Cancer Angiogenesis and Opportunity of Influence on Tumor by Changing Vascularization
Abstract
1. Introduction
2. Vascularization as a Predictor at Cancer
3. Influence of VEGF on Tumor Growth and Vascularization
4. Results of Tumor Angiogenesis Inhibition
4.1. Effects of VEGF Suppression and Inactivation of Its Receptors
4.2. Suppression of Factors Indirectly Associated with Angiogenesis
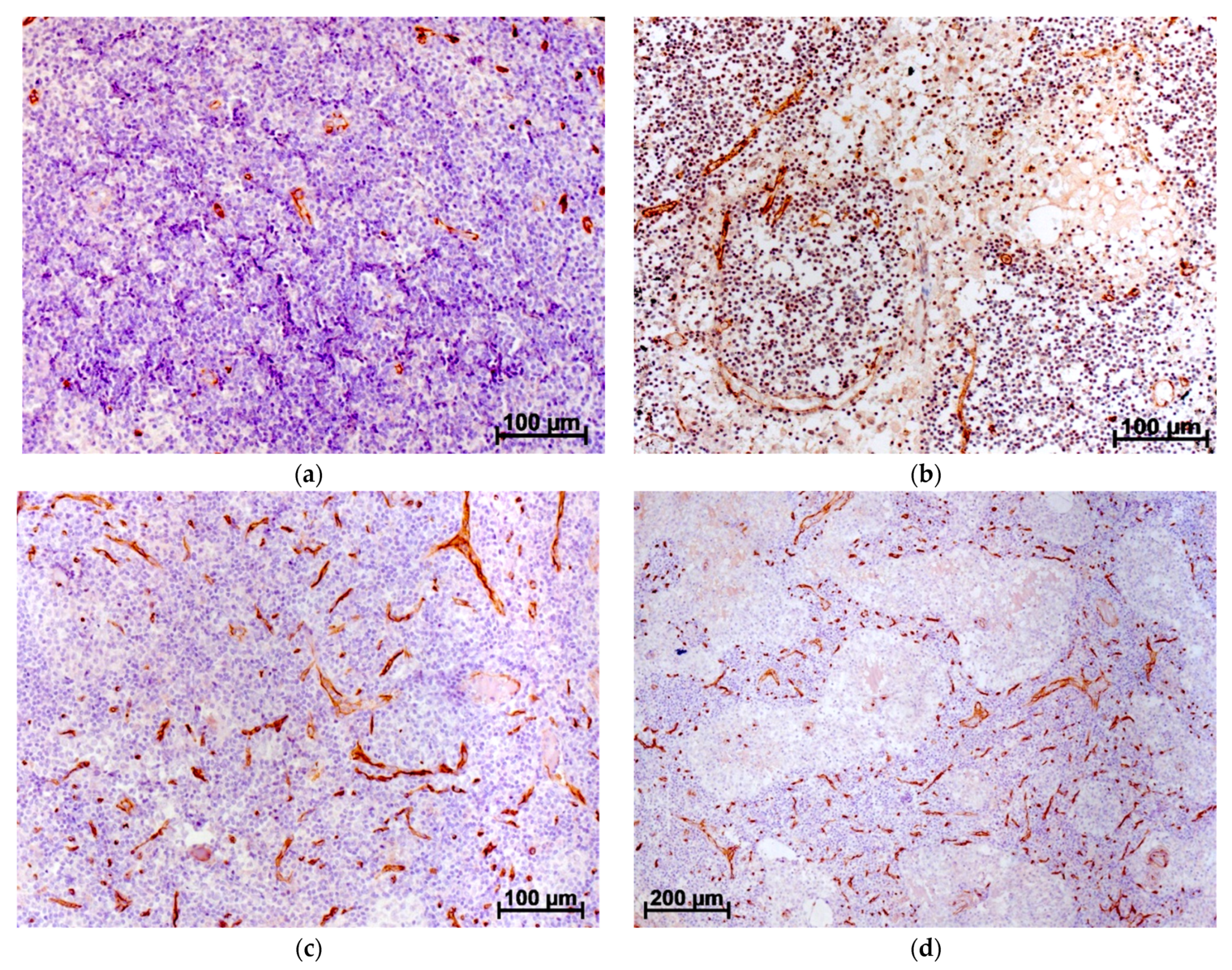
5. Tumor Angiogenesis and Stromal Cells
6. Features of Angiogenesis in Ovarian Cancer and Other Tumors of the Female Reproductive System
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lorusso, D.; Ceni, V.; Muratore, M.; Salutari, V.; Nero, C.; Pietragalla, A.; Ciccarone, F.; Carbone, V.; Daniele, G.; Scambia, G. Emerging role of immune checkpoint inhibitors in the treatment of ovarian cancer. Expert. Opin. Emerg. Drugs 2020, 25, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Rashid, M.H.; Liu, M.; Angara, K.; Mivechi, N.F.; Maihle, N.J.; Arbab, A.S.; Ko, L. Malignant pericytes expressing GT198 give rise to tumor cells through angiogenesis. Oncotarget 2017, 8, 51591–51607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, X.; Ji, J.; Zhang, G.; Fang, C.; Jiang, F.; Ma, S.; Hou, J. Expression and significance of B7-H3 and Tie-2 in the tumor vasculature of clear cell renal carcinoma. Onco-Targets Ther. 2017, 10, 5417–5424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Chen, W.L.; Zhang, F.; Wei, X.L.; Zeng, D.; Liang, Y.K.; Wu, J.D.; Zhang, L.Y.; Guo, C.P.; Zeng, H.C.; et al. Over-expression of both VEGF-C and Twist predicts poor prognosis in human breast cancer. Clin. Transl. Oncol. 2019, 21, 1250–1259. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Crivellato, E. Cross talk between natural killer cells and mast cells in tumor angiogenesis. Inflamm. Res. 2019, 68, 19–23. [Google Scholar] [CrossRef]
- Xing, C.; Li, Y.; Ding, C.; Wang, S.; Zhang, H.; Chen, L.; Li, P.; Dai, M. CD44+ Circulating Tumor Endothelial Cells Indicate Poor Prognosis in Pancreatic Ductal Adenocarcinoma After Radical Surgery: A Pilot Study. Cancer Manag. Res. 2021, 13, 4417–4431. [Google Scholar] [CrossRef]
- Farnsworth, R.H.; Achen, M.G.; Stacker, S.A. The evolving role of lymphatics in cancer metastasis. Curr. Opin. Immunol. 2018, 53, 64–73. [Google Scholar] [CrossRef]
- Maiborodin, I.V.; Kozjakov, A.E.; Babayants, E.V.; Krasilnikov, S.E. Angiogenesis in Lymph Nodes in the Development of Cancer in the Region of Lymph Collection. Novosti Khirurgii 2016, 24, 579–585. [Google Scholar] [CrossRef]
- Maiborodin, I.V.; Kozyakov, A.E.; Babayants, E.V.; Krasil’nikov, S.E. Features of Blood Supply to Axillary Lymph Nodes in Breast Cancer Patients. Bull. Exp. Biol. Med. 2017, 163, 82–86. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, C.; Guan, W.; Chen, L.; Guo, K.; Yu, A.; Xie, K. Clinicopathological and prognostic significance of nestin expression in patients with breast cancer: A systematic review and meta-analysis. Cancer Cell Int. 2020, 20, 169. [Google Scholar] [CrossRef]
- Barlingay, G.; Findakly, D.; Hartmann, C.; Amar, S. The Potential Clinical Benefit of Tocilizumab Therapy for Patients with HHV-8-infected AIDS-related Multicentric Castleman Disease: A Case Report and Literature Review. Cureus 2020, 12, e7589. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Bal, A.; Kashyap, D.; Kumar, S.; Shrivastav, S.; Das, A.; Singh, G. Tumour angiogenesis and c-Met pathway activation—Implications in breast cancer. APMIS 2020, 128, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Seabra, M.A.L.; Cândido, E.B.; Vidigal, P.V.T.; Lamaita, R.M.; Rodrigues, A.N.; Silva Filho, A.L.D. Immunohistochemical WWOX Expression and Association with Angiogenesis, p53 Expression, Cell Proliferation and Clinicopathological Parameters in Cervical Cancer. Rev. Bras. Ginecol. Obstet. 2018, 40, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Meng, G.; Tan, B.; Chen, Z.; Ji, Q.; Wang, X.; Liu, C.; Niu, S.; Li, Y.; Liu, Y. Relationship between HER2 expression and tumor interstitial angiogenesis in primary gastric cancer and its effect on prognosis. Pathol. Res. Pract. 2021, 217, 153280. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, P.; Lee, J.L.; Hubel, N.E.; Hu, D.; Yerneni, S.; Campbell, P.G.; Pollock, N.; Klei, L.R.; Concel, V.J.; Delekta, P.C.; et al. The CARMA3-Bcl10-MALT1 Signalosome Drives NFκB Activation and Promotes Aggressiveness in Angiotensin II Receptor-Positive Breast Cancer. Cancer Res. 2018, 78, 1225–1240. [Google Scholar] [CrossRef]
- Kim, M.C.; Park, M.H.; Kang, S.H.; Bae, Y.K. NDRG3 protein expression is associated with aggressive biologic phenotype and unfavorable outcome in patients with invasive breast cancer. Int. J. Clin. Exp. Pathol. 2019, 12, 3886–3893. [Google Scholar]
- Kato, F.; Wada, N.; Hayashida, T.; Fukuda, K.; Nakamura, R.; Takahashi, T.; Kawakubo, H.; Takeuchi, H.; Kitagawa, Y. Experimental and clinicopathological analysis of HOXB9 in gastric cancer. Oncol. Lett. 2019, 17, 3097–3102. [Google Scholar] [CrossRef]
- Wang, F.W.; Cao, C.H.; Han, K.; Zhao, Y.X.; Cai, M.Y.; Xiang, Z.C.; Zhang, J.X.; Chen, J.W.; Zhong, L.P.; Huang, Y.; et al. APC-activated long noncoding RNA inhibits colorectal carcinoma pathogenesis through reduction of exosome production. J. Clin. Investig. 2019, 129, 727–743. [Google Scholar] [CrossRef]
- Shin, D.H.; Jo, J.Y.; Kim, S.H.; Choi, M.; Han, C.; Choi, B.K.; Kim, S.S. Midkine Is a Potential Therapeutic Target of Tumorigenesis, Angiogenesis, and Metastasis in Non-Small Cell Lung Cancer. Cancers 2020, 12, 2402. [Google Scholar] [CrossRef]
- Ma, T.H.; Gao, C.C.; Xie, R.; Yang, X.Z.; Dai, W.J.; Zhang, J.L.; Yan, W.; Wu, S.N. Predictive values of FAP and HGF for tumor angiogenesis and metastasis in colorectal cancer. Neoplasma 2017, 64, 880–886. [Google Scholar] [CrossRef]
- Lin, Q.; Xiao, G.; Wang, G.; He, Q.; Xu, L.; Qiu, P.; Tan, S.; Gong, M.; Wen, J.; Xiao, X. Heat Shock Factor 1 in Relation to Tumor Angiogenesis and Disease Progression in Patients with Pancreatic Cancer. Pancreas 2020, 49, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.; Bach, D.H.; Choi, H.J.; Kim, M.S.; Wu, S.Y.; Pradeep, S.; Ivan, C.; Cho, M.S.; Bayraktar, E.; Rodriguez-Aguayo, C.; et al. The hidden role of paxillin: Localization to nucleus promotes tumor angiogenesis. Oncogene 2021, 40, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Payan, N.; Presles, B.; Brunotte, F.; Coutant, C.; Desmoulins, I.; Vrigneaud, J.M.; Cochet, A. Biological correlates of tumor perfusion and its heterogeneity in newly diagnosed breast cancer using dynamic first-pass 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, C.; Hu, W.; Zhong, C.; Fan, L.; Song, X.; Gai, Z. Low expression level of ASK1-interacting protein-1 correlated with tumor angiogenesis and poor survival in patients with esophageal squamous cell cancer. Onco-Targets Ther. 2018, 11, 7699–7707. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Gong, W.; Kong, X.; Wang, C.; Wang, S.; Liu, A. Prognostic value of insulin-like growth factor 2 mRNA-binding protein 3 and vascular endothelial growth factor-A in patients with primary non-small-cell lung cancer. Oncol. Lett. 2019, 18, 4744–4752. [Google Scholar] [CrossRef]
- Mohamed, A.H.; Said, N.M. Immunohistochemical Expression of Fatty Acid Synthase and Vascular Endothelial Growth Factor in Primary Colorectal Cancer: A Clinicopathological Study. J. Gastrointest Cancer 2019, 50, 485–492. [Google Scholar] [CrossRef]
- Shamloo, N.; Taghavi, N.; Yazdani, F.; Azimian, P.; Ahmadi, S. Evaluation of VEGF expression correlates with COX-2 expression in pleomorphic adenoma, mucoepidermoid carcinoma and adenoid cystic carcinoma. Dent. Res. J. 2020, 17, 100–106. [Google Scholar]
- Tian, Y.; Cheng, X.; Li, Y. Chemotherapy combined with apatinib for the treatment of desmoplastic small round cell tumors: A case report. J. Cancer Res. Ther. 2020, 16, 1177–1181. [Google Scholar] [CrossRef]
- Yan, H.; Li, X.; Peng, Y.; Zhang, P.; Zou, N.; Liu, X. Apatinib and fractionated stereotactic radiotherapy for the treatment of limited brain metastases from primary lung mucoepidermoid carcinoma: A case report. Medicine 2020, 99, e22925. [Google Scholar] [CrossRef]
- García, M.; Palma, M.B.; Verine, J.; Miriuka, S.; Inda, A.M.; Errecalde, A.L.; Desgrandchamps, F.; Carosella, E.D.; Tronik-Le Roux, D. The immune-checkpoint HLA-G/ILT4 is involved in the regulation of VEGF expression in clear cell renal cell carcinoma. BMC Cancer 2020, 20, 624. [Google Scholar] [CrossRef]
- Wang, H.; Zhan, M.; Yang, R.; Shi, Y.; Liu, Q.; Wang, J. Elevated expression of NFE2L3 predicts the poor prognosis of pancreatic cancer patients. Cell Cycle 2018, 17, 2164–2174. [Google Scholar] [CrossRef]
- Liu, L.B.; Huang, J.; Zhong, J.P.; Ye, G.L.; Xue, L.; Zhou, M.H.; Huang, G.; Li, S.J. High Expression of CCDC34 Is Associated with Poor Survival in Cervical Cancer Patients. Med. Sci. Monit. 2018, 24, 8383–8390. [Google Scholar] [CrossRef] [PubMed]
- Lien, M.Y.; Chang, A.C.; Tsai, H.C.; Tsai, M.H.; Hua, C.H.; Cheng, S.P.; Wang, S.W.; Tang, C.H. Monocyte Chemoattractant Protein 1 Promotes VEGF-A Expression in OSCC by Activating ILK and MEK1/2 Signaling and Downregulating miR-29c. Front. Oncol. 2020, 10, 592415. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chen, Y.; Dong, S.; Xu, X.; Liu, J.; Song, P.; Yu, C.; Dai, L. AEG-1 mRNA expression in non-small cell lung cancer is associated with increased tumor angiogenesis. Pathol. Res. Pract. 2017, 213, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Chen, Y.; Dong, S.; Xu, X.; Liu, J.; Song, P.; Yu, C.; Ma, Z. Astrocyte elevated gene-1 is overexpressed in non-small-cell lung cancer and associated with increased tumour angiogenesis. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Rofstad, E.K. Integrins as therapeutic targets in the organ-specific metastasis of human malignant melanoma. J. Exp. Clin. Cancer Res. 2018, 37, 92. [Google Scholar] [CrossRef]
- Cheng, X.K.; Lin, W.R.; Jiang, H.; Su, Z.H.; Li, L.; Wang, J. MicroRNA-129-5p inhibits invasiveness and metastasis of lung cancer cells and tumor angiogenesis via targeting VEGF. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2827–2837. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, W.; Bai, Y.; Wan, L.; Sun, X.; Liu, Y.; Xiong, W.; Zhang, Y.Y.; Zhou, L. Oxyresveratrol prevents murine H22 hepatocellular carcinoma growth and lymph node metastasis via inhibiting tumor angiogenesis and lymphangiogenesis. J. Nat. Med. 2018, 72, 481–492. [Google Scholar] [CrossRef]
- Xia, Y.J.; Jiang, X.T.; Jiang, S.B.; He, X.J.; Luo, J.G.; Liu, Z.C.; Wang, L.; Tao, H.Q.; Chen, J.Z. PHD3 affects gastric cancer progression by negatively regulating HIF1A. Mol. Med. Rep. 2017, 16, 6882–6889. [Google Scholar] [CrossRef]
- Atanasov, G.; Dietel, C.; Feldbrügge, L.; Benzing, C.; Krenzien, F.; Brandl, A.; Katou, S.; Schierle, K.; Robson, S.C.; Splith, K.; et al. Angiogenic miRNAs, the angiopoietin axis and related TIE2-expressing monocytes affect outcomes in cholangiocarcinoma. Oncotarget 2018, 9, 29921–29933. [Google Scholar] [CrossRef]
- Cheng, R.; Chen, Y.; Zhou, H.; Wang, B.; Du, Q.; Chen, Y. B7-H3 expression and its correlation with clinicopathologic features, angiogenesis, and prognosis in intrahepatic cholangiocarcinoma. APMIS 2018, 126, 396–402. [Google Scholar] [CrossRef]
- Yuan, D.; Wang, W.; Su, J.; Zhang, Y.; Luan, B.; Rao, H.; Cheng, T.; Zhang, W.; Xiao, S.; Zhang, M.; et al. SOCS6 Functions as a Tumor Suppressor by Inducing Apoptosis and Inhibiting Angiogenesis in Human Prostate Cancer. Curr. Cancer Drug Targets 2018, 18, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, D.H.; Park, S.Y.; Seol, J.W. Diosmetin inhibits tumor development and block tumor angiogenesis in skin cancer. Biomed. Pharmacother. 2019, 117, 109091. [Google Scholar] [CrossRef]
- Park, Y.G.; Choi, J.; Jung, H.K.; Kim, B.; Kim, C.; Park, S.Y.; Seol, J.W. Baicalein inhibits tumor progression by inhibiting tumor cell growth and tumor angiogenesis. Oncol. Rep. 2017, 38, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Portier, L.; Desterke, C.; Chaker, D.; Oudrhiri, N.; Asgarova, A.; Dkhissi, F.; Turhan, A.G.; Bennaceur-Griscelli, A.; Griscelli, F. iPSC-Derived Hereditary Breast Cancer Model Reveals the BRCA1-Deleted Tumor Niche as a New Culprit in Disease Progression. Int. J. Mol. Sci. 2021, 22, 1227. [Google Scholar] [CrossRef] [PubMed]
- Gills, J.; Moret, R.; Zhang, X.; Nelson, J.; Maresh, G.; Hellmers, L.; Canter, D.; Hudson, M.; Halat, S.; Matrana, M.; et al. A patient-derived orthotopic xenograft model enabling human high-grade urothelial cell carcinoma of the bladder tumor implantation, growth, angiogenesis, and metastasis. Oncotarget 2018, 9, 32718–32729. [Google Scholar] [CrossRef]
- An, D.; Banerjee, S.; Lee, J.M. Recent advancements of antiangiogenic combination therapies in ovarian cancer. Cancer Treat. Rev. 2021, 98, 102224. [Google Scholar] [CrossRef]
- Sopo, M.; Anttila, M.; Muukkonen, O.T.; YlÄ-Herttuala, S.; Kosma, V.M.; Keski-Nisula, L.; Sallinen, H. Microvessels in Epithelial Ovarian Tumors: High Microvessel Density Is a Significant Feature of Malignant Ovarian Tumors. Anticancer Res. 2020, 40, 6923–6931. [Google Scholar] [CrossRef]
- Perez-Fidalgo, J.A.; Ortega, B.; Simon, S.; Samartzis, E.P.; Boussios, S. NOTCH signalling in ovarian cancer angiogenesis. Ann. Transl. Med. 2020, 8, 1705. [Google Scholar] [CrossRef]
- Sharma, V.M.; Draheim, K.M.; Kelliher, M.A. The Notch1/c-Myc pathway in T cell leukemia. Cell Cycle 2007, 6, 927–930. [Google Scholar] [CrossRef]
- Arora, P.S.; Ansari, A.Z. Chemical biology: A Notch above other inhibitors. Nature 2009, 462, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Moellering, R.E.; Cornejo, M.; Davis, T.N.; Del Bianco, C.; Aster, J.C.; Blacklow, S.C.; Kung, A.L.; Gilliland, D.G.; Verdine, G.L.; Bradner, J.E. Direct inhibition of the NOTCH transcription factor complex. Nature 2009, 462, 182–188. [Google Scholar] [CrossRef]
- Bhandari, D.R.; Seo, K.W.; Roh, K.H.; Jung, J.W.; Kang, S.K.; Kang, K.S. REX-1 expression and p38 MAPK activation status can determine proliferation/differentiation fates in human mesenchymal stem cells. PLoS ONE 2010, 5, e10493. [Google Scholar] [CrossRef] [PubMed]
- Sopo, M.; Sallinen, H.; Hämäläinen, K.; Kivelä, A.; Ylä-Herttuala, S.; Kosma, V.M.; Keski-Nisula, L.; Anttila, M. High expression of Tie-2 predicts poor prognosis in primary high grade serous ovarian cancer. PLoS ONE 2020, 15, e0241484. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Mirza, M.R.; Pignata, S.; Walther, A.; Romero, I.; du Bois, A. Therapeutic options following second-line platinum-based chemotherapy in patients with recurrent ovarian cancer: Comparison of active surveillance and maintenance treatment. Cancer Treat. Rev. 2020, 90, 102107. [Google Scholar] [CrossRef]
- Tanigawa, T.; Matoda, M.; Omi, M.; Aoki, Y.; Netsu, S.; Nomura, H.; Okamoto, S.; Omatsu, K.; Yunokawa, M.; Kanao, H.; et al. Continuous Administration of Bevacizumab After Disease Progression in Recurrent Ovarian Cancer: A Retrospective Observational Study. Anticancer Res. 2020, 40, 5285–5290. [Google Scholar] [CrossRef]
- Elyashiv, O.; Ledermann, J.; Parmar, G.; Farrelly, L.; Counsell, N.; Feeney, A.; El-Khouly, F.; Macdonald, I.; Neto, A.; Arthur-Darkwa, E.; et al. ICON 9-an international phase III randomized study to evaluate the efficacy of maintenance therapy with olaparib and cediranib or olaparib alone in patients with relapsed platinum-sensitive ovarian cancer following a response to platinum-based chemotherapy. Int. J. Gynecol. Cancer 2021, 31, 134–138. [Google Scholar] [CrossRef]
- Guo, C.; Yan, C.; Qu, L.; Du, R.; Lin, J. The efficacy and toxicity of angiogenesis inhibitors for ovarian cancer: A meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 2021, 303, 285–311. [Google Scholar] [CrossRef]
- Hall, M.R.; Dehbi, H.M.; Banerjee, S.; Lord, R.; Clamp, A.; Ledermann, J.A.; Nicum, S.; Lilleywhite, R.; Bowen, R.; Michael, A.; et al. A phase II randomised, placebo-controlled trial of low dose (metronomic) cyclophosphamide and nintedanib (BIBF1120) in advanced ovarian, fallopian tube or primary peritoneal cancer. Gynecol. Oncol. 2020, 159, 692–698. [Google Scholar] [CrossRef]
- Lee, J.M.; Annunziata, C.M.; Hays, J.L.; Cao, L.; Choyke, P.; Yu, M.; An, D.; Turkbey, I.B.; Minasian, L.M.; Steinberg, S.M.; et al. Phase II trial of bevacizumab and sorafenib in recurrent ovarian cancer patients with or without prior-bevacizumab treatment. Gynecol. Oncol. 2020, 159, 88–94. [Google Scholar] [CrossRef]
- Li, B.; Tong, T.; Ren, N.; Rankin, G.O.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. Theasaponin E1 Inhibits Platinum-Resistant Ovarian Cancer Cells through Activating Apoptosis and Suppressing Angiogenesis. Molecules 2021, 26, 1681. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xin, H.; Lu, L. Extracellular vesicle-encapsulated microRNA-424 exerts inhibitory function in ovarian cancer by targeting MYB. J. Transl. Med. 2021, 19, 4. [Google Scholar] [CrossRef]
- Garrido, M.P.; Torres, I.; Avila, A.; Chnaiderman, J.; Valenzuela-Valderrama, M.; Aramburo, J.; Oróstica, L.; Durán-Jara, E.; Lobos-Gonzalez, L.; Romero, C. NGF/TRKA Decrease miR-145-5p Levels in Epithelial Ovarian Cancer Cells. Int. J. Mol. Sci. 2020, 21, 7657. [Google Scholar] [CrossRef]
- Yuan, J.; Yi, K.; Yang, L. LncRNA NEAT1 promotes proliferation of ovarian cancer cells and angiogenesis of co-incubated human umbilical vein endothelial cells by regulating FGF9 through sponging miR-365: An experimental study. Medicine 2021, 100, e23423. [Google Scholar] [CrossRef] [PubMed]
- Bekes, I.; Löb, S.; Holzheu, I.; Janni, W.; Baumann, L.; Wöckel, A.; Wulff, C. Nectin-2 in ovarian cancer: How is it expressed and what might be its functional role? Cancer Sci. 2019, 110, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Brook, N.; Brook, E.; Dass, C.R.; Chan, A.; Dharmarajan, A. Pigment Epithelium-Derived Factor and Sex Hormone-Responsive Cancers. Cancers 2020, 12, 3483. [Google Scholar] [CrossRef]
- Barwal, T.S.; Sharma, U.; Bazala, S.; Singh, I.; Jain, M.; Prakash, H.; Shekhar, S.; Sandberg, E.N.; Bishayee, A.; Jain, A. MicroRNAs and Long Noncoding RNAs as Novel Therapeutic Targets in Estrogen Receptor-Positive Breast and Ovarian Cancers. Int. J. Mol. Sci. 2021, 22, 4072. [Google Scholar] [CrossRef]
- Carey, P.; Low, E.; Harper, E.; Stack, M.S. Metalloproteinases in Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 3403. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiborodin, I.; Mansurova, A.; Chernyavskiy, A.; Romanov, A.; Voitcitctkii, V.; Kedrova, A.; Tarkhov, A.; Chernyshova, A.; Krasil’nikov, S. Cancer Angiogenesis and Opportunity of Influence on Tumor by Changing Vascularization. J. Pers. Med. 2022, 12, 327. https://doi.org/10.3390/jpm12030327
Maiborodin I, Mansurova A, Chernyavskiy A, Romanov A, Voitcitctkii V, Kedrova A, Tarkhov A, Chernyshova A, Krasil’nikov S. Cancer Angiogenesis and Opportunity of Influence on Tumor by Changing Vascularization. Journal of Personalized Medicine. 2022; 12(3):327. https://doi.org/10.3390/jpm12030327
Chicago/Turabian StyleMaiborodin, Igor, Alfija Mansurova, Alexander Chernyavskiy, Alexander Romanov, Vladimir Voitcitctkii, Anna Kedrova, Alexander Tarkhov, Alena Chernyshova, and Sergey Krasil’nikov. 2022. "Cancer Angiogenesis and Opportunity of Influence on Tumor by Changing Vascularization" Journal of Personalized Medicine 12, no. 3: 327. https://doi.org/10.3390/jpm12030327
APA StyleMaiborodin, I., Mansurova, A., Chernyavskiy, A., Romanov, A., Voitcitctkii, V., Kedrova, A., Tarkhov, A., Chernyshova, A., & Krasil’nikov, S. (2022). Cancer Angiogenesis and Opportunity of Influence on Tumor by Changing Vascularization. Journal of Personalized Medicine, 12(3), 327. https://doi.org/10.3390/jpm12030327






