Reward Deficiency Syndrome (RDS) Surprisingly Is Evolutionary and Found Everywhere: Is It “Blowin’ in the Wind”?
Abstract
:1. Introduction
1.1. Summary Preamble
1.2. “Blowin’ in the Wind”—Bob Dylan, 1962
2. Genes and Evolution for the Survival of Our Species
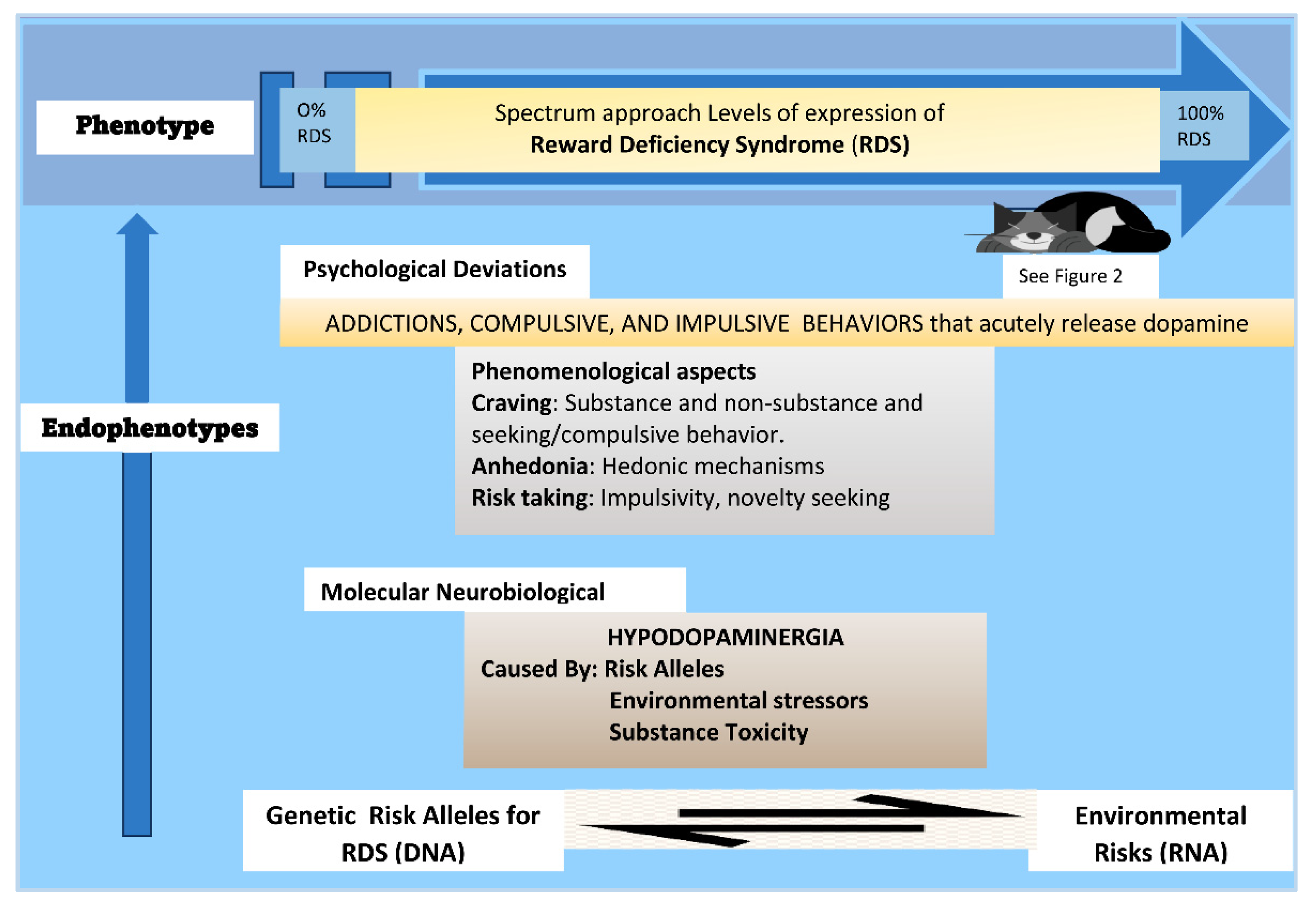
3. Reward Deficiency Syndrome
4. Prevalence of Addiction-Related Gene Polymorphisms
5. The Reward Deficiency Syndrome (RDS) Model
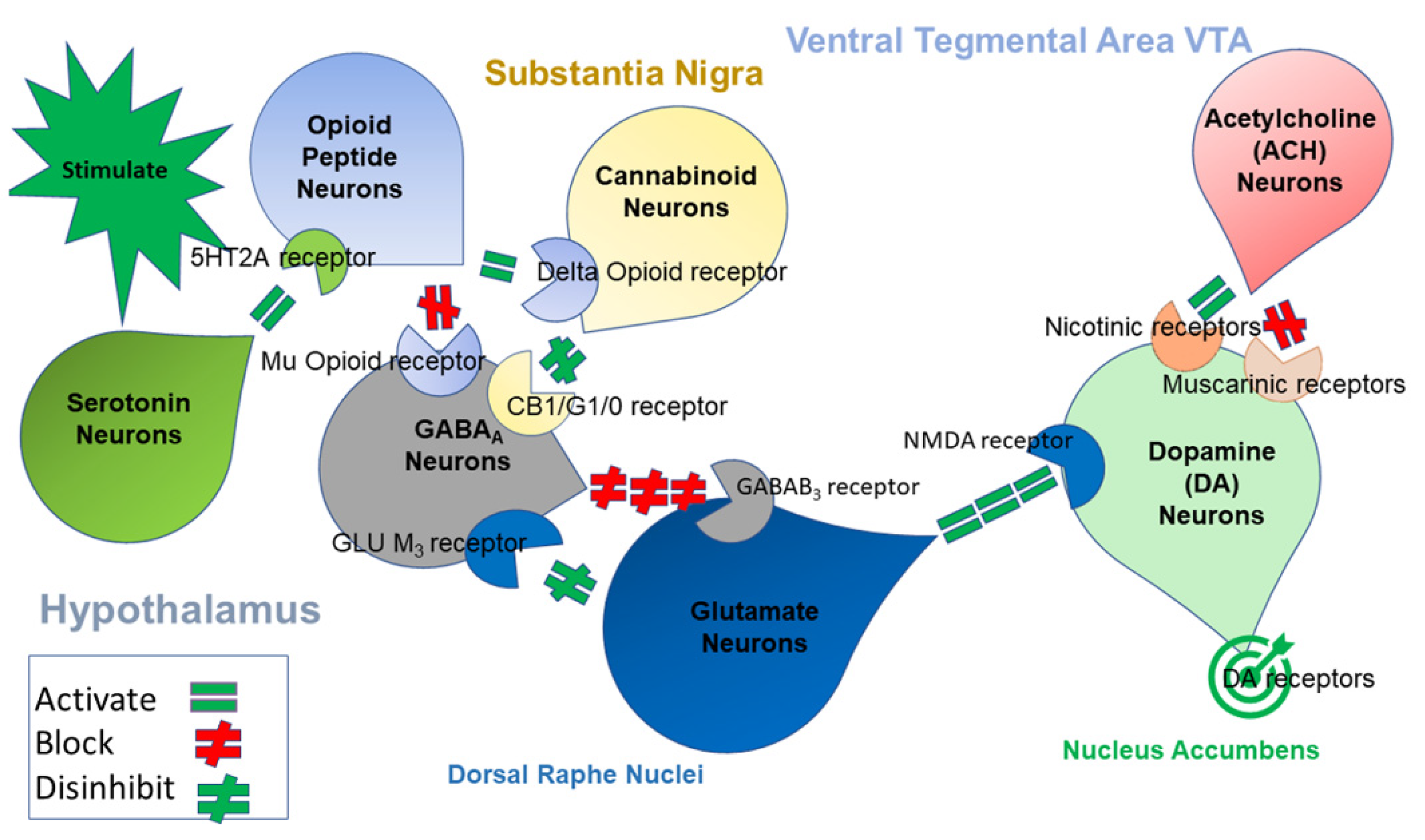
6. The Neuro-Genetic Background of the RDS
6.1. Neurotransmitters and Mental Illness
6.2. Genetic Associations
7. Phenomenological Aspects of the RDS
8. Evaluation of the RDS Model
9. The RDS Model: Clinical Applications
10. Mental Illness and RDS
11. Conclusions
12. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blum, K.; Noble, E.P.; Sheridan, P.J.; Montgomery, A.; Ritchie, T.; Jagadeeswaran, P.; Nogami, H.; Briggs, A.H.; Cohn, J.B. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA 1990, 263, 2055–2060. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Gold, M.; Demetrovics, Z.; Archer, T.; Thanos, P.K.; Baron, D.; Badgaiyan, R.D. Substance use disorder a bio-directional subset of reward deficiency syndrome. Front. Biosci. 2017, 22, 1534–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, K.; Thanos, P.K.; Gold, M.S. Dopamine and glucose, obesity, and reward deficiency syndrome. Front. Psychol. 2014, 5, 919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, K. Reward Deficiency Syndrome. In The Sage Encyclopedia of Abnormal Clinical Psychology; Wenzel, A., Ed.; Sage Publications: Conshohocken, PA, USA, 2017. [Google Scholar]
- Dawkins, R. Should doctors be Darwinian? Trans. Med. Soc. Lond. 2002, 119, 15–30. [Google Scholar]
- Comings, D.E. Evidence for ancient tetraploidy and conservation of linkage groups in mammalian chromosomes. Nature 1972, 238, 455–457. [Google Scholar] [CrossRef]
- Blum, K.; Gondré-Lewis, M.; Steinberg, B.; Elman, I.; Baron, D.; Modestino, E.J.; Badgaiyan, R.D.; Gold, M.S. Our evolved unique pleasure circuit makes humans different from apes: Reconsideration of data derived from animal studies. J. Syst. Integr. Neurosci. 2018, 4. [Google Scholar] [CrossRef]
- Kirk, U.; Brown, K.W.; Downar, J. Adaptive neural reward processing during anticipation and receipt of monetary rewards in mindfulness meditators. Soc. Cogn. Affect. Neurosci. 2015, 10, 752–759. [Google Scholar] [CrossRef]
- Esch, T.; Stefano, G.B. The neurobiology of pleasure, reward processes, addiction and their health implications. Neuro Endocrinol. Lett. 2004, 25, 235–251. [Google Scholar]
- Bressan, R.A.; Crippa, J.A. The role of dopamine in reward and pleasure behaviour—Review of data from preclinical research. Acta Psychiatr. Scand. Suppl. 2005, 427, 14–21. [Google Scholar] [CrossRef]
- Barbano, M.F.; Cador, M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology 2007, 191, 497–506. [Google Scholar] [CrossRef]
- Smith, K.S.; Berridge, K.C.; Aldridge, J.W. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc. Natl. Acad. Sci. USA 2011, 108, E255–E264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharot, T.; Shiner, T.; Brown, A.C.; Fan, J.; Dolan, R.J. Dopamine enhances expectation of pleasure in humans. Curr. Biol. 2009, 19, 2077–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liggins, J.; Pihl, R.O.; Benkelfat, C.; Leyton, M. The dopamine augmenter L-DOPA does not affect positive mood in healthy human volunteers. PLoS ONE 2012, 7, e28370. [Google Scholar] [CrossRef] [PubMed]
- D’Amour-Horvat, V.; Leyton, M. Impulsive actions and choices in laboratory animals and humans: Effects of high vs. low dopamine states produced by systemic treatments given to neurologically intact subjects. Front. Behav. Neurosci. 2014, 8, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamura, N.; Nakagawa, S.; Masuda, T.; Boku, S.; Kato, A.; Song, N.; An, Y.; Kitaichi, Y.; Inoue, T.; Koyama, T.; et al. The effect of dopamine on adult hippocampal neurogenesis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 50, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Chenu, A.; Tassin, J.P. Pleasure: Neurobiological conception and Freudian conception. Encephale 2014, 40, 100–107. [Google Scholar] [CrossRef]
- Branković, S. Boredom, dopamine, and the thrill of psychosis: Psychiatry in a new key. Psychiatr. Danub. 2015, 27, 126–137. [Google Scholar]
- Settle, J.E.; Dawes, C.T.; Christakis, N.A.; Fowler, J.H. Friendships Moderate an Association between a Dopamine Gene Variant and Political Ideology. J. Polit. 2010, 72, 1189–1198. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Blum, K.; Chen, T.J.; Downs, B.W.; Bowirrat, A.; Waite, R.L.; Braverman, E.R.; Madigan, M.; Oscar-Berman, M.; DiNubile, N.; Stice, E.; et al. Neurogenetics of dopaminergic receptor supersensitivity in activation of brain reward circuitry and relapse: Proposing “deprivation-amplification relapse therapy” (DART). Postgrad. Med. 2009, 121, 176–196. [Google Scholar] [CrossRef] [Green Version]
- Jayanthi, S.; Torres, O.V.; Ladenheim, B.; Cadet, J.L. A Single Prior Injection of Methamphetamine Enhances Methamphetamine Self-Administration (SA) and Blocks SA-Induced Changes in DNA Methylation and mRNA Expression of Potassium Channels in the Rat Nucleus Accumbens. Mol. Neurobiol. 2020, 57, 1459–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehmler, W.; Obrecht-Pflumio, S.; Canfield, V.; Thisse, C.; Thisse, B.; Levenson, R. Evolution and expression of D2 and D3 dopamine receptor genes in zebrafish. Dev. Dyn. 2004, 230, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Kotyuk, E.; Magi, A.; Eisinger, A.; Király, O.; Vereczkei, A.; Barta, C.; Griffiths, M.D.; Székely, A.; Kökönyei, G.; Farkas, J.; et al. Co-occurrences of substance use and other potentially addictive behaviors: Epidemiological results from the Psychological and Genetic Factors of the Addictive Behaviors (PGA) Study. J. Behav. Addict. 2020, 9, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Bowirrat, A.; Baron, D.; Lott, L.; Ponce, J.V.; Brewer, R.; Siwicki, D.; Boyett, B.; Gondre-Lewis, M.C.; Smith, D.E.; et al. Biotechnical development of genetic addiction risk score (GARS) and selective evidence for inclusion of polymorphic allelic risk in substance use disorder (SUD). J. Syst. Integr. Neurosci. 2020, 6. [Google Scholar] [CrossRef]
- Gondré-Lewis, M.C.; Bassey, R.; Blum, K. Pre-clinical models of reward deficiency syndrome: A behavioral octopus. Neurosci. Biobehav. Rev. 2020, 115, 164–188. [Google Scholar] [CrossRef]
- Blum, K.; Cadet, J.L.; Gold, M.S. Psychostimulant use disorder emphasizing methamphetamine and the opioid -dopamine connection: Digging out of a hypodopaminergic ditch. J. Neurol. Sci. 2021, 420, 117252. [Google Scholar] [CrossRef]
- Blum, K.; Baron, D.; McLaughlin, T.; Gold, M.S. Molecular neurological correlates of endorphinergic/dopaminergic mechanisms in reward circuitry linked to endorphinergic deficiency syndrome (EDS). J. Neurol. Sci. 2020, 411, 116733. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Sheridan, P.J.; Wood, R.C.; Braverman, E.R.; Chen, T.J.; Cull, J.G.; Comings, D.E. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J. R. Soc. Med. 1996, 89, 396–400. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Cull, J.; Braverman, E.; Comings, D.E. Reward deficiency syndrome. Am. Sci. 1996, 84, 132–145. [Google Scholar]
- Blum, K.; Chen, A.L.; Oscar-Berman, M.; Chen, T.J.; Lubar, J.; White, N.; Lubar, J.; Bowirrat, A.; Braverman, E.; Schoolfield, J.; et al. Generational association studies of dopaminergic genes in reward deficiency syndrome (RDS) subjects: Selecting appropriate phenotypes for reward dependence behaviors. Int. J. Environ. Res. Public Health 2011, 8, 4425–4459. [Google Scholar] [CrossRef] [Green Version]
- Bacon, S.D. E. M. JELLINEK. The disease concept of alcoholism. Pp. 246. New Haven, Conn.: Hillhouse Press on behalf of the Christopher J. Smithers Foundation, 1960. $6.00. ANNALS Am. Acad. Political Soc. Sci. 1961, 336, 212. [Google Scholar] [CrossRef]
- Blum, K.; Gondre-Lewis, M.C.; Baron, D.; Thanos, P.K.; Braverman, E.R.; Neary, J.; Elman, I.; Badgaiyan, R.D. Introducing Precision Addiction Management of Reward Deficiency Syndrome, the Construct That Underpins All Addictive Behaviors. Front. Psychiatry 2018, 9, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, K.; Wood, R.C.; Braverman, E.R.; Chen, T.J.; Sheridan, P.J. The D2 dopamine receptor gene as a predictor of compulsive disease: Bayes’ theorem. Funct. Neurol. 1995, 10, 37–44. [Google Scholar] [PubMed]
- Blum, K.; Chen, A.L.C.; Thanos, P.K.; Febo, M.; Demetrovics, Z.; Dushaj, K.; Kovoor, A.; Baron, D.; Smith, D.E.; Roy, A.K., III; et al. Genetic addiction risk score (GARS)™, a predictor of vulnerability to opioid dependence. Front. Biosci. 2018, 10, 175–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, L.; Modestino, E.J.; Siwicki, D.; Lott, L.; Thanos, P.K.; Baron, D.; Badgaiyan, R.D.; Ponce, J.V.; Giordano, J.; Downs, B.W.; et al. Hypodopaminergia and “Precision Behavioral Management” (PBM): It is a Generational Family Affair. Curr. Pharm. Biotechnol. 2019, 21, 528–541. [Google Scholar] [CrossRef]
- Blum, K.; Simpatico, T.; Badgaiyan, R.D.; Demetrovics, Z.; Fratantonio, J.; Agan, G.; Febo, M.; Gold, M.S. Coupling Neurogenetics (GARS) and a Nutrigenomic Based Dopaminergic Agonist to Treat Reward Deficiency Syndrome (RDS): Targeting Polymorphic Reward Genes for Carbohydrate Addiction Algorithms. J. Reward Defic. Syndr. 2015, 1, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, M.; Blum, K.; Ponce, J.V.; Lott, L.; Gondré-Lewis, M.C.; Badgaiyan, S.; Brewer, R.; Downs, B.W.; Fynman, P.; Weingarten, A.; et al. High Genetic Addiction Risk Score (GARS) in Chronically Prescribed Severe Chronic Opioid Probands Attending Multi-pain Clinics: An Open Clinical Pilot Trial. Mol. Neurobiol. 2021, 58, 3335–3346. [Google Scholar] [CrossRef]
- Blum, K.; Febo, M.; Badgaiyan, R.D.; Demetrovics, Z.; Simpatico, T.; Fahlke, C.; Oscar-Berman, M.; Li, M.; Dushaj, K.; Gold, M.S. Common Neurogenetic Diagnosis and Meso-Limbic Manipulation of Hypodopaminergic Function in Reward Deficiency Syndrome (RDS): Changing the Recovery Landscape. Curr. Neuropharmacol. 2017, 15, 184–194. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Green, R.; Smith, J.; Llanos-Gomez, L.; Baron, D.; Badgaiyan, R.D. Hypothesizing High Negative Emotionality as a Function of Genetic Addiction Risk Severity (GARS) Testing in Alcohol Use Disorder (AUD). Syst. Integr. Neurosci. 2021, 7. [Google Scholar] [CrossRef]
- Brancato, A.; Castelli, V.; Lavanco, G.; Tringali, G.; Micale, V.; Kuchar, M.; D’Amico, C.; Pizzolanti, G.; Feo, S.; Cannizzaro, C. Binge-like Alcohol Exposure in Adolescence: Behavioural, Neuroendocrine and Molecular Evidence of Abnormal Neuroplasticity… and Return. Biomedicines 2021, 9, 1161. [Google Scholar] [CrossRef]
- Blomqvist, O.; Gelernter, J.; Kranzler, H.R. Family-based study of DRD2 alleles in alcohol and drug dependence. Am. J. Med. Genet. 2000, 96, 659–664. [Google Scholar] [CrossRef]
- Berggren, U.; Fahlke, C.; Aronsson, E.; Karanti, A.; Eriksson, M.; Blennow, K.; Thelle, D.; Zetterberg, H.; Balldin, J. The taqI DRD2 A1 allele is associated with alcohol-dependence although its effect size is small. Alcohol. Alcohol. 2006, 41, 479–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorwood, P.; Batel, P.; Gouya, L.; Courtois, F.; Feingold, J.; Adès, J. Reappraisal of the association between the DRD2 gene, alcoholism and addiction. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2000, 15, 90–96. [Google Scholar] [CrossRef]
- Duan, J.; Wainwright, M.S.; Comeron, J.M.; Saitou, N.; Sanders, A.R.; Gelernter, J.; Gejman, P.V. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum. Mol. Genet. 2003, 12, 205–216. [Google Scholar] [CrossRef]
- Wendt, F.R.; Muniz Carvalho, C.; Pathak, G.A.; Gelernter, J.; Polimanti, R. Deciphering the Biological Mechanisms Underlying the Genome-Wide Associations between Computerized Device Use and Psychiatric Disorders. J. Clin. Med. 2019, 8, 2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.Z.; Kranzler, H.R.; Zhao, H.; Gruen, J.R.; Luo, X.; Gelernter, J. Association of haplotypic variants in DRD2, ANKK1, TTC12 and NCAM1 to alcohol dependence in independent case control and family samples. Hum. Mol. Genet. 2007, 16, 2844–2853. [Google Scholar] [CrossRef] [Green Version]
- Gelernter, J.; Yu, Y.; Weiss, R.; Brady, K.; Panhuysen, C.; Yang, B.Z.; Kranzler, H.R.; Farrer, L. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum. Mol. Genet. 2006, 15, 3498–3507. [Google Scholar] [CrossRef] [Green Version]
- Kranzler, H.R.; Zhou, H.; Kember, R.L.; Smith, R.V.; Justice, A.C.; Damrauer, S.; Tsao, P.S.; Klarin, D.; Baras, A.; Reid, J.; et al. Author Correction: Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat. Commun. 2019, 10, 4050. [Google Scholar] [CrossRef] [Green Version]
- Levey, D.F.; Stein, M.B.; Wendt, F.R.; Pathak, G.A.; Zhou, H.; Aslan, M.; Quaden, R.; Harrington, K.M.; Nuñez, Y.Z.; Overstreet, C.; et al. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat. Neurosci. 2021, 24, 954–963. [Google Scholar] [CrossRef]
- Benton, D.; Young, H.A. A meta-analysis of the relationship between brain dopamine receptors and obesity: A matter of changes in behavior rather than food addiction? Int. J. Obes. 2016, 40 (Suppl. 1), S12–S21. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.; Thomas, N.; Singleton, A.; Piggott, M.; Lloyd, S.; Perry, E.K.; Morris, C.M.; Perry, R.H.; Ferrier, I.N.; Court, J.A. D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: Reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics 1997, 7, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.P.; Blum, K.; Ritchie, T.; Montgomery, A.; Sheridan, P.J. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch. Gen. Psychiatry 1991, 48, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.R.; Braverman, E.R. Measuring adiposity in patients: The utility of body mass index (BMI), percent body fat, and leptin. PLoS ONE 2012, 7, e33308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, C.; Loxton, N.J.; Levitan, R.D.; Kaplan, A.S.; Carter, J.C.; Kennedy, J.L. ‘Food addiction’ and its association with a dopaminergic multilocus genetic profile. Physiol. Behav. 2013, 118, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Obregón, A.M.; Oyarce, K.; García-Robles, M.A.; Valladares, M.; Pettinelli, P.; Goldfield, G.S. Association of the dopamine D2 receptor rs1800497 polymorphism with food addiction, food reinforcement, and eating behavior in Chilean adults. Eat Weight Disord. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, L.; Micioni Di Bonaventura, E.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Romano, A.; Quaglia, W.; Cifani, C.; Micioni Di Bonaventura, M.V. Underlying Susceptibility to Eating Disorders and Drug Abuse: Genetic and Pharmacological Aspects of Dopamine D4 Receptors. Nutrients 2020, 12, 2288. [Google Scholar] [CrossRef]
- Paquet, C.; Portella, A.K.; Moore, S.; Ma, Y.; Dagher, A.; Meaney, M.J.; Kennedy, J.L.; Levitan, R.D.; Silveira, P.P.; Dube, L. Dopamine D4 receptor gene polymorphism (DRD4 VNTR) moderates real-world behavioural response to the food retail environment in children. BMC Public Health 2021, 21, 145. [Google Scholar] [CrossRef]
- Espeso-Gil, S.; Halene, T.; Bendl, J.; Kassim, B.; Ben Hutta, G.; Iskhakova, M.; Shokrian, N.; Auluck, P.; Javidfar, B.; Rajarajan, P.; et al. A chromosomal connectome for psychiatric and metabolic risk variants in adult dopaminergic neurons. Genome Med. 2020, 12, 19. [Google Scholar] [CrossRef]
- Carpenter, C.L.; Wong, A.M.; Li, Z.; Noble, E.P.; Heber, D. Association of dopamine D2 receptor and leptin receptor genes with clinically severe obesity. Obesity 2013, 21, E467–E473. [Google Scholar] [CrossRef]
- Pedram, P.; Zhai, G.; Gulliver, W.; Zhang, H.; Sun, G. Two novel candidate genes identified in adults from the Newfoundland population with addictive tendencies towards food. Appetite 2017, 115, 71–79. [Google Scholar] [CrossRef]
- Silveira, P.P.; Pokhvisneva, I.; Gaudreau, H.; Atkinson, L.; Fleming, A.S.; Sokolowski, M.B.; Steiner, M.; Kennedy, J.L.; Dubé, L.; Levitan, R.D.; et al. Fetal growth interacts with multilocus genetic score reflecting dopamine signaling capacity to predict spontaneous sugar intake in children. Appetite 2018, 120, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, M. Genetic determinants of the alcohol dependence syndrome: Searching for an endophenotype associated with sweet liking in families with alcohol addiction. Ann. Acad. Med. Stetin. 2011, 57, 79–87. [Google Scholar] [PubMed]
- Jasiewicz, A.; Grzywacz, A.; Jabłoński, M.; Bieńkowski, P.; Samochowiec, A.; Samochowiec, J. The analysis of the polymorphic variations of the dopamine gen transporter (DAT1) and the serotonin transporter (5-HTTLPR) in patients with alcohol dependence syndrome with inclusion of the phenotypic feature of sweet liking preference. Psychiatr. Pol. 2014, 48, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Aliasghari, F.; Nazm, S.A.; Yasari, S.; Mahdavi, R.; Bonyadi, M. Associations of the ANKK1 and DRD2 gene polymorphisms with overweight, obesity and hedonic hunger among women from the Northwest of Iran. Eat Weight Disord. 2021, 26, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.W.; Shott, M.E.; DeGuzman, M.C.; Smolen, A. Dopamine D2 -141C Ins/Del and Taq1A polymorphisms, body mass index, and prediction error brain response. Transl. Psychiatry 2018, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Lin, Y.C.; Chao, W.C.; Chung, H.K.; Chi, S.S.; Liu, W.S.; Wu, W.T. Association of genetic polymorphisms of glutamate decarboxylase 2 and the dopamine D2 receptor with obesity in Taiwanese subjects. Ann. Saudi Med. 2012, 32, 121–126. [Google Scholar] [CrossRef]
- Sikora, M.; Gese, A.; Czypicki, R.; Gąsior, M.; Tretyn, A.; Chojnowski, J.; Bieliński, M.; Jaracz, M.; Kamińska, A.; Junik, R.; et al. Correlations between polymorphisms in genes coding elements of dopaminergic pathways and body mass index in overweight and obese women. Endokrynol. Pol. 2013, 64, 101–107. [Google Scholar]
- Davis, C.A.; Levitan, R.D.; Reid, C.; Carter, J.C.; Kaplan, A.S.; Patte, K.A.; King, N.; Curtis, C.; Kennedy, J.L. Dopamine for “wanting” and opioids for “liking”: A comparison of obese adults with and without binge eating. Obesity 2009, 17, 1220–1225. [Google Scholar] [CrossRef]
- Need, A.C.; Ahmadi, K.R.; Spector, T.D.; Goldstein, D.B. Obesity is associated with genetic variants that alter dopamine availability. Ann. Hum. Genet. 2006, 70, 293–303. [Google Scholar] [CrossRef]
- Lawford, B.R.; Barnes, M.; Morris, C.P.; Noble, E.P.; Nyst, P.; Heslop, K.; Young, R.M.; Voisey, J.; Connor, J.P. Dopamine 2 Receptor Genes Are Associated with Raised Blood Glucose in Schizophrenia. Can. J. Psychiatry 2016, 61, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Wilkinson, A.; Shen, J.; Wu, X.; Chow, W.H. Genetic polymorphisms in genes related to risk-taking behaviours predicting body mass index trajectory among Mexican American adolescents. Pediatr. Obes. 2017, 12, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.D.; Riou, M.; Tesson, F.; Goldfield, G.S.; Rabasa-Lhoret, R.; Brochu, M.; Doucet, É. The TaqIA RFLP is associated with attenuated intervention-induced body weight loss and increased carbohydrate intake in post-menopausal obese women. Appetite 2013, 60, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comings, D.E.; Gade, R.; MacMurray, J.P.; Muhleman, D.; Peters, W.R. Genetic variants of the human obesity (OB) gene: Association with body mass index in young women, psychiatric symptoms, and interaction with the dopamine D2 receptor (DRD2) gene. Mol. Psychiatry 1996, 1, 325–335. [Google Scholar] [PubMed]
- Guo, G.; North, K.; Choi, S. DRD4 gene variant associated with body mass: The National Longitudinal Study of Adolescent Health. Hum. Mutat. 2006, 27, 236–241. [Google Scholar] [CrossRef]
- Stice, E.; Yokum, S.; Bohon, C.; Marti, N.; Smolen, A. Reward circuitry responsivity to food predicts future increases in body mass: Moderating effects of DRD2 and DRD4. NeuroImage 2010, 50, 1618–1625. [Google Scholar] [CrossRef] [Green Version]
- Levitan, R.D.; Masellis, M.; Lam, R.W.; Kaplan, A.S.; Davis, C.; Tharmalingam, S.; Mackenzie, B.; Basile, V.S.; Kennedy, J.L. A birth-season/DRD4 gene interaction predicts weight gain and obesity in women with seasonal affective disorder: A seasonal thrifty phenotype hypothesis. Neuropsychopharmacology 2006, 31, 2498–2503. [Google Scholar] [CrossRef] [Green Version]
- Leyton, M. What’s deficient in reward deficiency? J. Psychiatry Neurosci. JPN 2014, 39, 291–293. [Google Scholar] [CrossRef] [Green Version]
- Richter, A.; Richter, S.; Barman, A.; Soch, J.; Klein, M.; Assmann, A.; Libeau, C.; Behnisch, G.; Wüstenberg, T.; Seidenbecher, C.I.; et al. Motivational salience and genetic variability of dopamine D2 receptor expression interact in the modulation of interference processing. Front. Hum. Neurosci. 2013, 7, 250. [Google Scholar] [CrossRef] [Green Version]
- Koob, G.F.; Le Moal, M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2008, 363, 3113–3123. [Google Scholar] [CrossRef] [Green Version]
- Stice, E.; Spoor, S.; Bohon, C.; Small, D.M. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 2008, 322, 449–452. [Google Scholar] [CrossRef] [Green Version]
- Nutt, D.J.; Lingford-Hughes, A.; Erritzoe, D.; Stokes, P.R. The dopamine theory of addiction: 40 years of highs and lows. Nature reviews. Neuroscience 2015, 16, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dockstader, C.L.; Rubinstein, M.; Grandy, D.K.; Low, M.J.; van der Kooy, D. The D2 receptor is critical in mediating opiate motivation only in opiate-dependent and withdrawn mice. Eur. J. Neurosci. 2001, 13, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Laviolette, S.R.; Nader, K.; van der Kooy, D. Motivational state determines the functional role of the mesolimbic dopamine system in the mediation of opiate reward processes. Behav. Brain Res. 2002, 129, 17–29. [Google Scholar] [CrossRef]
- Bechara, A.; Nader, K.; van der Kooy, D. A two-separate-motivational-systems hypothesis of opioid addiction. Pharmacol. Biochem. Behav. 1998, 59, 1–17. [Google Scholar] [CrossRef]
- Stringer, S.; Minică, C.C.; Verweij, K.J.; Mbarek, H.; Bernard, M.; Derringer, J.; van Eijk, K.R.; Isen, J.D.; Loukola, A.; Maciejewski, D.F.; et al. Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Transl. Psychiatry 2016, 6, e769. [Google Scholar] [CrossRef]
- Potenza, M.N. How central is dopamine to pathological gambling or gambling disorder? Front. Behav. Neurosci. 2013, 7, 206. [Google Scholar] [CrossRef] [Green Version]
- Potenza, M.N. Searching for Replicable Dopamine-Related Findings in Gambling Disorder. Biol. Psychiatry 2018, 83, 984–986. [Google Scholar] [CrossRef]
- Muntean, B.S.; Dao, M.T.; Martemyanov, K.A. Allostatic Changes in the cAMP System Drive Opioid-Induced Adaptation in Striatal Dopamine Signaling. Cell Rep. 2019, 29, 946–960.e2. [Google Scholar] [CrossRef] [Green Version]
- da Silva Lobo, D.S.; Vallada, H.P.; Knight, J.; Martins, S.S.; Tavares, H.; Gentil, V.; Kennedy, J.L. Dopamine genes and pathological gambling in discordant sib-pairs. J. Gambl. Stud. 2007, 23, 421–433. [Google Scholar] [CrossRef]
- Pettorruso, M.; Zoratto, F.; Miuli, A.; De Risio, L.; Santorelli, M.; Pierotti, A.; Martinotti, G.; Adriani, W.; di Giannantonio, M. Exploring dopaminergic transmission in gambling addiction: A systematic translational review. Neurosci. Biobehav. Rev. 2020, 119, 481–511. [Google Scholar] [CrossRef]
- Potenza, M.N.; Steinberg, M.A.; Skudlarski, P.; Fulbright, R.K.; Lacadie, C.M.; Wilber, M.K.; Rounsaville, B.J.; Gore, J.C.; Wexler, B.E. Gambling urges in pathological gambling: A functional magnetic resonance imaging study. Arch. Gen. Psychiatry 2003, 60, 828–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glazer, J.; King, A.; Yoon, C.; Liberzon, I.; Kitayama, S. DRD4 polymorphisms modulate reward positivity and P3a in a gambling task: Exploring a genetic basis for cultural learning. Psychophysiology 2020, 57, e13623. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Z.; Balodis, I.M.; Lacadie, C.M.; Xu, J.; Potenza, M.N. A Preliminary Study of DBH (Encoding Dopamine Beta-Hydroxylase) Genetic Variation and Neural Correlates of Emotional and Motivational Processing in Individuals with and without Pathological Gambling. J. Behav. Addict. 2016, 5, 282–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagundo, A.B.; Fernández-Aranda, F.; de la Torre, R.; Verdejo-García, A.; Granero, R.; Penelo, E.; Gené, M.; Barrot, C.; Sánchez, C.; Alvarez-Moya, E.; et al. Dopamine DRD2/ANKK1 Taq1A and DAT1 VNTR polymorphisms are associated with a cognitive flexibility profile in pathological gamblers. J. Psychopharmacol. 2014, 28, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Voigt, G.; Montag, C.; Markett, S.; Reuter, M. On the genetics of loss aversion: An interaction effect of BDNF Val66Met and DRD2/ANKK1 Taq1a. Behav. Neurosci. 2015, 129, 801–811. [Google Scholar] [CrossRef]
- Verdejo-Garcia, A.; Clark, L.; Verdejo-Román, J.; Albein-Urios, N.; Martinez-Gonzalez, J.M.; Gutierrez, B.; Soriano-Mas, C. Neural substrates of cognitive flexibility in cocaine and gambling addictions. Br. J. Psychiatry J. Ment. Sci. 2015, 207, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Hillemacher, T.; Frieling, H.; Buchholz, V.; Hussein, R.; Bleich, S.; Meyer, C.; John, U.; Bischof, A.; Rumpf, H.J. Alterations in DNA-methylation of the dopamine-receptor 2 gene are associated with abstinence and health care utilization in individuals with a lifetime history of pathologic gambling. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 63, 30–34. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D.; Telang, F. Addiction: Beyond dopamine reward circuitry. Proc. Natl. Acad. Sci. USA 2011, 108, 15037–15042. [Google Scholar] [CrossRef] [Green Version]
- Ariza, M.; Garolera, M.; Jurado, M.A.; Garcia-Garcia, I.; Hernan, I.; Sánchez-Garre, C.; Vernet-Vernet, M.; Sender-Palacios, M.J.; Marques-Iturria, I.; Pueyo, R.; et al. Dopamine genes (DRD2/ANKK1-TaqA1 and DRD4-7R) and executive function: Their interaction with obesity. PLoS ONE 2012, 7, e41482. [Google Scholar] [CrossRef] [Green Version]
- Ikemoto, S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci. Biobehav. Rev. 2010, 35, 129–150. [Google Scholar] [CrossRef] [Green Version]
- Taber, K.H.; Black, D.N.; Porrino, L.J.; Hurley, R.A. Neuroanatomy of dopamine: Reward and addiction. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 1–4. [Google Scholar] [CrossRef] [PubMed]
- MacKillop, J.; Munafò, M.R. An intermediate phenotype approach to addiction genetics. In Genetic Influences on Addiction: An Intermediate Phenotype Approach; MacKillop, J., Munafò, M.R., Eds.; The MIT Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Blum, K.; Febo, M.; McLaughlin, T.; Cronje, F.J.; Han, D.; Gold, S.M. Hatching the behavioral addiction egg: Reward Deficiency Solution System (RDSS) as a function of dopaminergic neurogenetics and brain functional connectivity linking all addictions under a common rubric. J. Behav. Addict. 2014, 3, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowirrat, A.; Oscar-Berman, M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2005, 132b, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; González-Giraldo, Y.; Wegman-Ostrosky, T.; Forero, D.A. Molecular genetics of substance use disorders: An umbrella review. Neurosci. Biobehav. Rev. 2021, 124, 358–369. [Google Scholar] [CrossRef]
- Deak, J.D.; Johnson, E.C. Genetics of substance use disorders: A review. Psychol. Med. 2021, 51, 2189–2200. [Google Scholar] [CrossRef]
- Palmer, R.H.; Brick, L.; Nugent, N.R.; Bidwell, L.C.; McGeary, J.E.; Knopik, V.S.; Keller, M.C. Examining the role of common genetic variants on alcohol, tobacco, cannabis and illicit drug dependence: Genetics of vulnerability to drug dependence. Addiction 2015, 110, 530–537. [Google Scholar] [CrossRef]
- Jung, Y.; Montel, R.A.; Shen, P.H.; Mash, D.C.; Goldman, D. Assessment of the Association of D2 Dopamine Receptor Gene and Reported Allele Frequencies with Alcohol Use Disorders: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1914940. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Baron, D.; Lott, L.; Ponce, J.V.; Siwicki, D.; Boyett, B.; Steinberg, B.; Modestino, E.J.; Fried, L.; Hauser, M.; et al. In Search of Reward Deficiency Syndrome (RDS)-free Controls: The “Holy Grail” in Genetic Addiction Risk Testing. Curr. Psychopharmacol. 2020, 9, 7–21. [Google Scholar] [CrossRef]
- Stanfill, A.G.; Cao, X. Expression of Dopamine-Related Genes in Four Human Brain Regions. Brain Sci. 2020, 10, 567. [Google Scholar] [CrossRef]
- Zhou, H.; Sealock, J.M.; Sanchez-Roige, S.; Clarke, T.K.; Levey, D.F.; Cheng, Z.; Li, B.; Polimanti, R.; Kember, R.L.; Smith, R.V.; et al. Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat. Neurosci. 2020, 23, 809–818. [Google Scholar] [CrossRef]
- Crews, F.T.; Robinson, D.L.; Chandler, L.J.; Ehlers, C.L.; Mulholland, P.J.; Pandey, S.C.; Rodd, Z.A.; Spear, L.P.; Swartzwelder, H.S.; Vetreno, R.P. Mechanisms of Persistent Neurobiological Changes Following Adolescent Alcohol Exposure: NADIA Consortium Findings. Alcohol. Clin. Exp. Res. 2019, 43, 1806–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, L.E.; Ratanatharathorn, A.; Aiello, A.E.; Almli, L.M.; Amstadter, A.B.; Ashley-Koch, A.E.; Baker, D.G.; Beckham, J.C.; Bierut, L.J.; Bisson, J.; et al. Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol. Psychiatry 2018, 23, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Rentsch, C.T.; Cheng, Z.; Kember, R.L.; Nunez, Y.Z.; Sherva, R.M.; Tate, J.P.; Dao, C.; Xu, K.; Polimanti, R.; et al. Association of OPRM1 Functional Coding Variant With Opioid Use Disorder: A Genome-Wide Association Study. JAMA Psychiatry 2020, 77, 1072–1080. [Google Scholar] [CrossRef]
- Gelernter, J.; Kranzler, H.R. Genetics of drug dependence. Dialogues Clin. Neurosci. 2010, 12, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Z.; Kranzler, H.R.; Zhao, H.; Gruen, J.R.; Luo, X.; Gelernter, J. Haplotypic variants in DRD2, ANKK1, TTC12, and NCAM1 are associated with comorbid alcohol and drug dependence. Alcohol. Clin. Exp. Res. 2008, 32, 2117–2127. [Google Scholar] [CrossRef] [Green Version]
- Jagannathan, K.; Calhoun, V.D.; Gelernter, J.; Stevens, M.C.; Liu, J.; Bolognani, F.; Windemuth, A.; Ruaño, G.; Assaf, M.; Pearlson, G.D. Genetic associations of brain structural networks in schizophrenia: A preliminary study. Biol. Psychiatry 2010, 68, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, L.K.; Pugh, K.R.; Mencl, W.E.; Gelernter, J. C957T polymorphism of the dopamine D2 receptor gene modulates the effect of nicotine on working memory performance and cortical processing efficiency. Psychopharmacology 2006, 188, 530–540. [Google Scholar] [CrossRef]
- Gold, M.S.; Baron, D.; Bowirrat, A.; Blum, K. Neurological correlates of brain reward circuitry linked to opioid use disorder (OUD): Do homo sapiens acquire or have a reward deficiency syndrome? J. Neurol. Sci. 2020, 418, 117137. [Google Scholar] [CrossRef]
- Berridge, K.C.; Kringelbach, M.L. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology 2008, 199, 457–480. [Google Scholar] [CrossRef] [Green Version]
- Wise, R.A. Dopamine and reward: The anhedonia hypothesis 30 years on. Neurotox. Res. 2008, 14, 169–183. [Google Scholar] [CrossRef] [Green Version]
- Elman, I.; Borsook, D. Common Brain Mechanisms of Chronic Pain and Addiction. Neuron 2016, 89, 11–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elman, I.; Borsook, D.; Volkow, N.D. Pain and suicidality: Insights from reward and addiction neuroscience. Prog. Neurobiol. 2013, 109, 1–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borsook, D.; Linnman, C.; Faria, V.; Strassman, A.M.; Becerra, L.; Elman, I. Reward deficiency and anti-reward in pain chronification. Neurosci. Biobehav. Rev. 2016, 68, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Rommelse, N.N.; Altink, M.E.; Martin, N.C.; Buschgens, C.J.; Faraone, S.V.; Buitelaar, J.K.; Sergeant, J.A.; Oosterlaan, J. Relationship between endophenotype and phenotype in ADHD. Behav. Brain Funct. BBF 2008, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Atzmon, G.; Baron, D.; Badgaiyan, R.D. Hypothesizing Molecular Genetics of the Holocaust: Were Dopaminergic Genes Involved or Brain Wash? SOJ Psychol. 2016, 13, 1–5. [Google Scholar] [CrossRef] [Green Version]

| Gene with Most Common Risk Allele | Global Heterozygous Prevalence (%) | Percent of Subjects with Variant * |
|---|---|---|
| Dopamine D2 Receptor (DRD2): ** rs1800497—risk allele A1 | 46 | Asian 33%, Black or African American 55%, Hispanic or Latino 59%, Mixed Race 100%, Other 22%, Unknown 31%, White or Caucasian 34% |
| Dopamine D3 Receptor (DRD3): rs6280—risk allele C (Ser9Gly) | 41 | Asian 56%, Black or African American 93%, Hispanic or Latino 52%, Mixed Race 100%, Other 67%, Unknown 56%, White or Caucasian 54% |
| Dopamine D4 Receptor (DRD4): rs1800955—risk allele C (48bp repeat VNTR) | 42 | Asian 44%, Black or African American 57%, Hispanic or Latino 55%, Mixed Race 100%, Other 78%, Unknown 58%, White or Caucasian 70% |
| µ-Opioid Receptor (OPRM1): rs1799971—risk allele G (A118G) | 29 | Asian 56%, Black or African American 2%, Hispanic or Latino 28%, Mixed Race 0%, Other 0%, Unknown 39%, White or Caucasian 21% |
| Serotonin Transporter Receptor (5HTT) Linked Promoter Region (5HTTLPR) in SLC6A4: rs25531—risk allele S’ | 43 * | Asian 100%, Black or African American 71%, Hispanic or Latino 76%, Mixed Race 100%, Other 56%, Unknown 81%, White or Caucasian 76% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blum, K.; McLaughlin, T.; Bowirrat, A.; Modestino, E.J.; Baron, D.; Gomez, L.L.; Ceccanti, M.; Braverman, E.R.; Thanos, P.K.; Cadet, J.L.; et al. Reward Deficiency Syndrome (RDS) Surprisingly Is Evolutionary and Found Everywhere: Is It “Blowin’ in the Wind”? J. Pers. Med. 2022, 12, 321. https://doi.org/10.3390/jpm12020321
Blum K, McLaughlin T, Bowirrat A, Modestino EJ, Baron D, Gomez LL, Ceccanti M, Braverman ER, Thanos PK, Cadet JL, et al. Reward Deficiency Syndrome (RDS) Surprisingly Is Evolutionary and Found Everywhere: Is It “Blowin’ in the Wind”? Journal of Personalized Medicine. 2022; 12(2):321. https://doi.org/10.3390/jpm12020321
Chicago/Turabian StyleBlum, Kenneth, Thomas McLaughlin, Abdalla Bowirrat, Edward J. Modestino, David Baron, Luis Llanos Gomez, Mauro Ceccanti, Eric R. Braverman, Panayotis K. Thanos, Jean Lud Cadet, and et al. 2022. "Reward Deficiency Syndrome (RDS) Surprisingly Is Evolutionary and Found Everywhere: Is It “Blowin’ in the Wind”?" Journal of Personalized Medicine 12, no. 2: 321. https://doi.org/10.3390/jpm12020321
APA StyleBlum, K., McLaughlin, T., Bowirrat, A., Modestino, E. J., Baron, D., Gomez, L. L., Ceccanti, M., Braverman, E. R., Thanos, P. K., Cadet, J. L., Elman, I., Badgaiyan, R. D., Jalali, R., Green, R., Simpatico, T. A., Gupta, A., & Gold, M. S. (2022). Reward Deficiency Syndrome (RDS) Surprisingly Is Evolutionary and Found Everywhere: Is It “Blowin’ in the Wind”? Journal of Personalized Medicine, 12(2), 321. https://doi.org/10.3390/jpm12020321














