Personalized Assessment of Insomnia and Sleep Quality in Patients with Parkinson’s Disease
Abstract
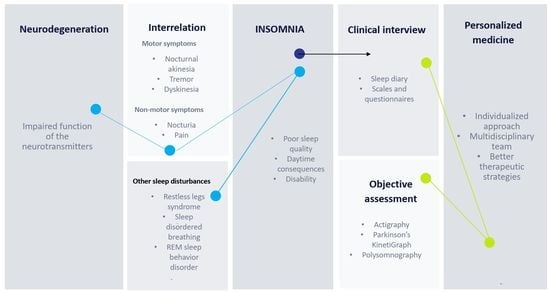
:1. Introduction
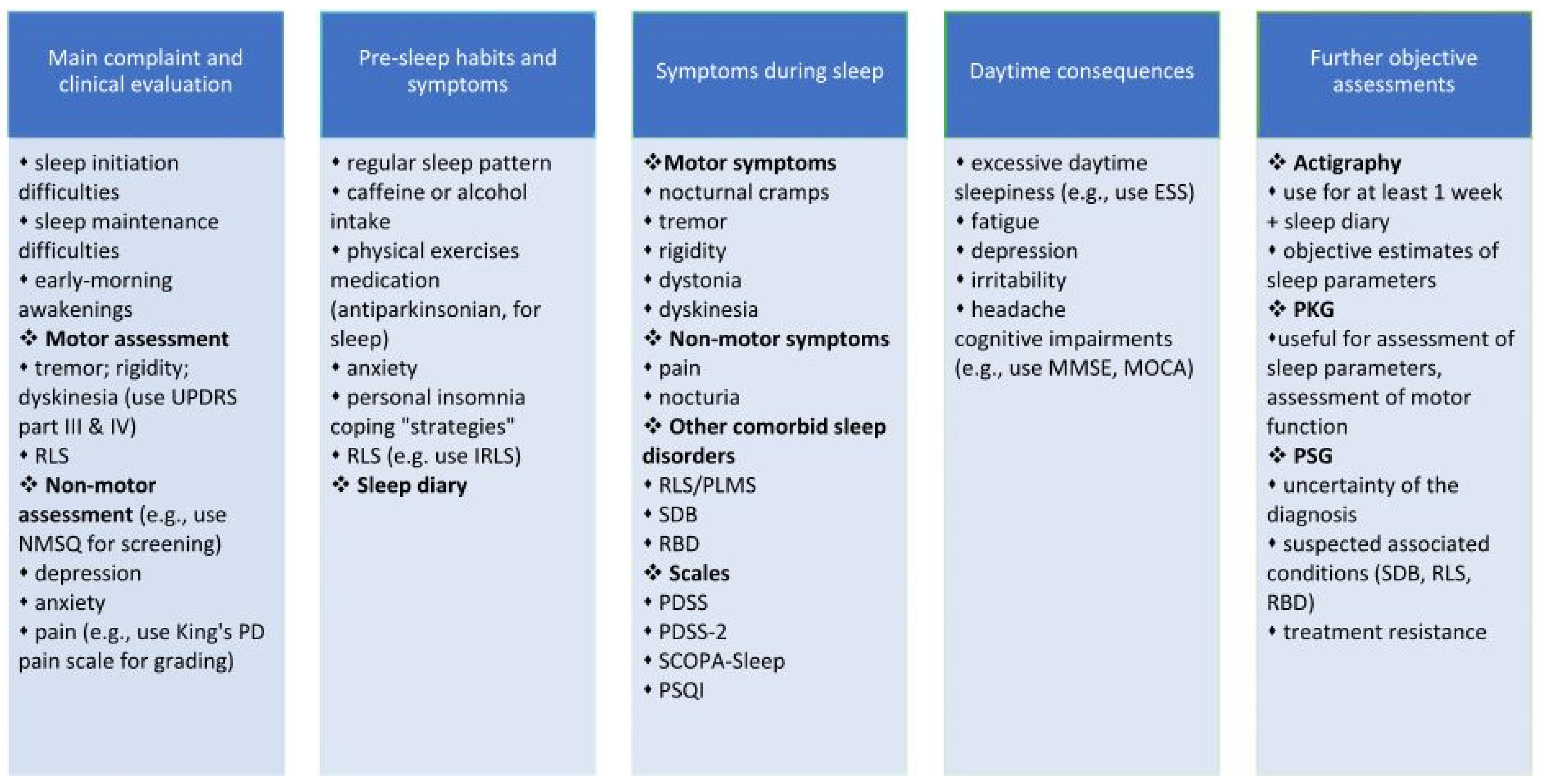
2. Assessment
2.1. Clinical Interview
2.2. The Assessment of Specific Risk Factors for Insomnia Associated with PD
2.2.1. Motor Symptoms
2.2.2. Non-Motor Symptoms
2.2.3. Other Associated Sleep Disorders
2.3. Rating Scales and Objective Assessment of Insomnia and Quality of Sleep in PD Patients
2.3.1. Multidomain Scales or Questionnaires Designed to Evaluate Non-Motor Symptoms in PD, including Insomnia
2.3.2. Specific Scales or Questionnaires Designed to Evaluate Insomnia and Sleep Quality
2.4. Objective Methods to Assess Insomnia
3. Personalized Medicine and the Assessment of the PD Patient with Insomnia and Impaired Quality of Sleep
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falup-Pecurariu, C.; Diaconu, Ș.; Țînț, D.; Falup-Pecurariu, O. Neurobiology of sleep (Review). Exp. Ther. Med. 2021, 21, 272. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; van Hilten, J.J.; Marinus, J. The course of insomnia in Parkinson’s disease. Parkinsonism Relat. Disord. 2016, 33, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Darien, I. The International Classification of Sleep Disorders (ICSD-3); American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Barone, P.; Antonini, A.; Colosimo, C.; Marconi, R.; Morgante, L.; Avarello, T.P.; Bottacchi, E.; Cannas, A.; Ceravolo, G.; Ceravolo, R.; et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov. Disord. 2009, 24, 1641-9. [Google Scholar] [CrossRef] [PubMed]
- Gjerstad, M.D.; Wentzel-Larsen, T.; Aarsland, D.; Larsen, J.P. Insomnia in Parkinson’s disease: Frequency and progression over time. J. Neurol. Neurosurg. Psychiatry 2007, 78, 476–479. [Google Scholar] [CrossRef] [Green Version]
- Yong, M.H.; Fook-Chong, S.; Pavanni, R.; Lim, L.L.; Tan, E.K. Case control polysomnographic studies of sleep disorders in Parkinson’s disease. PLoS ONE 2011, 6, e22511. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, V.K.; Keshavamurthy, B. Sleep dysfunction in Parkinson’s disease. J. Clin. Diagn. Res. 2016, 10, OC09–OC12. [Google Scholar] [CrossRef]
- Bolitho, S.J.; Naismith, S.L.; Salahuddin, P.; Terpening, Z.; Grunstein, R.R.; Lewis, S.J.G. Objective measurement of daytime napping, cognitive dysfunction and subjective sleepiness in Parkinson’s disease. PLoS ONE 2013, 8, e81233. [Google Scholar] [CrossRef] [Green Version]
- Tholfsen, L.K.; Larsen, J.P.; Schulz, J.; Tysnes, O.B.; Gjerstad, M.D. Changes in insomnia subtypes in early Parkinson disease. Neurology 2017, 88, 352–358. [Google Scholar] [CrossRef]
- Ylikoski, A.; Martikainen, K.; Sieminski, M.; Partinen, M. Parkinson’s disease and insomnia. Neurol. Sci. 2015, 36, 2003–2010. [Google Scholar] [CrossRef]
- Shafazand, S.; Wallace, D.M.; Arheart, K.L.; Vargas, S.; Luca, C.C.; Moore, H.; Katzen, H.; Levin, B.; Singer, C. Insomnia, sleep quality, and quality of life in mild to moderate parkinson’s disease. Ann. Am. Thorac. Soc. 2017, 14, 412–419. [Google Scholar] [CrossRef]
- Santos-García, D.; de Deus, T.; Cores, C.; Canfield, H.; Paz González, J.M.; Martínez Miró, C.; Valdés Aymerich, L.; Suárez, E.; Jesús, S.; Aguilar, M.; et al. Predictors of global non-motor symptoms burden progression in Parkinson’s disease. Results from the COPPADIS Cohort at 2-year follow-up. J. Pers. Med. 2021, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Ivan, I.-F.; Irincu, V.-L.; Diaconu, Ș.; Falup-Pecurariu, O.; Ciopleiaș, B.; Falup-Pecurariu, C. Gastro-intestinal dysfunctions in Parkinson’s disease (Review). Exp. Ther. Med. 2021, 22, 1083. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Okuma, Y.; Uchiyama, T.; Miyamoto, M.; Sakakibara, R.; Shimo, Y.; Hattori, N.; Kuwabara, S.; Yamamoto, T.; Kaji, Y.; et al. Impact of sleep-related symptoms on clinical motor subtypes and disability in Parkinson’s disease: A multicentre cross-sectional study. J. Neurol. Neurosurg. Psychiatry 2017, 88, 953–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falup-Pecurariu, C.; Diaconu, Ş. Sleep dysfunction in Parkinson’s disease. Int. Rev. Neurobiol. 2017, 133, 719–742. [Google Scholar] [CrossRef] [PubMed]
- Paus, S.; Brecht, H.M.; Köster, J.; Seeger, G.; Klockgether, T.; Wüllner, U. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson’s disease. Mov. Disord. 2003, 18, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Doufas, A.G.; Panagiotou, O.A.; Panousis, P.; Wong, S.S.; Ioannidis, J.P. Insomnia from drug treatments: Evidence from meta-analyses of randomized trials and concordance with prescribing information. Mayo Clin. Proc. 2017, 92, 72–87. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Martinez-Martin, P.; Schapira, A.H.V.; Stocchi, F.; Sethi, K.; Odin, P.; Brown, R.G.; Koller, W.; Barone, P.; MacPhee, G.; et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: The NMSQuest study. Mov. Disord. 2006, 21, 916–923. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, J.Y.; Li, J.; Liu, C.F. Excessive daytime sleepiness in Parkinson’s disease: Clinical implications and management. Chin. Med. J. 2018, 34, 180–198. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Högl, B.; Arnulf, I.; Comella, C.; Ferreira, J.; Iranzo, A.; Tilley, B.; Trenkwalder, C.; Poewe, W.; Rascol, O.; Sampaio, C.; et al. Scales to assess sleep impairment in Parkinson’s disease: Critique and recommendations. Mov. Disord. 2010, 25, 2704–2716. [Google Scholar] [CrossRef]
- Huang, J.; Zhuo, W.; Zhang, Y.; Sun, H.; Chen, H.; Zhu, P.; Pan, X.; Yang, J.; Wang, L. Cognitive function characteristics of Parkinson’s disease with sleep disorders. Parkinson’s Dis. 2017, 79, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Hoops, S.; Nazem, S.; Siderowf, A.D.; Duda, J.E.; Xie, S.X.; Stern, M.B.; Weintraub, D. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009, 73, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Fiorenzato, E.; Weis, L.; Falup-Pecurariu, C.; Diaconu, S.; Siri, C.; Reali, E.; Pezzoli, G.; Bisiacchi, P.; Antonini, A.; Biundo, R. Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) performance in progressive supranuclear palsy and multiple system atrophy. J. Neural Transm. 2016, 123, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Greene, D. Dementia Rating Scale-2 (DRS-2) By P.J. Jurica, C.L. Leitten, and S. Mattis: Psychological assessment resources, 2001. Arch. Clin. Neuropsychol. 2004, 19, 145–147. [Google Scholar] [CrossRef] [Green Version]
- Pagonabarraga, J.; Kulisevsky, J.; Llebaria, G.; García-Sánchez, C.; Pascual-Sedano, B.; Gironell, A. Parkinson’s disease-cognitive rating scale: A new cognitive scale specific for Parkinson’s disease. Mov. Disord. 2008, 23, 998–1005. [Google Scholar] [CrossRef]
- Ibáñez, V.; Silva, J.; Cauli, O. A survey on sleep questionnaires and diaries. Sleep Med. 2018, 42, 90–96. [Google Scholar] [CrossRef]
- Stavitsky, K.; Saurman, J.L.; McNamara, P.; Cronin-Golomb, A. Sleep in Parkinson’s disease: A comparison of actigraphy and subjective measures. Parkinsonism Relat. Disord. 2010, 16, 280–283. [Google Scholar] [CrossRef] [Green Version]
- Videnovic, A.; Klerman, E.B.; Wang, W.; Marconi, A.; Kuhta, T.; Zee, P.C. Timed light therapy for sleep and daytime sleepiness associated with Parkinson disease a randomized clinical trial. JAMA Neurol. 2017, 74, 411–418. [Google Scholar] [CrossRef]
- Bollu, P.C.; Kaur, H. Sleep medicine: Insomnia and sleep. Mo. Med. 2019, 116, 68–75. [Google Scholar]
- Wade, R.; Pachana, N.A.; Mellick, G.; DIssanayaka, N. Factors related to sleep disturbances for individuals with Parkinson’s disease: A regional perspective. Int. Psychogeriatr. 2020, 32, 827–838. [Google Scholar] [CrossRef]
- Sobreira-Neto, M.A.; Pena-Pereira, M.A.; Sobreira, E.S.T.; Chagas, M.H.N.; de Almeida, C.M.O.; Fernandes, R.M.F.; Tumas, V.; Eckeli, A.L. Chronic insomnia in patients with Parkinson disease: Which associated factors are relevant? J. Geriatr. Psychiatry Neurol. 2020, 33, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Wang, F.Y.; Mao, C.J.; Guo, S.P.; Chen, J.; Li, J.; Wang, Q.J.; Bei, H.Z.; Yu, Q.; Liu, C.F. Analysis of nocturnal hypokinesia and sleep quality in Parkinson’s disease. J. Clin. Neurosci. 2018, 54, 96–101. [Google Scholar] [CrossRef]
- Bhidayasiri, R.; Trenkwalder, C. Getting a good night sleep? The importance of recognizing and treating nocturnal hypokinesia in Parkinson’s disease. Parkinsonism Relat. Disord. 2018, 50, 10–18. [Google Scholar] [CrossRef]
- Mao, C.J.; Yang, Y.P.; Chen, J.P.; Wang, F.; Chen, J.; Zhang, J.R.; Zhang, H.J.; Zhuang, S.; Xiong, Y.T.; Gu, C.C.; et al. Poor nighttime sleep is positively associated with dyskinesia in Parkinson’s disease patients. Parkinsonism Relat. Disord. 2018, 48, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Dhawan, V.; Dhoat, S.; Williams, A.J.; DiMarco, A.; Pal, S.; Forbes, A.; Tobías, A.; Martinez-Martin, P.; Chaudhuri, K.R. The range and nature of sleep dysfunction in untreated Parkinson’s disease (PD). A comparative controlled clinical study using the Parkinson’s disease sleep scale and selective polysomnography. J. Neurol. Sci. 2006, 248, 158–162. [Google Scholar] [CrossRef]
- Norlinah, M.I.; Afidah, K.N.; Noradina, A.T.; Shamsul, A.S.; Hamidon, B.B.; Sahathevan, R.; Raymond, A.A. Sleep disturbances in Malaysian patients with Parkinson’s disease using polysomnography and PDSS. Parkinsonism Relat. Disord. 2009, 15, 670–674. [Google Scholar] [CrossRef]
- Cai, G.E.; Luo, S.; Chen, L.N.; Lu, J.P.; Huang, Y.J.; Ye, Q.Y. Sleep fragmentation as an important clinical characteristic of sleep disorders in Parkinson’s disease: A preliminary study. Chin. Med. J. 2019, 132, 1788–1795. [Google Scholar] [CrossRef]
- Vaughan, C.P.; Bliwise, D.L. Sleep and nocturia in older adults. Sleep Med. Clin. 2018, 13, 107–116. [Google Scholar] [CrossRef]
- Chung, S.; Bohnen, N.I.; Albin, R.L.; Frey, K.A.; Müller, M.L.T.M.; Chervin, R.D. Insomnia and sleepiness in Parkinson disease: Associations with symptoms and comorbidities. J. Clin. Sleep Med. 2013, 9, 1131–1137. [Google Scholar] [CrossRef]
- Fu, Y.T.; Mao, C.J.; Ma, L.J.; Zhang, H.J.; Wang, Y.; Li, J.; Huang, J.Y.; Liu, J.Y.; Liu, C.F. Pain correlates with sleep disturbances in Parkinson’s disease patients. Pain Pract. 2018, 18, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Martin, P.; Rizos, A.M.; Wetmore, J.B.; Antonini, A.; Odin, P.; Pal, S.; Sophia, R.; Carroll, C.; Martino, D.; Falup-Pecurariu, C.; et al. Relationship of nocturnal sleep dysfunction and pain subtypes in Parkinson’s disease. Mov. Disord. Clin. Pract. 2019, 6, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, K.R.; Rizos, A.; Trenkwalder, C.; Rascol, O.; Pal, S.; Martino, D.; Carroll, C.; Paviour, D.; Falup-Pecurariu, C.; Kessel, B.; et al. King’s Parkinson’s disease pain scale, the first scale for pain in PD: An international validation. Mov. Disord. 2015, 30, 1623–1631. [Google Scholar] [CrossRef] [Green Version]
- Kay, D.B.; Tanner, J.J.; Bowers, D. Sleep disturbances and depression severity in patients with Parkinson’s disease. Brain Behav. 2018, 8, e00967. [Google Scholar] [CrossRef]
- Rana, A.Q.; Qureshi, A.R.M.; Kachhvi, H.B.; Rana, M.A.; Chou, K.L. Increased likelihood of anxiety and poor sleep quality in Parkinson’s disease patients with pain. J. Neurol. Sci. 2016, 369, 212–215. [Google Scholar] [CrossRef]
- Rana, A.Q.; Qureshi, A.R.M.; Shamli Oghli, Y.; Saqib, Y.; Mohammed, B.; Sarfraz, Z.; Rana, R. Decreased sleep quality in Parkinson’s patients is associated with higher anxiety and depression prevalence and severity, and correlates with pain intensity and quality. Neurol. Res. 2018, 40, 696–701. [Google Scholar] [CrossRef]
- Ferini-Strambi, L.; Carli, G.; Casoni, F.; Galbiati, A. Restless legs syndrome and Parkinson disease: A causal relationship between the two disorders? Front. Neurol. 2018, 9, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonakis, A.; Androutsou, A.; Koloutsou, M.E.; Vagiakis, E. Restless Legs Syndrome masquerades as chronic insomnia. Sleep Medicine 2020, 75, 106–111. [Google Scholar] [CrossRef]
- Allen, R.P.; Picchietti, D.L.; Garcia-Borreguero, D.; Ondo, W.G.; Walters, A.S.; Winkelman, J.W.; Zucconi, M.; Ferri, R.; Trenkwalder, C.; Lee, H.B. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria—History, rationale, description, and significance. Sleep Med. 2014, 15, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Walters, A.S.; LeBrocq, C.; Dhar, A.; Hening, W.; Rosen, R.; Allen, R.P.; Trenkwalder, C.; The International Restless Legs Syndrome Study Group. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003, 4, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Björnsdóttir, E.; Janson, C.; Gíslason, T.; Sigurdsson, J.F.; Pack, A.I.; Gehrman, P.; Benediktsdóttir, B. Insomnia in untreated sleep apnea patients compared to controls. J. Sleep Res. 2012, 21, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krell, S.B.; Kapur, V.K. Insomnia complaints in patients evaluated for obstructive sleep apnea. Sleep Breath. 2005, 9, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Sobreira-Neto, M.A.; Pena-Pereira, M.A.; Sobreira, E.S.T.; Chagas, M.H.N.; Fernandes, R.M.F.; Tumas, V.; Eckeli, A.L. High frequency of sleep disorders in Parkinson’s disease and its relationship with quality of life. Eur. Neurol. 2017, 78, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Sobreira-Neto, M.A.; Pena-Pereira, M.A.; Sobreira, E.S.T.; Chagas, M.H.N.; De Almeida, C.M.O.; Fernandes, R.M.F.; Tumas, V.; Eckeli, A.L. Obstructive sleep apnea and Parkinson’s disease: Characteristics and associated factors. Arq. Neuro-Psiquiatr. 2019, 77, 609–616. [Google Scholar] [CrossRef]
- St Louis, E.K.; Boeve, B.F. REM sleep behavior disorder: Diagnosis, clinical implications, and future directions. Mayo Clin. Proc. 2017, 92, 1723–1736. [Google Scholar] [CrossRef]
- Kamble, N.; Yadav, R.; Lenka, A.; Kumar, K.; Nagaraju, B.C.; Pal, P.K. Impaired sleep quality and cognition in patients of Parkinson’s disease with REM sleep behavior disorder: A comparative study. Sleep Med. 2019, 62, 1–5. [Google Scholar] [CrossRef]
- Diaconu, Ș.; Falup-Pecurariu, O.; Țînț, D.; Falup-Pecurariu, C. REM sleep behaviour disorder in Parkinson’s disease (Review). Exp. Ther. Med. 2021, 22, 812. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.; Ye, J.; Fu, Y.; Wang, J.; Su, L.; Zhu, X.; Zhang, M.; Cheng, Y.; Wu, W.; et al. Polysomnographic and neuropsychological characteristics of rapid eye movement sleep behavior disorder patients. Brain Behav. 2019, 9, e01220. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Martinez-Martin, P.; Brown, R.G.; Sethi, K.; Stocchi, F.; Odin, P.; Ondo, W.; Abe, K.; MacPhee, G.; MacMahon, D.; et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot study. Mov. Disord. 2007, 22, 1901–1911. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Schrag, A.; Weintraub, D.; Rizos, A.; Rodriguez-Blazquez, C.; Mamikonyan, E.; Martinez-Martin, P. The movement disorder society nonmotor rating scale: Initial validation study. Mov. Disord. 2020, 35, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Pal, S.; DiMarco, A.; Whately-Smith, C.; Bridgman, K.; Mathew, R.; Pezzela, F.R.; Forbes, A.; Högl, B.; Trenkwalder, C. The Parkinson’s disease sleep scale: A new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 73, 629–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Martin, P.; Visser, M.; Rodriguez-Blazquez, C.; Marinus, J.; Chaudhuri, K.R.; van Hilten, J.J. SCOPA-sleep and PDSS: Two scales for assessment of sleep disorder in Parkinson’s disease. Mov. Disord. 2008, 23, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martín, P.; Salvador, C.; Menéndez-Guisasola, L.; González, S.; Tobías, A.; Almazán, J.; Chaudhuri, K.R. Parkinson’s disease sleep scale: Validation study of a Spanish version. Mov. Disord. 2004, 19, 1226–1232. [Google Scholar] [CrossRef]
- Abe, K.; Hikita, T.; Sakoda, S. Sleep disturbances in Japanese patients with Parkinson’s disease—Comparing with patients in the UK. J. Neurol. Sci. 2005, 234, 73–78. [Google Scholar] [CrossRef]
- Margis, R.; Donis, K.; Schönwald, S.V.; Fagondes, S.C.; Monte, T.; Martín-Martínez, P.; Chaudhuri, K.R.; Kapczinski, F.; Rieder, C.R.M. Psychometric properties of the Parkinson’s disease sleep scale—Brazilian version. Parkinsonism Relat. Disord. 2009, 15, 495–499. [Google Scholar] [CrossRef]
- Pierantozzi, M.; Placidi, F.; Liguori, C.; Albanese, M.; Imbriani, P.; Marciani, M.G.; Mercuri, N.B.; Stanzione, P.; Stefani, A. Rotigotine may improve sleep architecture in Parkinson’s disease: A double-blind, Randomized, Placebo-controlled polysomnographic study. Sleep Med. 2016, 21, 140–144. [Google Scholar] [CrossRef]
- Trenkwalder, C.; Kohnen, R.; Högl, B.; Metta, V.; Sixel-Döring, F.; Frauscher, B.; Hülsmann, J.; Martinez-Martin, P.; Chaudhuri, K.R. Parkinson’s disease sleep scale—Validation of the revised version PDSS-2. Mov. Disord. 2011, 26, 644–652. [Google Scholar] [CrossRef]
- Suzuki, K.; Miyamoto, T.; Miyamoto, M.; Suzuki, S.; Numao, A.; Watanabe, Y.; Tatsumoto, M.; Sakuta, H.; Watanabe, Y.; Fujita, H.; et al. Evaluation of cutoff scores for the Parkinson’s disease sleep scale-2. Acta Neurol. Scand. 2015, 131, 426–430. [Google Scholar] [CrossRef]
- Horváth, K.; Aschermann, Z.; Ács, P.; Deli, G.; Janszky, J.; Karádi, K.; Komoly, S.; Faludi, B.; Kovács, N. Test-retest validity of Parkinson’s disease sleep scale 2nd version (PDSS-2). J. Parkinson’s Dis. 2014, 4, 687–691. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Wetmore, J.B.; Rodríguez-Blázquez, C.; Arakaki, T.; Bernal, O.; Campos-Arillo, V.; Cerda, C.; Estrada-Bellmann, I.; Garretto, N.; Ginsburg, L.; et al. The Parkinson’s Disease Sleep Scale–2 (PDSS-2): Validation of the spanish version and its relationship with a roommate-based version. Mov. Disord. Clin. Pract. 2019, 6, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Arnaldi, D.; Cordano, C.; De Carli, F.; Accardo, J.; Ferrara, M.; Picco, A.; Tamburini, T.; Brugnolo, A.; Abbruzzese, G.; Nobili, F. Parkinson’s disease sleep scale 2: Application in an Italian population. Neurol. Sci. 2016, 37, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, R.; Du, Y.; Mou, Y.; Li, N.; Cheng, L. Reliability and validity of Parkinson’s disease sleep scale-Chinese version in the south west of China. Natl. Med. J. China 2016, 96, 3294–3299. [Google Scholar] [CrossRef]
- Radziunas, A.; Deltuva, V.P.; Tamasauskas, A.; Gleizniene, R.; Pranckeviciene, A.; Petrikonis, K.; Bunevicius, A. Brain MRI morphometric analysis in Parkinson’s disease patients with sleep disturbances. BMC Neurol. 2018, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, R.K.; Martinez-Martin, P.; Antonini, A.; Brown, R.G.; Friedman, J.H.; Onofrj, M.; Surmann, E.; Ghys, L.; Trenkwalder, C. Rotigotine and specific non-motor symptoms of Parkinson’s disease: Post hoc analysis of RECOVER. Parkinsonism Relat. Disord. 2013, 19, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Marinus, J.; Visser, M.; Van Hilten, J.J.; Lammers, G.J.; Stiggelbout, A.M. Assessment of sleep and sleepiness in parkinson disease. Sleep 2003, 26, 1049–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, N.; Lewitt, P.; Neikrug, A.B.; Kesslak, P.; Coate, B.; Ancoli-Israel, S. Nighttime sleep and daytime sleepiness improved with pimavanserin during treatment of Parkinson’s disease psychosis. Clin. Neuropharmacol. 2018, 41, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Backhaus, J.; Junghanns, K.; Broocks, A.; Riemann, D.; Hohagen, F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J. Psychosom. Res. 2002, 53, 737–740. [Google Scholar] [CrossRef]
- Kurtis, M.M.; Balestrino, R.; Rodriguez-Blazquez, C.; Forjaz, M.J.; Martinez-Martin, P. A review of scales to evaluate sleep disturbances in movement disorders. Front. Neurol. 2018, 9, 369. [Google Scholar] [CrossRef] [Green Version]
- Mao, Z.; Liu, C.; Ji, S.; Yang, Q.; Ye, H.; Han, H.; Xue, Z. Clinical characteristics of sleep disorders in patients with Parkinson’s disease. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2017, 37, 100–104. [Google Scholar] [CrossRef]
- Medeiros, C.A.M.; Carvalhedo De Bruin, P.F.; Lopes, L.A.; Magalhães, M.C.; De Lourdes Seabra, M.; Sales De Bruin, V.M. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson’s disease: A randomized, double blind, placebo-controlled study. J. Neurol. 2007, 254, 459–464. [Google Scholar] [CrossRef]
- Hadoush, H.; Al-Sharman, A.; Khalil, H.; Banihani, S.A.; Al-Jarrah, M. Sleep quality, depression, and quality of life after bilateral anodal transcranial direct current stimulation in patients with Parkinson’s disease. Med. Sci. Monit. Basic Res. 2018, 24, 198–205. [Google Scholar] [CrossRef]
- Martin, J.L.; Hakim, A.D. Wrist actigraphy. Chest 2011, 139, 1514–1527. [Google Scholar] [CrossRef]
- Harvey, A.G.; Tang, N.K.Y. (Mis)perception of sleep in insomnia: A puzzle and a resolution. Psychol. Bull. 2012, 138, 77–101. [Google Scholar] [CrossRef]
- Happe, S.; Klösch, G.; Lorenzo, J.; Kunz, D.; Penzel, T.; Röschke, J.; Himanen, S.L.; Gruber, G.; Zeitlhofer, J. Perception of sleep: Subjective versus objective sleep parameters in patients with Parkinson’s disease in comparison with healthy elderly controls—Sleep perception in Parkinson’s disease and controls. J. Neurol. 2005, 252, 936–943. [Google Scholar] [CrossRef]
- Marino, M.; Li, Y.; Rueschman, M.N.; Winkelman, J.W.; Ellenbogen, J.M.; Solet, J.M.; Dulin, H.; Berkman, L.F.; Buxton, O.M. Measuring sleep: Accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep 2013, 36, 1747–1755. [Google Scholar] [CrossRef]
- Mccall, C.; Mccall, W.V. Comparison of actigraphy with polysomnography and sleep logs in depressed insomniacs. J. Sleep Res. 2012, 21, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Withrow, D.; Roth, T.; Koshorek, G.; Roehrs, T. Relation between ambulatory actigraphy and laboratory polysomnography in insomnia practice and research. J. Sleep Res. 2019, 28, e12854. [Google Scholar] [CrossRef]
- Williams, J.M.; Taylor, D.J.; Slavish, D.C.; Gardner, C.E.; Zimmerman, M.R.; Patel, K.; Reichenberger, D.A.; Francetich, J.M.; Dietch, J.R.; Estevez, R. Validity of actigraphy in young adults with insomnia. Behav. Sleep Med. 2020, 18, 91–106. [Google Scholar] [CrossRef]
- Klingelhoefer, L.; Rizos, A.; Sauerbier, A.; McGregor, S.; Martinez-Martin, P.; Reichmann, H.; Horne, M.; Chaudhuri, K.R. Night-time sleep in Parkinson’s disease—the potential use of Parkinson’s KinetiGraph: A prospective comparative study. Eur. J. Neurol. 2016, 23, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Sundgren, M.; Andréasson, M.; Svenningsson, P.; Noori, R.-M.; Johansson, A. Does information from the Parkinson KinetiGraph™ (PKG) influence the neurologist’s treatment decisions?—An observational study in routine clinical care of people with Parkinson’s disease. J. Pers. Med. 2021, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Kotschet, K.; Johnson, W.; McGregor, S.; Kettlewell, J.; Kyoong, A.; O’Driscoll, D.M.; Turton, A.R.; Griffiths, R.I.; Horne, M.K. Daytime sleep in Parkinson’s disease measured by episodes of immobility. Parkinsonism Relat. Disord. 2014, 20, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Knudson, M.; Thomsen, T.H.; Kjaer, T.W. Comparing objective and subjective measures of Parkinson’s disease using the Parkinson’s KinetiGraph. Front. Neurol. 2020, 11, 570833. [Google Scholar] [CrossRef]
- Chen, L.; Cai, G.; Weng, H.; Yu, J.; Yang, Y.; Huang, X.; Chen, X.; Ye, Q. More sensitive identification for bradykinesia compared to tremors in Parkinson’s disease based on Parkinson’s KinetiGraph (PKG). Front. Aging Neurosci. 2020, 12, 594701. [Google Scholar] [CrossRef]
- McGregor, S.; Churchward, P.; Soja, K.; O’Driscoll, D.; Braybrook, M.; Khodakarami, H.; Evans, A.; Farzanehfar, P.; Hamilton, G.; Horne, M. The use of accelerometry as a tool to measure disturbed nocturnal sleep in Parkinson’s disease. NPJ Parkinson’s Dis. 2018, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Peeraully, T.; Yong, M.H.; Chokroverty, S.; Tan, E.K. Sleep and Parkinson’s disease: A review of case-control polysomnography studies. Mov. Disord. 2012, 27, 1729–1737. [Google Scholar] [CrossRef]
- Vaughan, C.P.; Juncos, J.L.; Trotti, L.M.; Johnson, T.M., 2nd; Bliwise, D.L. Nocturia and overnight polysomnography in Parkinson disease. Neurourol. Urodyn. 2013, 32, 1080–1085. [Google Scholar] [CrossRef] [Green Version]
- Eisensehr, I.; v Lindeiner, H.; Jäger, M.; Noachtar, S. REM sleep behavior disorder in sleep-disordered patients with versus without Parkinson’s disease: Is there a need for polysomnography? J. Neurol. Sci. 2001, 186, 7–11. [Google Scholar] [CrossRef]
- Littner, M.; Hirshkowitz, M.; Kramer, M.; Kapen, S.; McDowell Anderson, W.; Bailey, D.; Berry, R.B.; Davila, D.; Johnson, S.; Kushida, C.; et al. Practice parameters for using polysomnography to evaluate insomnia: An update. Sleep 2003, 26, 754–760. [Google Scholar] [CrossRef] [Green Version]
- Titova, N.; Chaudhuri, K.R. Personalized medicine in Parkinson’s disease: Time to be precise. Mov. Disord. 2017, 32, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Titova, N.; Chaudhuri, K.R. Personalized medicine and nonmotor symptoms in Parkinson’s disease. Int. Rev. Neurobiol. 2017, 134, 1257–1281. [Google Scholar] [CrossRef] [PubMed]
| Scale Name | Designed For | Nr. Items | Approximate Completion Time | Short Description | Time Frame | Cutoff | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|---|
| PDSS |
| 15 | 10 min |
| Previous week | 82/83 |
|
|
| PDSS-2 |
| 15 | 10 min |
| Previous week | ≥15 |
|
|
| SCOPA—sleep |
| 12 | 5–10 min |
| Previous month | 6/7 for night symptoms 4/5 for day symptoms |
|
|
| PSQI |
| 19 | 5–10 min for completing5 min for scoring |
| Previous month | >5 in the general population; >8 in PD patients |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaconu, Ş.; Falup-Pecurariu, C. Personalized Assessment of Insomnia and Sleep Quality in Patients with Parkinson’s Disease. J. Pers. Med. 2022, 12, 322. https://doi.org/10.3390/jpm12020322
Diaconu Ş, Falup-Pecurariu C. Personalized Assessment of Insomnia and Sleep Quality in Patients with Parkinson’s Disease. Journal of Personalized Medicine. 2022; 12(2):322. https://doi.org/10.3390/jpm12020322
Chicago/Turabian StyleDiaconu, Ştefania, and Cristian Falup-Pecurariu. 2022. "Personalized Assessment of Insomnia and Sleep Quality in Patients with Parkinson’s Disease" Journal of Personalized Medicine 12, no. 2: 322. https://doi.org/10.3390/jpm12020322
APA StyleDiaconu, Ş., & Falup-Pecurariu, C. (2022). Personalized Assessment of Insomnia and Sleep Quality in Patients with Parkinson’s Disease. Journal of Personalized Medicine, 12(2), 322. https://doi.org/10.3390/jpm12020322