






