IL4Rα and IL13Rα1 Are Involved in the Development of Human Gallbladder Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Gallbladder Carcinomas
2.2. Immunohistochemical Staining and Scoring
2.3. Reagents
2.4. Cell Culture
2.5. Transfection
2.6. Water-Soluble Tetrazolium Salt-1 (WST-1) Assay
2.7. Morphological Change Analysis
2.8. Cell Counting Assay
2.9. Colony Formation Assay
2.10. Annexin V Staining Analysis
2.11. Caspase-3/7 Activity Assay
2.12. Mitochondria Depolarization Assay
2.13. Cell Cycle Analysis
2.14. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
2.15. Comet Assay
2.16. Separation of Cytosol/Nuclear Proteins
2.17. Western Blotting
2.18. Statistical Analysis
3. Results
3.1. Expression of IL4Rα and IL13Rα1 in Gallbladder Carcinomas and Their Association with Clinicopathologic Variables
3.2. Prognostic Significance of the Expressions of IL4Rα and IL13Rα1 in Gallbladder Carcinomas
3.3. Knockdown of IL4Rα or IL13Rα1 Displays Anti-Proliferative Activity in SNU308 Cells
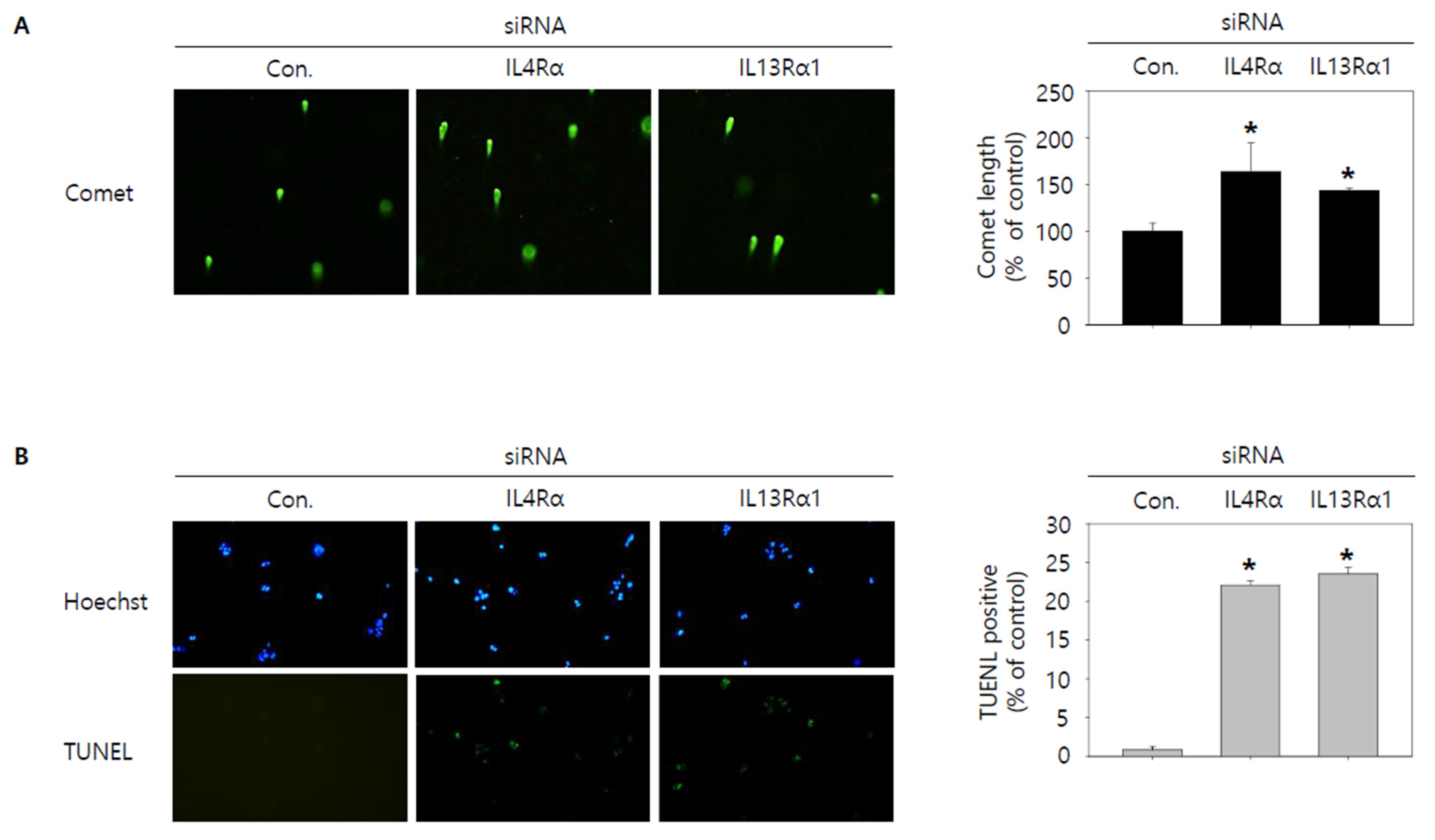
3.4. Knockdown of IL4Rα or IL13Rα1 Induces Apoptosis in SNU308 Cells via Regulation of JAK2/FOXO3 Pathways
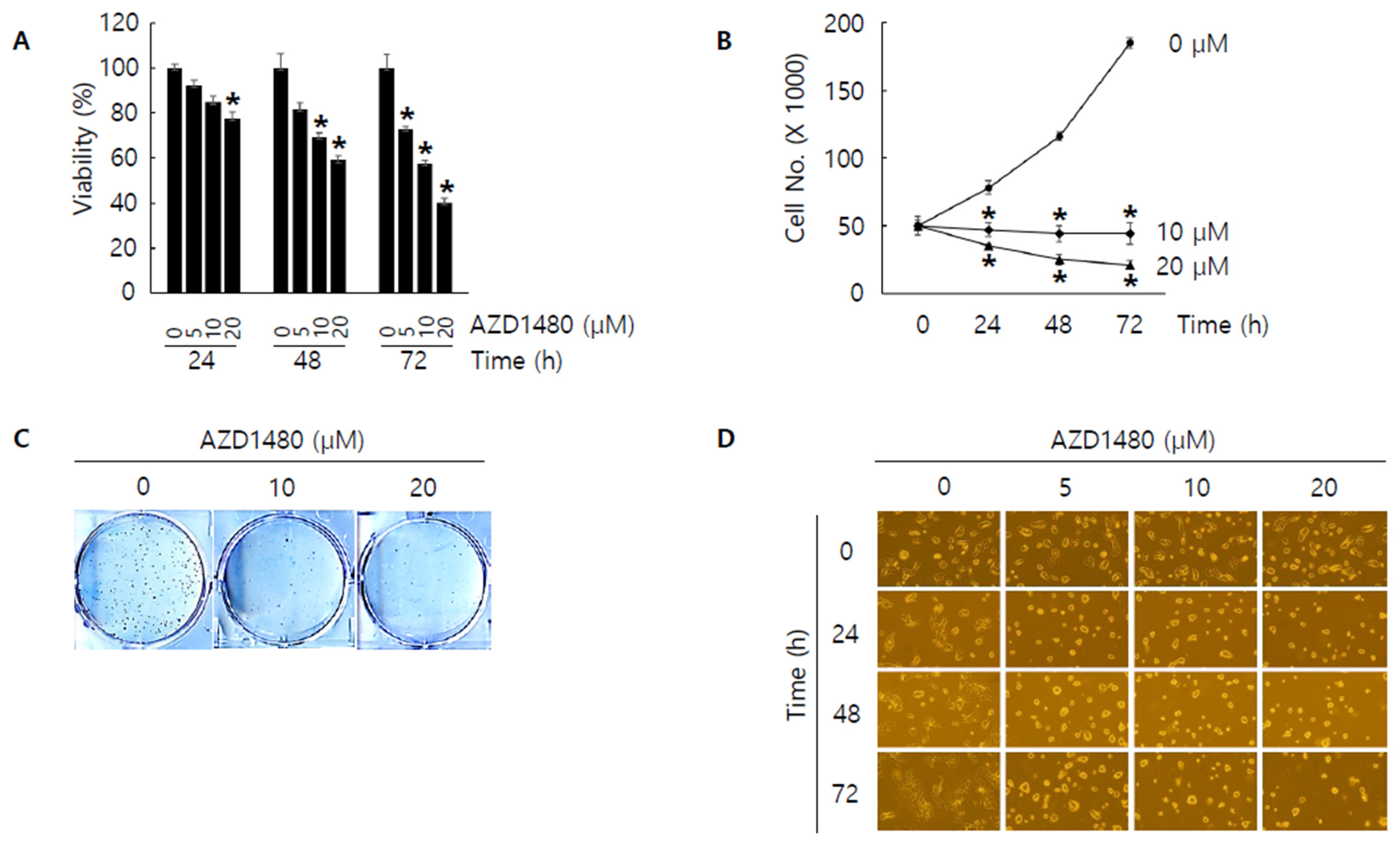
3.5. AZD1480 Displays Anti-Proliferative Activity in SNU308 Cells
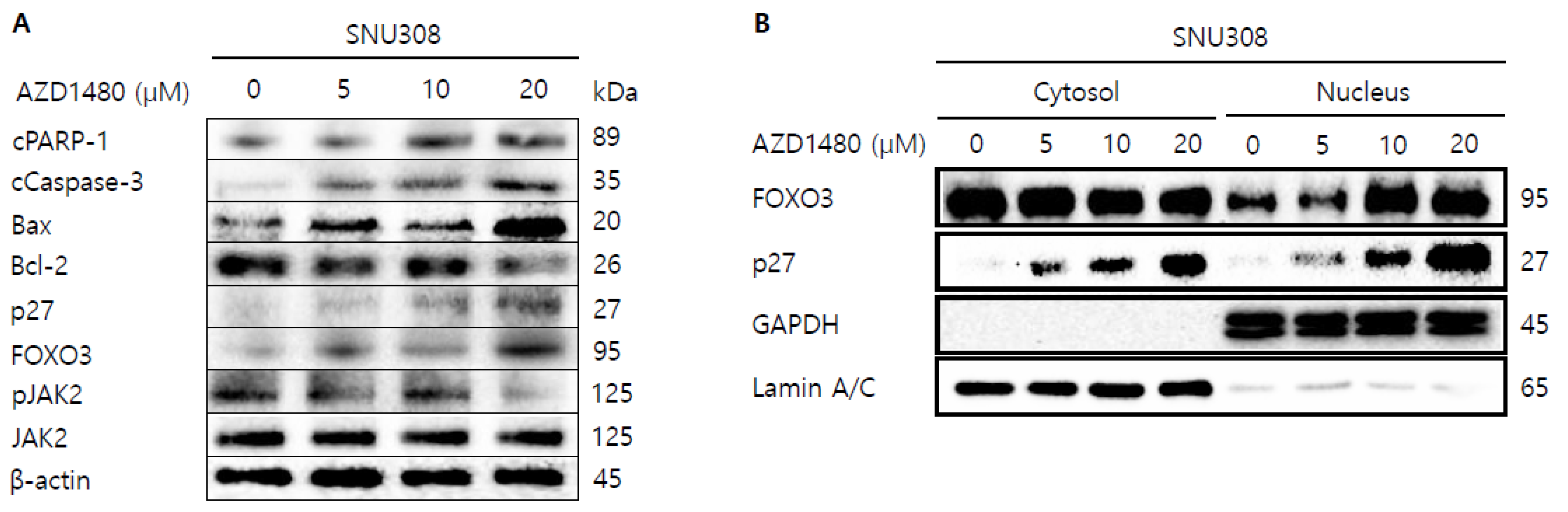
3.6. AZD1480 Induces Apoptosis in SNU308 Cells via Regulation of JAK2/FOXO3 Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Catalano, O.A.; Sahani, D.V.; Kalva, S.P.; Cushing, M.S.; Hahn, P.F.; Brown, J.J.; Edelman, R.R. MR imaging of the gallbladder: A pictorial essay. Radiographics 2008, 28, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Randi, G.; Franceschi, S.; La Vecchia, C. Gallbladder cancer worldwide: Geographical distribution and risk factors. Int. J. Cancer 2006, 118, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Thandra, K.C.; Barsouk, A. Epidemiology of gallbladder cancer. Clin. Exp. Hepatol. 2019, 5, 93–102. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, K.L.; Gupta, A.; Yadav, A.; Kumar, A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J. Gastroenterol. 2017, 23, 3978–3998. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wang, S.; Jin, L.; Weng, M.; Zhou, D.; Wang, J.; Tang, Z.; Quan, Z. Long non-coding RNA GBCDRlnc1 induces chemoresistance of gallbladder cancer cells by activating autophagy. Mol. Cancer 2019, 18, 82. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Hu, Y.; Li, Y.; Shao, R.; Liu, F.; Liu, Y. Overview of current targeted therapy in gallbladder cancer. Signal Transduct. Target. Ther. 2020, 5, 230. [Google Scholar] [CrossRef]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef]
- Dhanda, S.K.; Gupta, S.; Vir, P.; Raghava, G.P. Prediction of IL4 inducing peptides. Clin. Dev. Immunol. 2013, 2013, 263952. [Google Scholar] [CrossRef]
- Ierna, M.X.; Scales, H.E.; Saunders, K.L.; Lawrence, C.E. Mast cell production of IL-4 and TNF may be required for protective and pathological responses in gastrointestinal helminth infection. Mucosal Immunol. 2008, 1, 147–155. [Google Scholar] [CrossRef]
- Choi, P.; Reiser, H. IL-4: Role in disease and regulation of production. Clin. Exp. Immunol. 1998, 113, 317–319. [Google Scholar] [CrossRef]
- Gadani, S.P.; Cronk, J.C.; Norris, G.T.; Kipnis, J. IL-4 in the brain: A cytokine to remember. J. Immunol. 2012, 189, 4213–4219. [Google Scholar] [CrossRef] [PubMed]
- Rael, E.L.; Lockey, R.F. Interleukin-13 signaling and its role in asthma. World Allergy Organ. J. 2011, 4, 54–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wynn, T.A. IL-13 effector functions. Annu. Rev. Immunol. 2003, 21, 425–456. [Google Scholar] [CrossRef]
- de Vries, J.E. The role of IL-13 and its receptor in allergy and inflammatory responses. J. Allergy Clin. Immunol. 1998, 102, 165–169. [Google Scholar] [CrossRef]
- Matsunaga, M.C.; Yamauchi, P.S. IL-4 and IL-13 Inhibition in Atopic Dermatitis. J. Drugs Dermatol. 2016, 15, 925–929. [Google Scholar]
- Bagnasco, D.; Ferrando, M.; Varricchi, G.; Passalacqua, G.; Canonica, G.W. A Critical Evaluation of Anti-IL-13 and Anti-IL-4 Strategies in Severe Asthma. Int. Arch. Allergy Immunol. 2016, 170, 122–131. [Google Scholar] [CrossRef]
- Huang, X.L.; Wang, Y.J.; Yan, J.W.; Wan, Y.N.; Chen, B.; Li, B.Z.; Yang, G.J.; Wang, J. Role of anti-inflammatory cytokines IL-4 and IL-13 in systemic sclerosis. Inflamm. Res. 2015, 64, 151–159. [Google Scholar] [CrossRef]
- Suzuki, A.; Leland, P.; Joshi, B.H.; Puri, R.K. Targeting of IL-4 and IL-13 receptors for cancer therapy. Cytokine 2015, 75, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.A.; Lee, J.; Ha, S.H.; Lee, C.M.; Kim, K.M.; Jang, K.Y.; Park, S.H. Interleukin4Ralpha (IL4Ralpha) and IL13Ralpha1 Are Associated with the Progress of Renal Cell Carcinoma through Janus Kinase 2 (JAK2)/Forkhead Box O3 (FOXO3) Pathways. Cancers 2019, 11, 1394. [Google Scholar] [CrossRef] [Green Version]
- Burt, B.M.; Bader, A.; Winter, D.; Rodig, S.J.; Bueno, R.; Sugarbaker, D.J. Expression of interleukin-4 receptor alpha in human pleural mesothelioma is associated with poor survival and promotion of tumor inflammation. Clin. Cancer Res. 2012, 18, 1568–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.M.; Hussein, U.K.; Park, S.H.; Moon, Y.J.; Zhang, Z.; Ahmed, A.G.; Ahn, A.R.; Park, H.S.; Kim, J.R.; Jang, K.Y. Expression of IL4Ralpha and IL13Ralpha1 are associated with poor prognosis of soft-tissue sarcoma of the extremities, superficial trunk, and retroperitoneum. Diagn. Pathol. 2021, 16, 2. [Google Scholar] [CrossRef]
- Park, M.H.; Kwon, H.J.; Kim, J.R.; Lee, B.; Lee, S.J.; Bae, Y.K. Elevated Interleukin-13 Receptor Alpha 1 Expression in Tumor Cells Is Associated with Poor Prognosis in Patients with Invasive Breast Cancer. Ann. Surg. Oncol. 2017, 24, 3780–3787. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Puri, R.K. Analysis of the cancer genome atlas (TCGA) database identifies an inverse relationship between interleukin-13 receptor alpha1 and alpha2 gene expression and poor prognosis and drug resistance in subjects with glioblastoma multiforme. J. Neurooncol. 2018, 136, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, S.R. Mechanistic Insights into Regulation of JAK2 Tyrosine Kinase. Front. Endocrinol. 2017, 8, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihle, J.N.; Gilliland, D.G. Jak2: Normal function and role in hematopoietic disorders. Curr. Opin. Genet. Dev. 2007, 17, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.L.; Macdonald, A. JAK2 Inhibition Impairs Proliferation and Sensitises Cervical Cancer Cells to Cisplatin-Induced Cell Death. Cancers 2019, 11, 1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushansky, K. The chronic myeloproliferative disorders and mutation of JAK2: Dameshek’s 54 year old speculation comes of age. Best Pract. Res. Clin. Haematol. 2007, 20, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, C.; Birgens, H.S.; Nordestgaard, B.G.; Kjaer, L.; Bojesen, S.E. The JAK2 V617F somatic mutation, mortality and cancer risk in the general population. Haematologica 2011, 96, 450–453. [Google Scholar] [CrossRef]
- Allred, D.C.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar]
- Hundal, R.; Shaffer, E.A. Gallbladder cancer: Epidemiology and outcome. Clin. Epidemiol. 2014, 6, 99–109. [Google Scholar] [CrossRef]
- Kim, T.G. Patterns of initial failure after resection for gallbladder cancer: Implications for adjuvant radiotherapy. Radiat. Oncol. J. 2017, 35, 359–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppola, C.; Rienzo, A.; Piscopo, G.; Barbieri, A.; Arra, C.; Maurea, N. Management of QT prolongation induced by anti-cancer drugs: Target therapy and old agents. Different algorithms for different drugs. Cancer Treat. Rev. 2018, 63, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Pakkala, S.; Ramalingam, S.S. Personalized therapy for lung cancer: Striking a moving target. JCI Insight 2018, 3, e120858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, M.; Kim, J.W.; Roh, J.L.; Park, Y.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y.; Lee, B.H. Recurrence and cancer-specific survival according to the expression of IL-4Ralpha and IL-13Ralpha1 in patients with oral cavity cancer. Eur. J. Cancer 2015, 51, 177–185. [Google Scholar] [CrossRef]
- Todaro, M.; Lombardo, Y.; Francipane, M.G.; Alea, M.P.; Cammareri, P.; Iovino, F.; Di Stefano, A.B.; Di Bernardo, C.; Agrusa, A.; Condorelli, G.; et al. Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived interleukin-4. Cell Death Differ. 2008, 15, 762–772. [Google Scholar] [CrossRef] [Green Version]
- Thul, P.J.; Akesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Bjork, L.; Breckels, L.M.; et al. A subcellular map of the human proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Justiz Vaillant, A.A.; Qurie, A. Interleukin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Little, A.C.; Pathanjeli, P.; Wu, Z.; Bao, L.; Goo, L.E.; Yates, J.A.; Oliver, C.R.; Soellner, M.B.; Merajver, S.D. IL-4/IL-13 Stimulated Macrophages Enhance Breast Cancer Invasion Via Rho-GTPase Regulation of Synergistic VEGF/CCL-18 Signaling. Front. Oncol. 2019, 9, 456. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Song, X.; Traub, B.; Luxenhofer, M.; Kornmann, M. Involvement of IL-4, IL-13 and Their Receptors in Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22, 2998. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.X.; Lian, Q.W.; Pan, J.D.; Xu, Z.L.; Zhou, T.M.; Ye, B. JAK2 tyrosine kinase inhibitor AG490 suppresses cell growth and invasion of gallbladder cancer cells via inhibition of JAK2/STAT3 signaling. J. Biol. Regul. Homeost. Agents 2017, 31, 51–58. [Google Scholar]
- Akada, H.; Akada, S.; Hutchison, R.E.; Sakamoto, K.; Wagner, K.U.; Mohi, G. Critical role of Jak2 in the maintenance and function of adult hematopoietic stem cells. Stem Cells 2014, 32, 1878–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Jin, J.; Xu, J.; Shao, Y.W.; Fan, Y. JAK2 variations and functions in lung adenocarcinoma. Tumor Biol. 2017, 39, 1010428317711140. [Google Scholar] [CrossRef] [Green Version]
- Qureshy, Z.; Johnson, D.E.; Grandis, J.R. Targeting the JAK/STAT pathway in solid tumors. J. Cancer Metastasis Treat. 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Judd, L.M.; Menheniott, T.R.; Ling, H.; Jackson, C.B.; Howlett, M.; Kalantzis, A.; Priebe, W.; Giraud, A.S. Inhibition of the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and in vivo. PLoS ONE 2014, 9, e95993. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Liu, Z.; Guo, X.; Miao, Z.; Ma, S. Atractylenolide I Induces Apoptosis and Suppresses Glycolysis by Blocking the JAK2/STAT3 Signaling Pathway in Colorectal Cancer Cells. Front. Pharmacol. 2020, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Du, Y.; Liu, X.; Chen, H.; Weng, X.; Guo, J.; Wang, M.; Wang, X.; Wang, L. EZH2 inhibition suppresses bladder cancer cell growth and metastasis via the JAK2/STAT3 signaling pathway. Oncol. Lett. 2019, 18, 907–915. [Google Scholar] [CrossRef] [Green Version]
- Salcher, S.; Spoden, G.; Hagenbuchner, J.; Fuhrer, S.; Kaserer, T.; Tollinger, M.; Huber-Cantonati, P.; Gruber, T.; Schuster, D.; Gust, R.; et al. A drug library screen identifies Carbenoxolone as novel FOXO inhibitor that overcomes FOXO3-mediated chemoprotection in high-stage neuroblastoma. Oncogene 2020, 39, 1080–1097. [Google Scholar] [CrossRef] [Green Version]
- Birkenkamp, K.U.; Coffer, P.J. Regulation of cell survival and proliferation by the FOXO (Forkhead box, class O) subfamily of Forkhead transcription factors. Biochem. Soc. Trans. 2003, 31, 292–297. [Google Scholar] [CrossRef]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ma, W.; Cheng, X.; Zhang, X.; Xie, Y.; Ji, Z.; Wu, S. Activation of FOXO3 pathway is involved in polyphyllin I-induced apoptosis and cell cycle arrest in human bladder cancer cells. Arch. Biochem. Biophys. 2020, 687, 108363. [Google Scholar] [CrossRef]
- Yu, D.S.; Chen, Y.T.; Wu, C.L.; Yu, C.P. Expression of p-FOXO3/FOXO3 in bladder cancer and its correlation with clinicopathology and tumor recurrence. Int. J. Clin. Exp. Pathol. 2017, 10, 11069–11074. [Google Scholar] [PubMed]
- Wang, Y.; Kang, X.L.; Zeng, F.C.; Xu, C.J.; Zhou, J.Q.; Luo, D.N. Correlations of Foxo3 and Foxo4 expressions with clinicopathological features and prognosis of bladder cancer. Pathol. Res. Pract. 2017, 213, 766–772. [Google Scholar] [CrossRef] [PubMed]
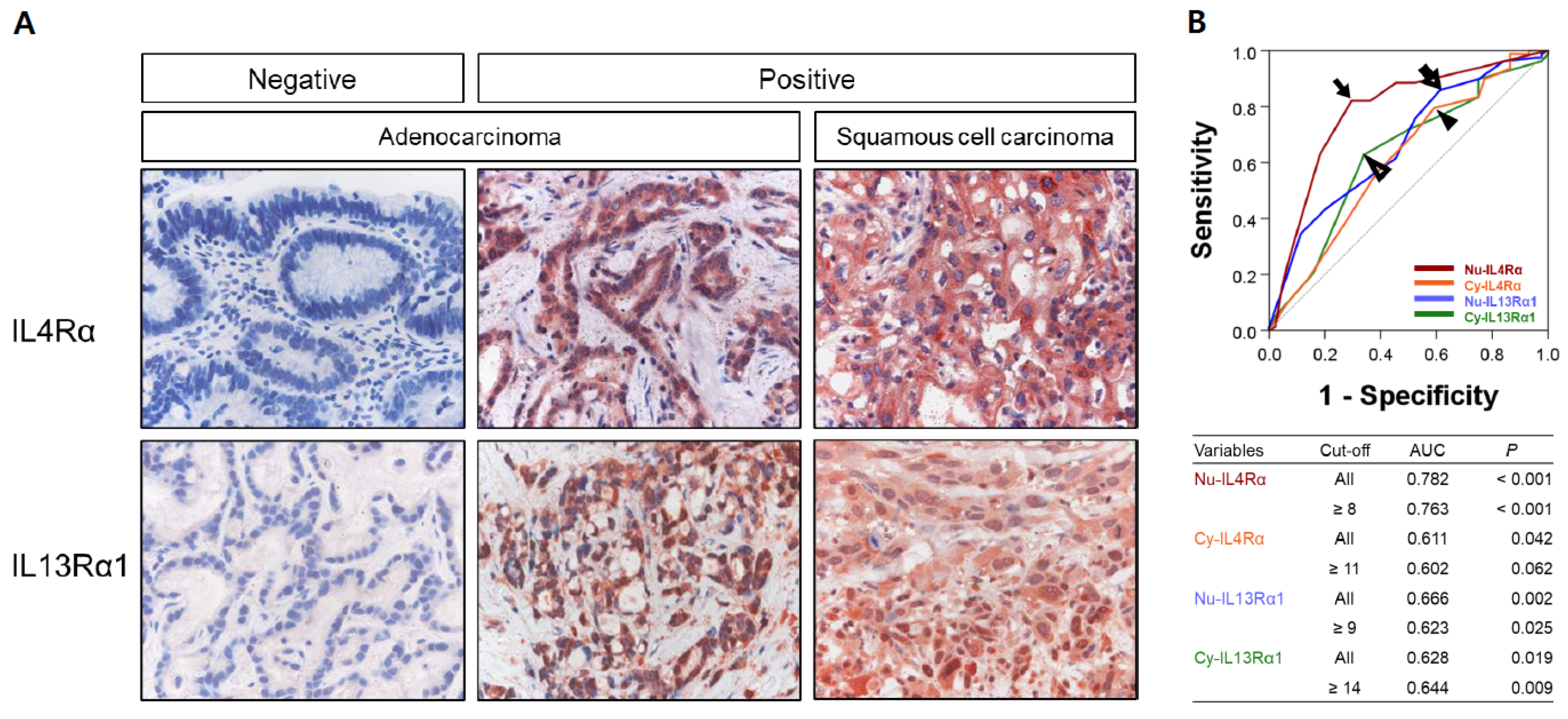

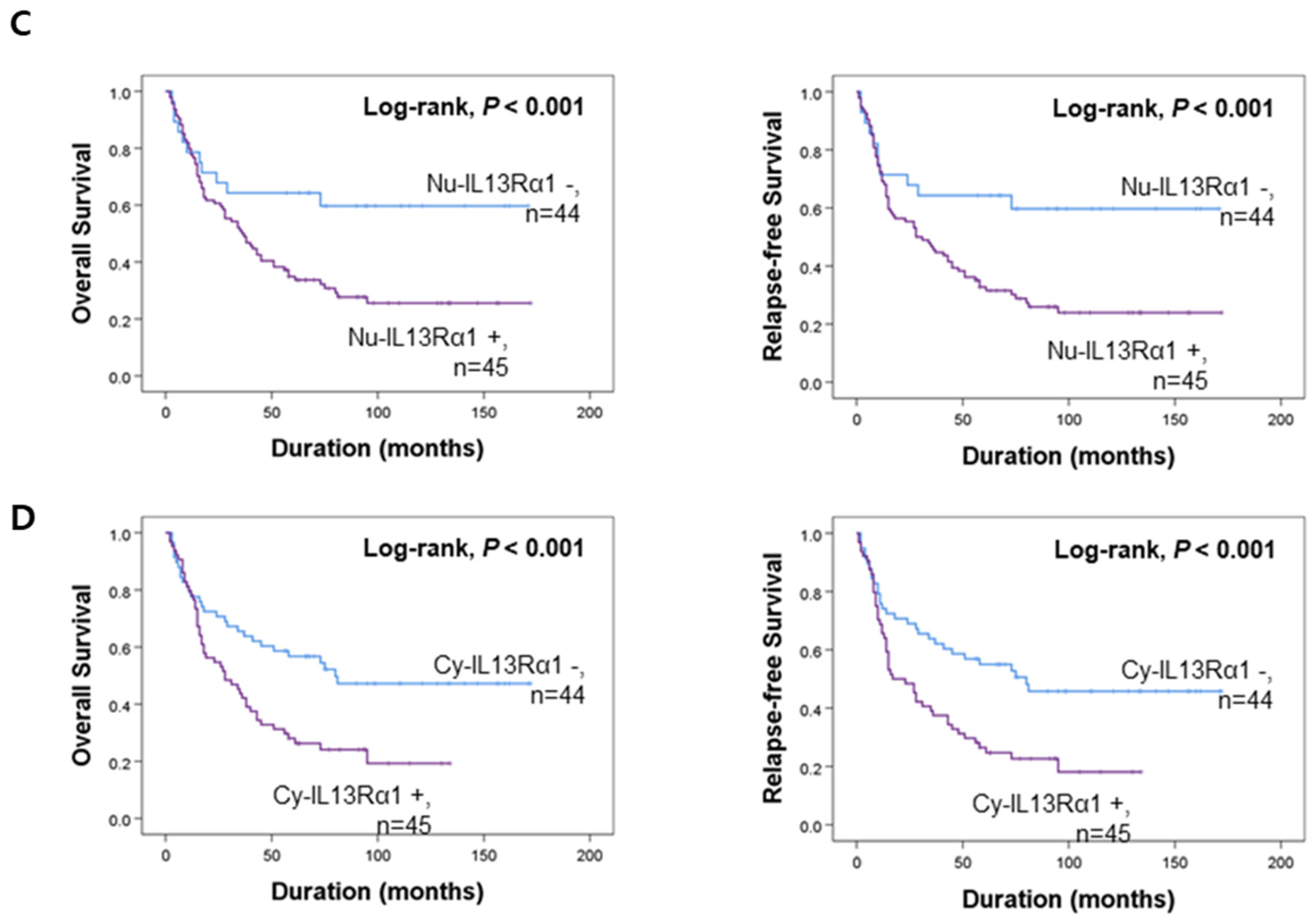
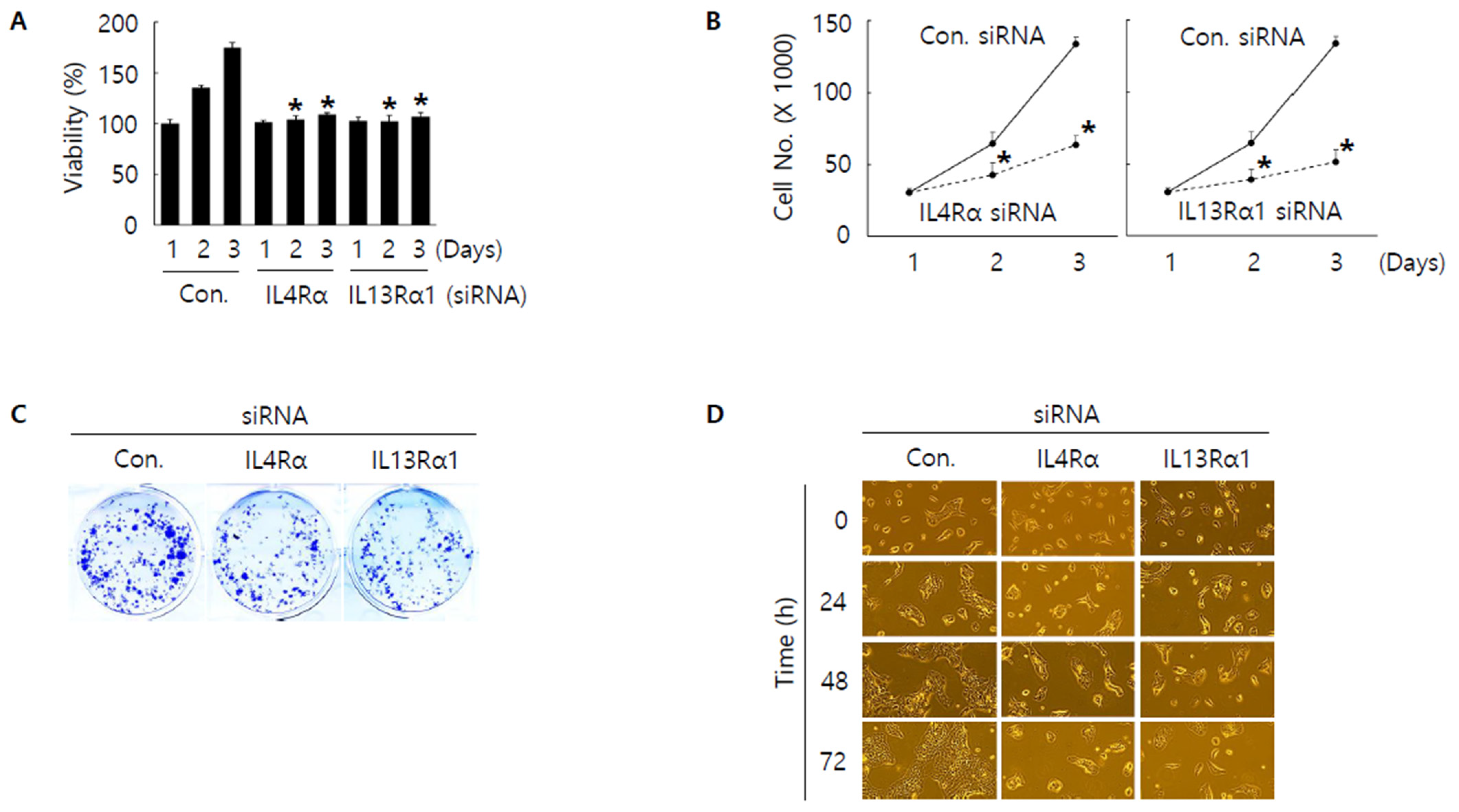
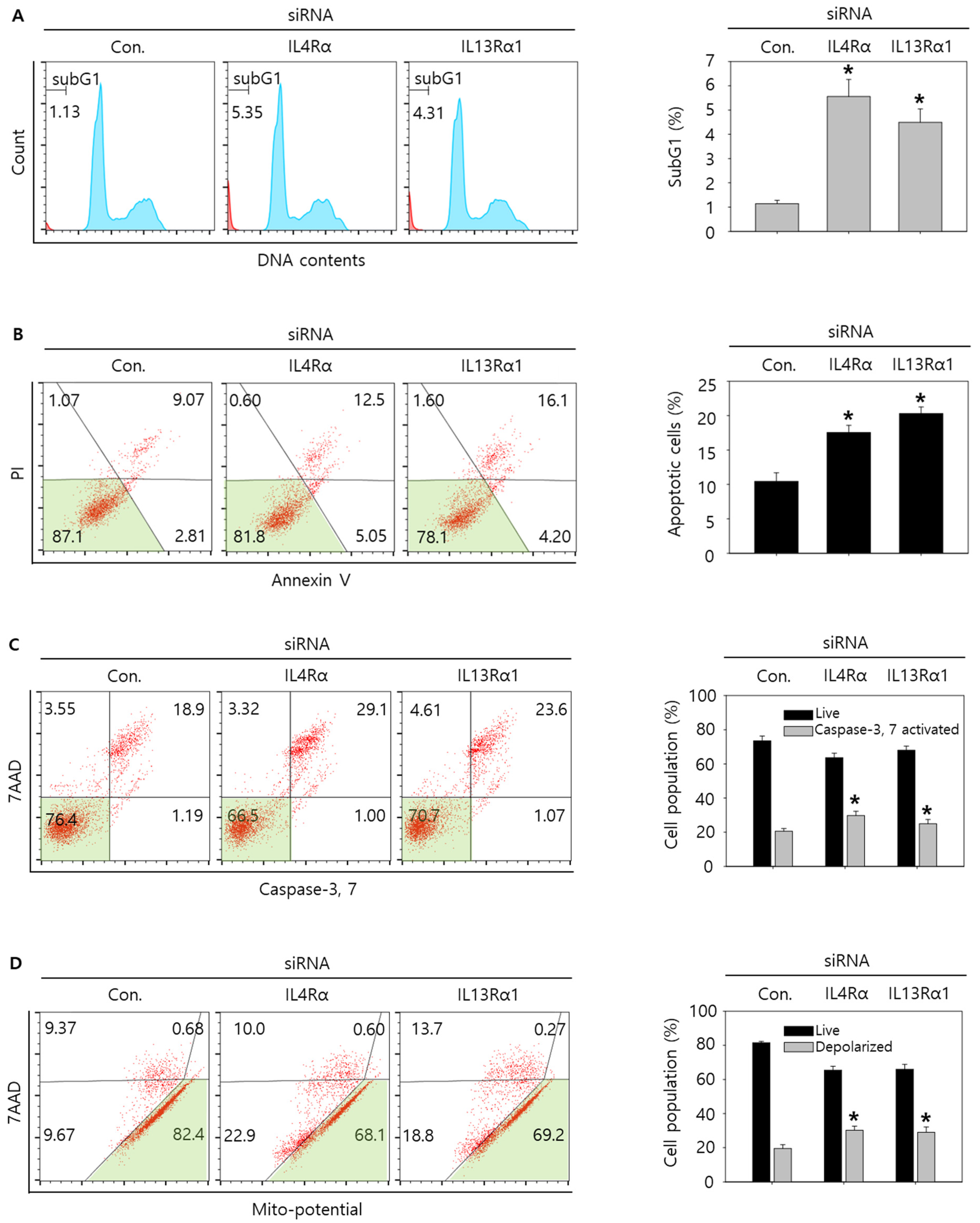



| Characteristics | No. | Nu-IL4Rα | Cy-IL4Rα | Nu-IL13Rα1 | Cy-IL13Rα1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | p | Positive | p | Positive | p | Positive | p | |||
| Age (years) | <65 y | 60 | 31 (52%) | 0.010 | 36 (60%) | 0.003 | 46 (77%) | 0.921 | 28 (47%) | 0.208 |
| ≥65 y | 62 | 46 (74%) | 52 (84%) | 48 (77%) | 36 (58%) | |||||
| Sex | Male | 62 | 42 (68%) | 0.282 | 51 (82%) | 0.011 | 41 (66%) | 0.004 | 33 (53%) | 0.863 |
| Female | 60 | 35 (58%) | 37 (62%) | 53 (88%) | 31 (52%) | |||||
| CEA | Normal | 98 | 60 (61%) | 0.195 | 70 (71%) | 0.658 | 74 (76%) | 0.594 | 52 (53%) | 0.651 |
| Elevated | 21 | 16 (76%) | 16 (76%) | 17 (81%) | 10 (48%) | |||||
| CA19-9 | Normal | 79 | 49 (62%) | 0.622 | 57 (72%) | 0.968 | 62 (78%) | 0.616 | 41 (52%) | 0.644 |
| Elevated | 39 | 26 (67%) | 28 (72%) | 29 (74%) | 22 (56%) | |||||
| TNM stage | I and II | 75 | 42 (56%) | 0.04 | 53 (71%) | 0.649 | 59 (79%) | 0.591 | 40 (53%) | 0.807 |
| III and IV | 47 | 35 (74%) | 35 (74%) | 35 (74%) | 24 (51%) | |||||
| T category | T1 | 32 | 16 (50%) | 0.035 | 21 (66%) | 0.595 | 21 (66%) | 0.214 | 12 (38%) | 0.219 |
| T2 | 59 | 35 (59%) | 42 (71%) | 50 (85%) | 34 (58%) | |||||
| T3 | 27 | 23 (85%) | 22 (81%) | 20 (74%) | 15 (56%) | |||||
| T4 | 4 | 3 (75%) | 3 (75%) | 3 (75%) | 3 (75%) | |||||
| LN metastasis | Absence | 93 | 58 (62%) | 0.759 | 69 (74%) | 0.363 | 73 (78%) | 0.497 | 50 954%) | 0.605 |
| Presence | 29 | 19 (66%) | 16 (55%) | 21 (72%) | 14 (48%) | |||||
| Distant metastasis | Absence | 114 | 69 (61%) | 0.025 | 80 (70%) | 0.069 | 88 (77%) | 0.887 | 60 (53%) | 0.885 |
| Presence | 8 | 8 (100%) | 8 (100%) | 6 (75%) | 4 (50%) | |||||
| Histologic type | Adenocarcinoma NOS | 118 | 73 (62%) | 0.299 | 84 (71%) | 0.450 | 92 (78%) | 0.166 | 62 (53%) | 0.510 |
| Adenosquamous carcinoma | 3 | 3 (100%) | 3 (100%) | 2 (67%) | 2 (67%) | |||||
| Squamous cell carcinoma NOS | 1 | 1 (100%) | 1 (100%) | 0 (0%) | 0 (0%) | |||||
| Histologic grade | Low | 50 | 25 (50%) | 0.012 | 36 (72%) | 0.979 | 35 (70%) | 0.123 | 19 (38%) | 0.008 |
| High | 72 | 52 (72%) | 52 (72%) | 59 (82%) | 45 (63%) | |||||
| Cy-IL13Rα1 | Negative | 58 | 29 (50%) | 0.004 | 34 (59%) | 0.002 | 33 (57%) | <0.001 | ||
| Positive | 64 | 48 (75%) | 54 (84%) | 61 (95%) | ||||||
| Nu-IL13Rα1 | Negative | 28 | 12 (43%) | 0.011 | 19 (68%) | 0.566 | ||||
| Positive | 94 | 65 (69%) | 69 (73%) | |||||||
| Cy-IL4Rα | Negative | 34 | 13 (38%) | <0.001 | ||||||
| Positive | 88 | 64 (73%) | ||||||||
| Characteristics | No. | OS | RFS | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||
| Age, y ≥ 65 (vs. <65) | 62/122 | 2.603 | 1.625–4.168 | <0.001 | 2.438 | 1.539–3.863 | <0.001 |
| Sex, female (vs. male) | 60/122 | 0.736 | 0.471–1.150 | 0.178 | 0.71 | 0.456–1.103 | 0.127 |
| CEA, elevated (vs. normal) | 21/119 | 1.487 | 0.856–2.584 | 0.159 | 1.394 | 0.804–2.416 | 0.237 |
| CA19-9, elevated (vs. normal) | 39/118 | 1.729 | 1.091–2.741 | 0.020 | 1.653 | 1.047–2.610 | 0.031 |
| TNM stage, I and II (vs. III and IV) | 47/122 | 3.658 | 2.318–5.773 | <0.001 | 3.21 | 2.045–5.039 | <0.001 |
| T category, T1 | 32/122 | 1 | <0.001 | 1 | <0.001 | ||
| T2 | 59/122 | 2.511 | 1.279–4.929 | 0.007 | 2.716 | 1.388–5.313 | 0.004 |
| T3 | 27/122 | 9.948 | 4.813–20.563 | <0.001 | 9.115 | 4.404–18.866 | <0.001 |
| T4 | 4/122 | 10.679 | 3.283–34.738 | <0.001 | 11.195 | 3.436–36.482 | <0.001 |
| LN metastasis, presence (vs. absence) | 29/122 | 1.978 | 1.217–3.215 | 0.006 | 1.857 | 1.145–3.011 | 0.012 |
| Distant metastasis, presence (vs. absence) | 8/122 | 6.288 | 2.849–13.878 | <0.001 | 5.039 | 2.301–11.034 | <0.001 |
| Histologic type, adenocarcinoma NOS | 118/122 | 1 | 0.002 | 1 | 0.005 | ||
| Adenosquamous carcinoma | 3/122 | 4.416 | 1.372–14.209 | 0.013 | 3.635 | 1.133–11.665 | 0.030 |
| Squamous cell carcinoma NOS | 1/122 | 15.704 | 1.960–125.812 | 0.009 | 13.854 | 1.752–109.584 | 0.013 |
| Histologic grade, high (vs. low) | 72/122 | 2.874 | 1.747–4.727 | <0.001 | 2.8 | 1.718–4.564 | <0.001 |
| Cy-IL13Rα1, positive (vs. negative) | 64/122 | 1.987 | 1.250–3.161 | 0.004 | 2.008 | 1.271–3.172 | 0.003 |
| Nu-IL13Rα1, positive (vs. negative) | 94/122 | 2.197 | 1.159–4.168 | 0.016 | 2.31 | 1.220–4.376 | 0.010 |
| Cy-IL4Rα, positive (vs. negative) | 88/122 | 1.895 | 1.092–3.288 | 0.023 | 2.048 | 1.183–3.548 | 0.011 |
| Nu-IL4Rα, positive (vs. negative) | 77/122 | 4.614 | 2.575–8.269 | <0.001 | 4.019 | 2.311–6.988 | <0.001 |
| Characteristics | OS | RFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age, y ≥ 65 (vs. <65) | 2.755 | 1.675–4.533 | <0.001 | 2.646 | 1.633–4.287 | <0.001 |
| TNM stage, I and II (vs. III and IV) | 2.605 | 1.260–5.386 | 0.010 | 2.098 | 1.023–4.300 | 0.043 |
| T category, T1 | 1 | 0.048 | 1 | 0.016 | ||
| T2 | 2.202 | 1.065–4.552 | 0.033 | 2.502 | 1.218–5.138 | 0.012 |
| T3 | 3.264 | 1.179–9.036 | 0.023 | 3.805 | 1.383–10.471 | 0.010 |
| T4 | 6.302 | 1.602–24.788 | 0.008 | 8.146 | 2.063–32.156 | 0.003 |
| Nu-IL4Rα, positive (vs. negative) | 3.379 | 1.825–6.254 | <0.001 | 2.919 | 1.622–5.253 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.W.; Lee, C.M.; Kang, M.-A.; Hussein, U.K.; Park, H.S.; Ahn, A.-R.; Yu, H.C.; Yang, J.D.; Yang, Y.-H.; Park, K.; et al. IL4Rα and IL13Rα1 Are Involved in the Development of Human Gallbladder Cancer. J. Pers. Med. 2022, 12, 249. https://doi.org/10.3390/jpm12020249
Ahn SW, Lee CM, Kang M-A, Hussein UK, Park HS, Ahn A-R, Yu HC, Yang JD, Yang Y-H, Park K, et al. IL4Rα and IL13Rα1 Are Involved in the Development of Human Gallbladder Cancer. Journal of Personalized Medicine. 2022; 12(2):249. https://doi.org/10.3390/jpm12020249
Chicago/Turabian StyleAhn, Sung Woo, Chang Min Lee, Mi-Ae Kang, Usama Khamis Hussein, Ho Sung Park, Ae-Ri Ahn, Hee Chul Yu, Jae Do Yang, Yung-Hun Yang, Kyungmoon Park, and et al. 2022. "IL4Rα and IL13Rα1 Are Involved in the Development of Human Gallbladder Cancer" Journal of Personalized Medicine 12, no. 2: 249. https://doi.org/10.3390/jpm12020249
APA StyleAhn, S. W., Lee, C. M., Kang, M. -A., Hussein, U. K., Park, H. S., Ahn, A. -R., Yu, H. C., Yang, J. D., Yang, Y. -H., Park, K., Lee, J., Jang, K. Y., & Park, S. -H. (2022). IL4Rα and IL13Rα1 Are Involved in the Development of Human Gallbladder Cancer. Journal of Personalized Medicine, 12(2), 249. https://doi.org/10.3390/jpm12020249









