Identification of Independent Risk Factors for Skin Complications in a Multifactorial Logistic Regression Analysis of Simultaneous Immediate Autologous Breast Reconstruction and Skin Reduction Mastectomy in Large and Ptotic Breasts Using an Inferiorly Based Deepithelialized Dermal Breast Flap
Abstract
:1. Introduction
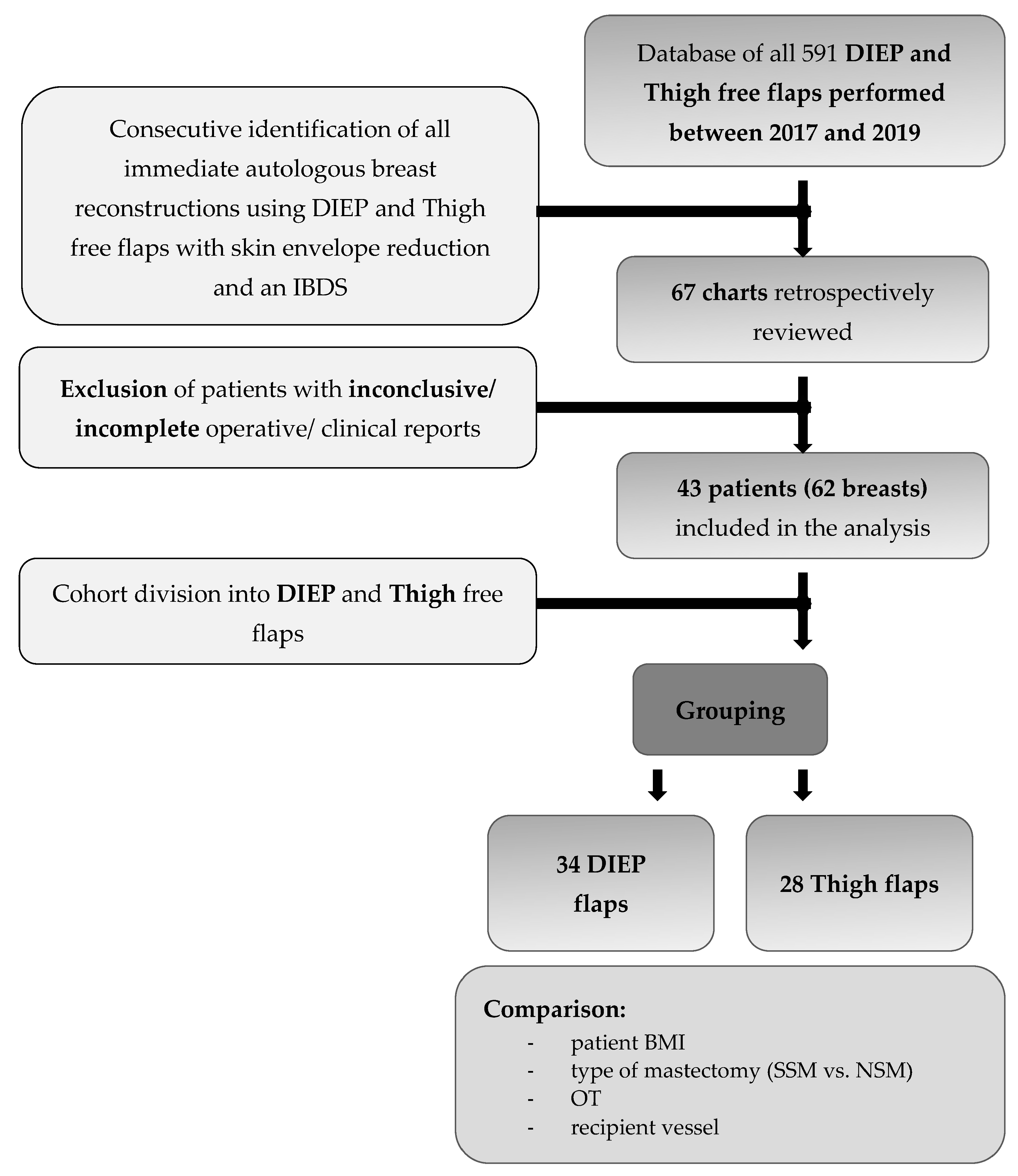
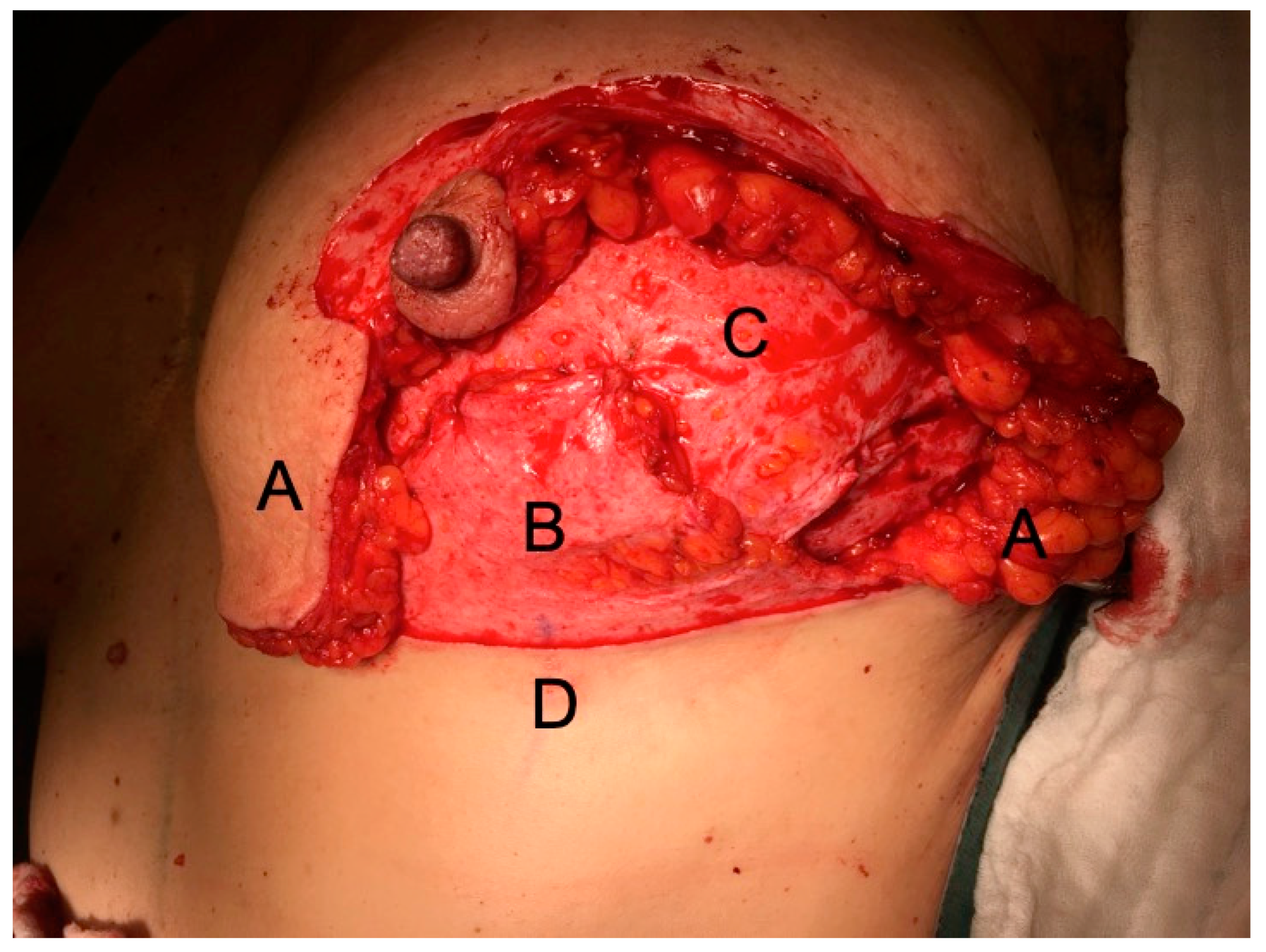
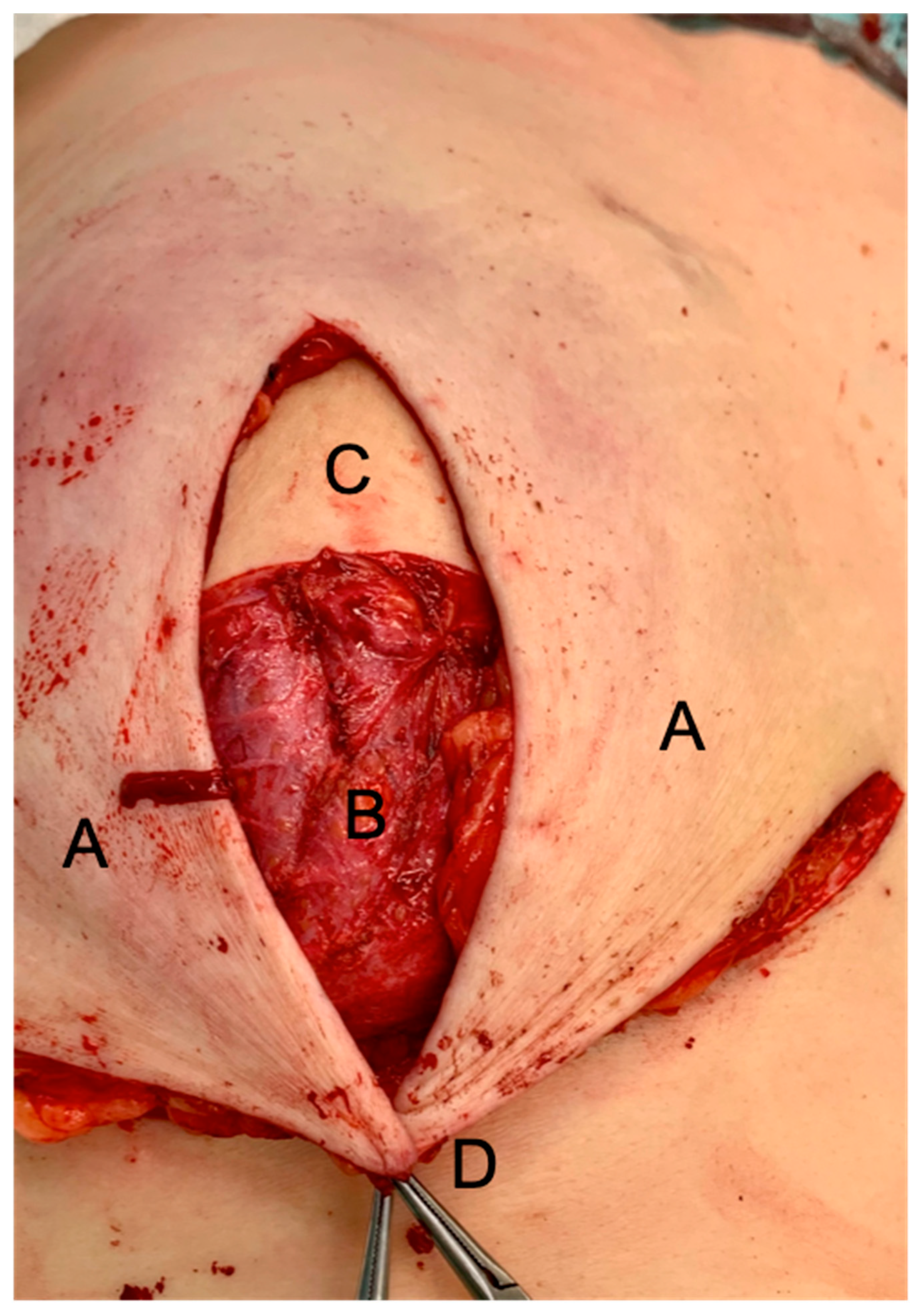
2. Patients and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Losken, A.; Collins, B.A.; Carlson, G.W. Dual-plane prosthetic reconstruction using the modified wise pattern mastectomy and fasciocutaneous flap in women with macromastia. Plast. Reconstr. Surg. 2010, 126, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, S. Does global warming contribute to the obesity epidemic? Environ Res. 2020, 182, 108962. [Google Scholar] [CrossRef] [PubMed]
- Rochlin, D.H.; Nguyen, D.H. Deepithelialized Skin Reduction Preserves Skin and Nipple Perfusion in Immediate Reconstruction of Large and Ptotic Breasts. Ann. Plast. Surg. 2018, 81, 22–27. [Google Scholar] [CrossRef]
- Tondu, T.; Hubens, G.; Tjalma, W.A.; Thiessen, F.E.; Vrints, I.; Van Thielen, J.; Verhoeven, V. Breast reconstruction after nipple-sparing mastectomy in the large and/or ptotic breast: A systematic review of indications, techniques, and outcomes. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 469–485. [Google Scholar] [CrossRef]
- Hunter, J.E.; Malata, C.M. Refinements of the LeJour vertical mammaplasty skin pattern for skin-sparing mastectomy and immediate breast reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2007, 60, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Vollbach, F.H.; Heitmann, C.D.; Fansa, H. An Appraisal of Internal Mammary Artery Perforators as Recipient Vessels in Microvascular Breast Reconstruction—An Analysis of 515 Consecutive Cases. Plast. Reconstr. Surg.-Glob. Open 2016, 4, e1144. [Google Scholar] [CrossRef]
- Fansa, H.; Heitmann, C. Breast Reconstruction with Autologous Tissue; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Danker, S.J.; Schmitt, M.; Smith, N.X.; Chong, H.J.; Sandholm, P.H.; Murphy, J.A.; Ladizinsky, D.A. Bostwick Autoderm and Implant Technique: Improved Outcomes for Obese Patients in Immediate Breast Reconstruction. Plast. Reconstr. Surg. 2021, 147, 187e–195e. [Google Scholar] [CrossRef]
- Ribeiro, L.; Accorsi, A.; Buss, A.; Marcal-Pessoa, M. Creation and Evolution of 30 Years of the Inferior Pedicle in Reduction Mammaplasties. Plast. Reconstr. Surg. 2002, 110, 960–970. [Google Scholar] [CrossRef] [Green Version]
- Ladizinsky, D.A.; Sandholm, P.H.; Jewett, S.T.; Shahzad, F.; Andrews, K. Breast reconstruction with the bostwick autoderm technique. Plast. Reconstr. Surg. 2013, 132, 261–270. [Google Scholar] [CrossRef]
- Di Candia, M.; Lie, K.H.; Forouhi, P.; Malata, C.M. Experience with the Wise mammaplasty skin resection pattern in skin-sparing mastectomy and immediate breast reconstruction for large breast volumes. Int. J. Surg. 2011, 9, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Lewin, R.; Jepsen, C.; Hallberg, H.; Hansson, E. Immediate breast reconstruction with a wise pattern mastectomy and NAC-sparing McKissock vertical bipedicle dermal flap. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Safran, T.; Al-Halabi, B.; Viezel-Mathieu, A.; Boileau, J.-F.; Dionisopoulos, T. Skin-Reducing Mastectomy with Immediate Prepectoral Reconstruction: Surgical, Aesthetic, and Patient-Reported Outcomes with and without Dermal Matrices. Plast. Reconstr. Surg. 2021, 147, 1046–1057. [Google Scholar] [CrossRef]
- Lanitis, S.; Kontos, M.; Chortis, P.; Gkanis, V.; Peristeraki, S.; Lainas, S.; Hadjiminas, D.J. De-epithelialized Skin Flaps to Minimize Complications in Large Breast Reconstruction. Ann. Plast. Surg. 2021, 87, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Frey, J.D.; Salibian, A.A.; Karp, N.S. Nipple-areola complex malposition in nipple-sparing mastectomy: A review of risk factors and corrective techniques from greater than 1000 reconstructions. Plast. Reconstr. Surg. 2017, 140, 247e–257e. [Google Scholar] [CrossRef] [PubMed]
- Lotan, A.M.; Tongson, K.C.; Police, A.M.; Dec, W. Mastectomy incision design to optimize aesthetic outcomes in breast reconstruction. Plast. Reconstr. Surg.-Glob. Open. 2020, 8, e3086. [Google Scholar] [CrossRef] [PubMed]
- Alperovich, M.; Tanna, N.; Samra, F.; Blechman, K.M.; Shapiro, R.L.; Guth, A.; Axelrod, D.; Choi, M.; Karp, N. Nipple-sparing mastectomy in patients with a history of reduction mammaplasty or mastopexy: How safe is it? Plast. Reconstr. Surg. 2013, 131, 962–967. [Google Scholar] [CrossRef] [Green Version]
- Dellacroce, F.J.; Blum, C.A.; Sullivan, S.K.; Stolier, A.; Trahan, C.; Wise, M.W.; Duracher, D. Nipple-Sparing Mastectomy and Ptosis: Perforator Flap Breast Reconstruction Allows Full Secondary Mastopexy with Complete Nipple Areolar Repositioning. Plast. Reconstr. Surg. 2015, 136, 1e–9e. [Google Scholar] [CrossRef] [Green Version]
- Chirappapha, P.; Petit, J.-Y.; Rietjens, M.; De Lorenzi, F.; Garusi, C.; Martella, S.; Barbieri, B.; Gottardi, A.; Andrea, M.; Giuseppe, L.; et al. Nipple sparing mastectomy: Does breast morphological factor related to necrotic complications? Plast. Reconstr. Surg. 2014, 2, 1–7. [Google Scholar] [CrossRef]
- Schneider, L.F.; Chen, C.M.; Stolier, A.J.; Shapiro, R.L.; Ahn, C.Y.; Allen, R.J. Nipple-sparing mastectomy and immediate free-flap reconstruction in the large ptotic breast. Ann. Plast. Surg. 2012, 69, 425–428. [Google Scholar] [CrossRef] [Green Version]
- Frey, J.D.; Salibian, A.A.; Karp, N.S.; Choi, M. The impact of mastectomy weight on reconstructive trends and outcomes in nipple-sparing mastectomy: Progressively greater complications with larger breast size. Plast. Reconstr. Surg. 2018, 141, 795e–804e. [Google Scholar] [CrossRef]
- Abedi, N.; Ho, A.L.; Knox, A.; Tashakkor, Y.; Omeis, T.; Van Laeken, N.; Lennox, P.; Macadam, S.A. Predictors of mastectomy flap necrosis in patients undergoing immediate breast reconstruction a review of 718 patients. Ann. Plast. Surg. 2016, 76, 629–634. [Google Scholar] [CrossRef]
- Davies, K.; Allan, L.; Roblin, P.; Ross, D.; Farhadi, J. Factors affecting post-operative complications following skin sparing mastectomy with immediate breast reconstruction. Breast 2011, 20, 21–25. [Google Scholar] [CrossRef]
- Lin, I.C.; Bergey, M.; Sonnad, S.S.; Serletti, J.M.; Wu, L.C. Management of the ptotic or hypertrophic breast in immediate autologous breast reconstruction: A comparison between the wise and vertical reduction patterns for mastectomy. Ann. Plast. Surg. 2013, 70, 264–270. [Google Scholar] [CrossRef]
- Dec, W. Optimizing aesthetic outcomes for breast reconstruction in patients with significant macromastia or ptosis. JPRAS Open 2018, 16, 24–30. [Google Scholar] [CrossRef]
- Gorai, K.; Inoue, K.; Saegusa, N.; Shimamoto, R.; Takeishi, M.; Okazaki, M.; Nakagawa, M. Prediction of skin necrosis after mastectomy for breast cancer using indocyanine green angiography imaging. Plast. Reconstr. Surg.-Glob. Open 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Momeni, A.; Ahdoot, M.A.; Kim, R.Y.; Leroux, E.; Galaiya, D.J.; Lee, G.K. Should we continue to consider obesity a relative contraindication for autologous microsurgical breast reconstruction? J. Plast. Reconstr. Aesthetic Surg. 2012, 65, 420–425. [Google Scholar] [CrossRef]
- Jensen, J.A.; Lin, J.H.; Kapoor, N.; Giuliano, A.E. Surgical delay of the nipple-A reolar complex: A powerful technique to maximize nipple viability following nipple-sparing mastectomy. Ann. Surg. Oncol. 2012, 19, 3171–3176. [Google Scholar] [CrossRef]
- Mitchell, S.D.; Willey, S.C.; Beitsch, P.; Feldman, S. Evidence based outcomes of the American Society of Breast Surgeons Nipple Sparing Mastectomy Registry. Gland Surg. 2018, 7, 247–257. [Google Scholar] [CrossRef]
| Flap Types | |||||
|---|---|---|---|---|---|
| Overall | DIEP | Thigh | p-Value | Significant | |
| Number of cases (%) | n = 62 (100%) | n = 34 (54.8%) | n = 28 (45.2%) | - | - |
| Patient Characteristics | - | - | - | - | - |
| Mean age ± SD [years] | 45.1 ± 10.0 | 46.9 ± 12.1 | 43 ± 6.1 | 0.1 | |
| BMI ± SD [kg/m2] | 25.5 ± 5.2 | 27.9 ± 5.4 | 22.5 ± 2.7 | <0.0001 | ** |
| Comorbidities | - | - | - | - | - |
| Smoking | 11 (100%) | 2 (18.2%) | 9 (81.8%) | 0.007 | ** |
| Alcohol | 7 (100%) | 1 (14.3%) | 6 (85.7%) | 0.02 | * |
| Diabetes | 0 | 0 | 0 | - | - |
| Operative Characteristics | - | - | - | - | - |
| Unilateral | 24 (100%) | 16 (66.7%) | 8 (33.3%) | 0.1 | |
| Bilateral | 19 (100%) | 9 (47.4%) | 10 (52.6%) | 0.1 | |
| SSM | 40 (100%) | 23 (57.5%) | 17 (42.5%) | 0.6 | |
| NSM | 22 (100%) | 11 (50%) | 11 (50%) | 0.6 | |
| Internal mammary artery/vein (IMA/V) | 30 (100%) | 15 (50%) | 15 (50%) | 0.6 | |
| Int. mam. artery/vein perf. (IMAP) | 32 (100%) | 19 (59.4%) | 13 (40.6%) | 0.5 | |
| Mean Coupler ± SD [mm] | 2.4 ± 0.3 | 2.4 ± 0.3 | 2.3 ± 0.3 | 0.2 | |
| Mean mastectomy weight ± SD [g] | 468.8 ± 214.7 | 544.9 ± 217.6 | 376.4 ± 170.4 | 0.002 | ** |
| Mean flap weight ± SD [g] | 438.5 ± 223.4 | 569.0 ± 212.9 | 280.1 ± 98.5 | <0.0001 | ** |
| Mean OT ± SD [minutes] † | 252.9 ± 60.1 | 258.1 ± 64.3 | 246.6 ± 53.9 | 0.5 | |
| Mean OT ± SD [minutes]—unilateral | 201.5 ± 43.8 | 208.3 ± 41.8 | 207.3 ± 45.6 | 0.3 | |
| Mean OT ± SD [minutes]—bilateral | 285.4 ± 44.2 | 302.4 ± 44.5 | 287.9 ± 36.7 | 0.03 | * |
| Mean ischemia time ± SD [minutes] | 39.9 ± 13.6 | 41.6 ± 14.4 | 37.7 ± 12.2 | 0.3 | |
| Adjuvant oncologic therapy | - | - | - | - | - |
| Invasive cancer | 28 (100%) | 18 (64.3%) | 10 (35.7%) | 0.2 | |
| DCIS | 9 (100%) | 6 (66.7%) | 3 (33.3%) | 0.4 | |
| Prophylactic surgery | 25 (100%) | 10 (40%) | 15 (60%) | 0.05 | * |
| Prev. breast conserving therapy | 13 (100%) | 11 (84.6%) | 2 (15.4%) | 0.02 | * |
| Prev. radiation therapy (prev. Rx) | 9 (100%) | 7 (77.8%) | 2 (22.2%) | 0.1 | |
| Prev. chemo therapy (prev. Cx) | 29 (100%) | 16 (55.2%) | 13 (44.8%) | 0.9 | |
| Postop. Radiation therapy (post. Rx) | 7 (100%) | 3 (42.9%) | 4 (57.1%) | 0.5 | |
| Postop. chemo therapy (post. Cx) | 10 (100%) | 5 (50%) | 5 (50%) | 0.7 | |
| Sentinel lymph node biopsy (SLN) | 24 (100%) | 15 (62.5%) | 9 (37.5%) | 0.3 | |
| Axillary lymph node dissection | 5 (100%) | 2 (40%) | 3 (60%) | 0.5 | |
| Flap Types | |||||
|---|---|---|---|---|---|
| Overall | DIEP | Thigh | p-Value | Significant | |
| Number of cases (%) | n = 62 (100%) | n = 34 (54.8%) | n = 28 (45.2%) | — | — |
| SSM | 40 (64.5%) | 23 (67.6%) | 17 (60.7%) | ||
| NSM | 22 (35.5%) | 11 (32.4%) | 11 (39.2%) | ||
| Any complication (% within flap type) | n = 20 (32.2%) | n = 11 (32.4%) | n = 9 (32.1%) | 0.99 | n.s. |
| Minor complications (no surgery) | — | — | — | — | — |
| Impaired WH breast skin | 16 (25.8%) | 8 (23.5%) | 8 (28.6%) | >0.99 | n.s. |
| Partial NAC necrosis | 9 (14.5%) | 5 (8.1%) | 4 (14.3%) | 0.963 | n.s. |
| Major complication (surg. treated) | — | — | — | — | — |
| Complete flap loss | 0 | 0 | 0 | — | — |
| Partial flap loss | 0 | 0 | 0 | — | — |
| Complete NAC necrosis | 1 (1.6%) | 0 | 1 (3.6%) | 0.267 | n.s. |
| Impaired WH breast | 4 (6.5%) | 3 (4.8%) | 1 (3.6%) | 0.402 | n.s. |
| Any Skin Complications | |||||
|---|---|---|---|---|---|
| Overall | Any Complication | No Complication | p-Values | Significant | |
| Number of cases (%) | n = 62 (100%) | n = 20 (32.3%) | n = 42 (67.7%) | - | - |
| Patient Characteristics | |||||
| Mean age ± SD [years] | 45.7 (±11.4) | 44.9 (±9.5) | 0.77 | n.s. | |
| Mean BMI ± SD [kg/m2] | 27.6 (±6.4) | 24.4 (±4.2) | 0.026 | * | |
| Comorbidities | |||||
| Active smoker/non-smoker (%) | 4 (20%)/16 (80%) | 7 (16.7%)/35 (83.3%) | 0.74 | n.s. | |
| Alcohol/no Alcohol (%) | 2 (10%)/18 (90%) | 5 (11.9%)/37 (88.1%) | 0.99 | n.s. | |
| Operative Characteristics | |||||
| Flap type (DIEP/Thigh) | 11 (55%)/9 (45%) | 23 (54.8%)/19 (45.2%) | 0.99 | n.s. | |
| Unilateral/bilateral reconstruction | 4 (20%)/16 (80%) | 20 (47.6%)/22 (52.4%) | 0.05 | * | |
| SSM/NSM | 9 (45%)/11 (55%) | 31 (73.8%)/11 (26.2%) | <0.05 | * | |
| Recipient Vessel (IMA/IMAP) | 11 (55%)/9 (45%) | 19 (45.2%)/23 (54.8%) | 0.59 | n.s. | |
| Coupler rank [1.5/2.0/2.5/3.0 mm] (%) |
(0/3/16/1) (0%/15%/80%/5%) | (2/13/25/2) (4.8%/31%/59.5%/4.8%) | 0.37 | n.s. | |
| Mean mastectomy weight ± SD [g] | 543.4 (±237) | 433.3 (±199.2) | 0.06 | n.s. | |
| Mean flap weight ± SD [g] | 505.5 (±264.8) | 406.7 (±199.3) | 0.11 | n.s. | |
| Mean operative time ± SD [minutes] † | 285.4 (±56.4) | 237.5 (±56.8) | 0.003 | ** | |
| Mean ischemia time ± SD [minutes] | 37.9 (±10.98) | 40.8 (±14.7) | 0.44 | n.s. | |
| Adjuvant oncologic therapy | |||||
| Invasive Cancer/no inv. Ca. | 8 (40%)/12 (60%) | 20 (47.6%)/22 (52.4%) | 0.6 | n.s. | |
| DCIS/no DCIS (%) | 3 (15%)/17 (85%) | 6 (14.3%)/36 (85.7%) | 0.99 | n.s. | |
| Prophylactic/non-prophylactic surgery (%) | 8 (40%)/12 (60%) | 14 (33.3%/28 (66.7%) | 0.78 | n.s. | |
| Prev. BCT/no BCT (%) | 4 (20%)/16 (80%) | 9 (21.4%)/33 (78.6%) | 0.99 | n.s. | |
| Prev. Rx/no Rx (%) | 3 (15%)/17 (85%) | 6 (14.3%)/36 (85.7%) | 0.99 | n.s. | |
| Prev. Cx/no Cx (%) | 8 (40%)/12 (60%) | 21 (50%)/21 (50%) | 0.59 | n.s. | |
| Postop. Rx (%) | 1 (5%) | 6 (14.3%) | 0.41 | n.s. | |
| Postop. Cx (%) | 2 (10%) | 8 (19%) | 0.48 | n.s. | |
| SLN/no SLN (%) | 8 (40%)/12 (60%) | 16 (38.1%)/26 (61.9%) | 0.99 | n.s. | |
| ALND/no ALND (%) | 0 (0%)/20 100%) | 5 (11.9%)/37 (88.1%) | 0.17 | n.s. | |
| Partial NAC Necrosis | |||||
|---|---|---|---|---|---|
| Overall | NAC Necrosis | No NAC Necrosis | p-Values | Significant | |
| Number of cases (%) | n = 62 (100%) | n = 9 (14.5%) | n = 53 (85.5%) | - | - |
| Patient Characteristics | |||||
| Mean age ± SD [years] | 38 (± 10.7) | 46.4 (±9.6) | 0.02 | * | |
| Mean BMI ± SD [kg/m2] | 24.9 (± 5.5 ) | 25.5 (±5.2) | 0.73 | n.s. | |
| Comorbidities | |||||
| Active smoker/non-smoker (%) | 2 (22.2%)/7 (77.8%) | 9 (17%)/44 (83%) | 0.66 | n.s. | |
| Alcohol/no Alcohol (%) | 2 (22.2%)/7 (77.8%) | 5 (9.4%)/48 (90.6%) | 0.27 | n.s. | |
| Operative Characteristics | |||||
| Flap type (DIEP/Thigh) (%) | 5 (55.6%)/4 (44.4%) | 29 (54.7%)/24 (45.3%) | 0.99 | n.s. | |
| Unilateral/bilateral reconstruction (%) | 0 (0%)/9 (100%) | 24 (45.3%)/29 (54.7%) | 0.009 | ** | |
| SSM/NSM (%) | 0 (0%)/9 (100%) | 40 (75.5%)/13 (24.5%) | < 0.001 | ** | |
| Recipient Vessel (IMA/IMAP) (%) | 4 (44.4%)/5 (55.6%) | 26 (49.1%)/27 (50.9%) | 0.99 | n.s. | |
| Coupler rank [1.5/2.0/2.5/3.0 mm] (%) |
(0/2/7/0) (0%/22.2%/77.8%/0%) | (2/14/34/3) (3.8%/26.4%/64.2%/5.7%) | 0.77 | n.s. | |
| Mean mastectomy weight ± SD [g] | 518.7 (± 292.9) | 460 (±203.1) | 0.58 | n.s. | |
| Mean flap weight ± SD [g] | 492.4 (± 324.2) | 429.4 (±206.7) | 0.59 | n.s. | |
| Mean operative time ± SD [minutes] † | 300.4 (± 43) | 244.9 (±59.7) | 0.01 | * | |
| Mean ischemia time ± SD [minutes] | 35.4 (± 7.96) | 40.6 (±14.2) | 0.3 | n.s. | |
| Adjuvant oncologic therapy | |||||
| Invasive Cancer/no inv. Ca. | 2 (22.2%)/7 (77.8%) | 26 (49.1%)/27 (50.9%) | 0.17 | n.s. | |
| DCIS/no DCIS (%) | 2 (22.2%)/7 (77.8%) | 7 (13.2%)/46 (86.8%) | 0.61 | n.s. | |
| Prophylactic/non-prophylactic surgery (%) | 6 (66.7%)/3 (33.3%) | 16 (30.2%)/37 (69.8%) | 0.057 | n.s. | |
| Prev. BCT/no BCT (%) | 0 (0%)/9 (100%) | 13 (24.5%)/40 (75.5%) | 0.18 | n.s. | |
| Prev. Rx/no Rx (%) | 1 (11.1%)/8 (88.9%) | 8 (15.1%)/45 (84.9%) | 0.99 | n.s. | |
| Prev. Cx/no Cx (%) | 2 (22.2%)/7 (77.8%) | 27 (50.9%)/26 (49.1%) | 0.16 | n.s. | |
| Postop. Rx (%) | 0 (0%) | 7 (13.2%) | 0.58 | n.s. | |
| Postop. Cx (%) | 1 (11.1%) | 9 (17%) | 0.99 | n.s. | |
| SLN/no SLN (%) | 2 (22.2%)/7 (77.8%) | 22 (41.5%)/31 (58.5%) | 0.46 | n.s. | |
| ALND/no ALND (%) | 0 (0%)/9 (100%) | 5 (9.4%)/48 (90.6%) | 0.99 | n.s. | |
| Impaired WH Treated Conservatively | |||||
|---|---|---|---|---|---|
| Overall | Impaired WH | Uneventful WH | p-Values | Significant | |
| Number of cases (%) | n = 62 (100%) | n = 14 (22.6%) | n = 48 (77.4%) | - | - |
| Patient Characteristics | |||||
| Mean age ± SD [years] | 49.1 ( ±10.7) | 44 (±9.6) | 0.09 | n.s. | |
| Mean BMI ± SD [kg/m2] | 28.1 ( ±6.98 ) | 24.7 (±4.4) | 0.03 | * | |
| Comorbidities | |||||
| Active smoker/non-smoker (%) | 2 (14.3%)/12 (85.7%) | 9 (18.8%)/39 (81.2%) | 0.99 | n.s. | |
| Alcohol/no Alcohol (%) | 0 (0%)/14 (100%) | 7 (14.6%)/41 (85.4%) | 0.33 | n.s. | |
| Operative Characteristics | |||||
| Flap type (DIEP/Thigh) (%) | 9 (64.3%)/5 (35.7%) | 25 (52.1%)/23 (47.9%) | 0.55 | n.s. | |
| Unilateral/bilateral (%) | 4 (28.6%)/10 (71.4%) | 20 (41.7%)/28 (58.3%) | 0.54 | n.s. | |
| SSM/NSM (%) | 9 (64.3%)/5 (35.7%) | 31 (64.6%)/17 (35.4%) | 0.99 | n.s. | |
| Recipient Ves. (IMA/IMAP)(%) | 8 (57.1%)/6 (42.9%) | 22 (45.8%)/26 (54.2%) | 0.55 | n.s. | |
| Coupler rank [1.5/2.0/2.5/3.0 mm] (%) |
(0/2/11/1) (0%/14.3%/78.6%/7.1%) | (2/14/30/2) (4.2%/29.2%/62.5%/4.2%) | 0.55 | n.s. | |
| Mean mastectomy weight ± SD [g] | 522.9 ( ±229.2) | 453.0 (±212.5) | 0.29 | n.s. | |
| Mean flap weight ± SD [g] | 501.6 ( ±224.6) | 420.1 (±224.3) | 0.24 | n.s. | |
| Mean operative time ± SD [minutes] † | 274.5 ( ±60.4) | 246.7 (±59.8) | 0.13 | n.s. | |
| Mean ischemia time ± SD [minutes] | 39.8 ( ±11.4) | 39.9 (±14.3) | 0.98 | n.s. | |
| oncologic characteristics | |||||
| Invasive Cancer/no inv. Ca. | 7 (50%)/7 (50%) | 21 (43.8%)/27 (56.2%) | 0.77 | n.s. | |
| DCIS/no DCIS (%) | 2 (14.3%)/12 (85.7%) | 7 (14.6%)/41 (85.4%) | 0.99 | n.s. | |
| Prophylactic/non-prophylac. (%) | 4 (28.6%)/10 (71.4%) | 18 (37.5%)/30 (62.5%) | 0.75 | n.s. | |
| Prev. BCT/no BCT (%) | 4 (28.6%)/10 (71.4%) | 9 (18.8%)/39 (81.2%) | 0.47 | n.s. | |
| Prev. Rx/no Rx (%) | 3 (21.4%)/11 (78.6%) | 6 (12.5%)/42 (87.5%) | 0.41 | n.s. | |
| Prev. Cx/no Cx (%) | 8 (57.1%)/6 (42.9%) | 23 (47.9%)/25 (52.1%) | 0.77 | n.s. | |
| Postop. Rx (%) | 1 (7.1%) | 6 (12.5%) | 0.99 | n.s. | |
| Postop. Cx (%) | 1 (7.1%) | 9 (18.8%) | 0.43 | n.s. | |
| SLN/no SLN (%) | 6 (42.9%)/8 (57.1%) | 18 (37.5%)/30 (62.5%) | 0.76 | n.s. | |
| ALND/no ALND (%) | 0 (0%)/14 (100%) | 5 (10.4%)/43 (89.6%) | 0.58 | n.s. | |
| Impaired WH with Surgical Treatment | |||||
|---|---|---|---|---|---|
| Overall | WH Disorder | No Surgery | p-Values | Significant | |
| Number of cases (%) | n = 62 (100%) | n = 4 (6.5%) | n = 58 (93.5%) | - | - |
| Patient Characteristics | |||||
| Mean age ± SD [years] | 44 ( ±15.3) | 45.2 (±9.8) | 0.82 | n.s. | |
| Mean BMI ± SD [kg/m2] | 33 ( ±7 ) | 24.9 (±4.7) | 0.002 | ** | |
| Comorbidities | |||||
| Active smoker/non-smoker (%) | 1 (25%)/3 (75%) | 10 (17.2%)/48 (82.8%) | 0.55 | n.s. | |
| Alcohol/no Alcohol (%) | 0 (0%)/4 (100%) | 7 (12.1%)/51 (87.9%) | 0.99 | n.s. | |
| Operative Characteristics | |||||
| Flap type (DIEP/Thigh) (%) | 3 (75%)/1 (25%) | 31 (53.4%)/27 (46.6%) | 0.62 | n.s. | |
| Unilateral/bilateral reconstruction (%) | 1 (25%)/3 (75%) | 23 (39.7%)/35 (60.3%) | 0.99 | n.s. | |
| SSM/NSM (%) | 1 (25%)/3 (75%) | 39 (67.2%)/19 (32.8%) | 0.12 | n.s. | |
| Recipient Vessel (IMA/IMAP) (%) | 3 (75%)/1 (25%) | 27 (46.6%)/31 (53.4%) | 0.35 | n.s. | |
| Coupler rank [1.5/2.0/2.5/3.0 mm] (%) |
(0/0/4/0) (0%/0%/100%/0%) | (2/16/37/3) (3.4%/27.6%/63.8%/5.2%) | 0.53 | n.s. | |
| Mean mastectomy weight ± SD [g] | 566.5 ( ±284.2) | 462.1 (±212.6) | 0.36 | n.s. | |
| Mean flap weight ± SD [g] | 739.0 (±319.2) | 417.8 (±205.3) | 0.005 | ** | |
| Mean operative time ± SD [minutes] † | 312.25 ( ±68.1) | 248.8 (±58.5) | 0.04 | * | |
| Mean ischemia time ± SD [minutes] | 34.5 ( ±11.1) | 40.2 (±13.7) | 0.42 | n.s. | |
| Adjuvant oncologic therapy | |||||
| Invasive Cancer/no inv. Ca. | 2 (50%)/2 (50%) | 26 (44.8%)/32 (55.2%) | 0.99 | n.s. | |
| DCIS/no DCIS (%) | 0 (0%)/4 (100%) | 9 (15.5%)/49 (84.5%) | 0.99 | n.s. | |
| Prophylactic/non-prophylactic surgery (%) | 1 (25%)/3 (75%) | 21 (36.2%/37 (63.8%) | 0.99 | n.s. | |
| Prev. BCT/no BCT (%) | 1 (25%)/3 (75%) | 12 (20.7%)/46 (79.3%) | 0.99 | n.s. | |
| Prev. Rx/no Rx (%) | 1 (25%)/3 (75%) | 8 (13.8%)/50 (86.2%) | 0.48 | n.s. | |
| Prev. Cx/no Cx (%) | 1 (25%)/3 (75%) | 26 (44.8%)/32 (55.2%) | 0.33 | n.s. | |
| Postop. Rx (%) | 0 (0%) | 7 (12.1%) | 0.99 | n.s. | |
| Postop. Cx (%) | 0 (0%) | 10 (17.2%) | 0.99 | n.s. | |
| SLN/no SLN (%) | 2 (50%)/2 (50%) | 22 (37.9%)/36 (62.1%) | 0.64 | n.s. | |
| ALND/no ALND (%) | 0 (0%)/4 (100%) | 5 (8.6%)/53 (91.4%) | 0.99 | n.s. | |
| Variable | Adjusted Odds Ratio | 95% CI | p-Values | Significant |
|---|---|---|---|---|
| Any Complication | ||||
| BMI | 1.48 | 1.06–2.05 | 0.019 | * |
| Mastectomy type SSM vs. NSM | 21.38 | 2.9–160.5 | 0.003 | ** |
| Impaired wound healing treated conservatively | ||||
| Operation time (OT) | 1.01 | 1.00–1.03 | 0.044 | * |
| Age | 1.06 | 1.00–1.13 | 0.045 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vollbach, F.H.; Thomas, B.F.; Fansa, H. Identification of Independent Risk Factors for Skin Complications in a Multifactorial Logistic Regression Analysis of Simultaneous Immediate Autologous Breast Reconstruction and Skin Reduction Mastectomy in Large and Ptotic Breasts Using an Inferiorly Based Deepithelialized Dermal Breast Flap. J. Pers. Med. 2022, 12, 332. https://doi.org/10.3390/jpm12030332
Vollbach FH, Thomas BF, Fansa H. Identification of Independent Risk Factors for Skin Complications in a Multifactorial Logistic Regression Analysis of Simultaneous Immediate Autologous Breast Reconstruction and Skin Reduction Mastectomy in Large and Ptotic Breasts Using an Inferiorly Based Deepithelialized Dermal Breast Flap. Journal of Personalized Medicine. 2022; 12(3):332. https://doi.org/10.3390/jpm12030332
Chicago/Turabian StyleVollbach, Felix H., Benjamin F. Thomas, and Hisham Fansa. 2022. "Identification of Independent Risk Factors for Skin Complications in a Multifactorial Logistic Regression Analysis of Simultaneous Immediate Autologous Breast Reconstruction and Skin Reduction Mastectomy in Large and Ptotic Breasts Using an Inferiorly Based Deepithelialized Dermal Breast Flap" Journal of Personalized Medicine 12, no. 3: 332. https://doi.org/10.3390/jpm12030332
APA StyleVollbach, F. H., Thomas, B. F., & Fansa, H. (2022). Identification of Independent Risk Factors for Skin Complications in a Multifactorial Logistic Regression Analysis of Simultaneous Immediate Autologous Breast Reconstruction and Skin Reduction Mastectomy in Large and Ptotic Breasts Using an Inferiorly Based Deepithelialized Dermal Breast Flap. Journal of Personalized Medicine, 12(3), 332. https://doi.org/10.3390/jpm12030332







