Personalized Preoperative Prediction of the Length of Hospital Stay after TAVI Using a Dedicated Decision Tree Algorithm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Collected Data
2.3.1. The TAVI Scorecard Database
2.3.2. Anthropometric Data
2.3.3. Comorbidities
2.3.4. Laboratory Investigations/Biomarkers
2.3.5. EuroQol-5 Dimension-5 Levels (EQ5D5L)
2.3.6. Kansas City Cardiomyopathy Questionnaire (KCCQ)
2.3.7. Clinical Frailty Scale (CFS)
2.3.8. EuroSCORE II
2.3.9. Contrast Agent Volume
2.4. Intervention—TAVI Procedure
2.5. Statistical Analysis
3. Results
3.1. Baseline Data
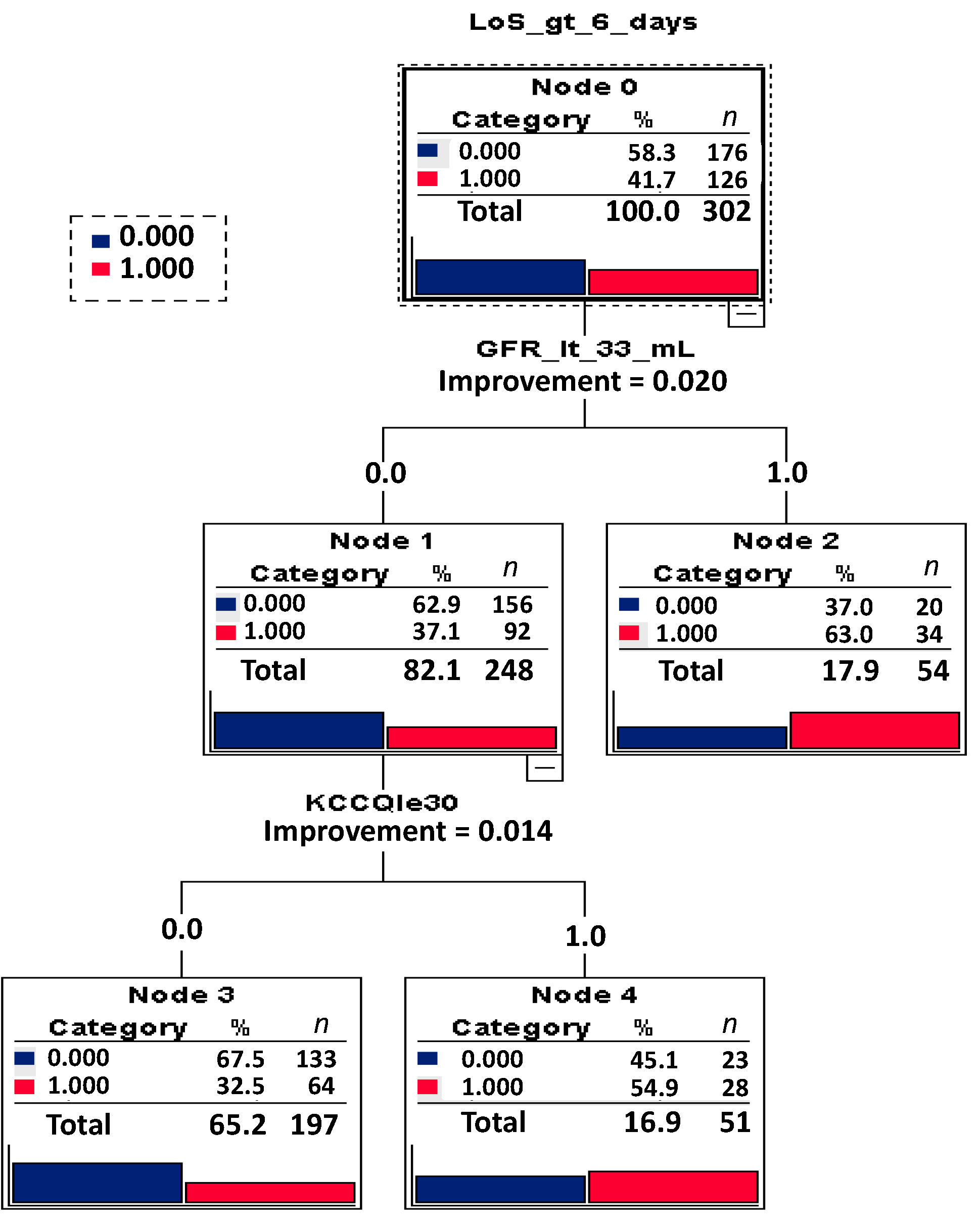
3.2. Analysis of LoS
4. Discussion
4.1. Strengths of the Study
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hachesu, P.R.; Ahmadi, M.; Alizadeh, S.; Sadoughi, F. Use of Data Mining Techniques to Determine and Predict Length of Stay of Cardiac Patients. Healthc. Inform. Res. 2013, 19, 121. [Google Scholar] [CrossRef] [PubMed]
- Alshakhs, F.; Alharthi, H.; Aslam, N.; Khan, I.U.; Elasheri, M. Predicting Postoperative Length of Stay for Isolated Coronary Artery Bypass Graft Patients Using Machine Learning. Int. J. Gen. Med. 2020, 13, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, B.; Yang, J.; Mo, X.; Zhang, L.; Li, M.; Chen, Z.; Fang, J.; Wang, F.; Huang, W.; et al. Predicting Intensive Care Unit Length of Stay After Acute Type A Aortic Dissection Surgery Using Machine Learning. Front. Cardiovasc. Med. 2021, 8, 675431. [Google Scholar] [CrossRef]
- Ma, F.; Yu, L.; Ye, L.; Yao, D.D.; Zhuang, W. Length-of-Stay Prediction for Pediatric Patients With Respiratory Diseases Using Decision Tree Methods. IEEE J. Biomed. Health Inform. 2020, 24, 2651–2662. [Google Scholar] [CrossRef]
- Lu, M.; Sajobi, T.; Lucyk, K.; Lorenzetti, D.; Quan, H. Systematic Review of Risk Adjustment Models of Hospital Length of Stay (LOS). Med. Care 2015, 53, 355–365. [Google Scholar] [CrossRef]
- Kaplan, R.S.; Norton, D.P. The Balanced Scorecard—Measures That Drive Performance. Harvard Business Review, January–February 1992. Available online: https://pubmed.ncbi.nlm.nih.gov/10119714/ (accessed on 26 January 2020).
- De Ronde-Tillmans, M.J.; de Jager, T.A.; Goudzwaard, J.A.; El Faquir, N.; van Mieghem, N.M.; Zijlstra, F.; Utens, E.M.; Mattace-Raso, F.U.; Lenzen, M.J.; de Jaegere, P.P. Long-Term Follow-up of Quality of Life in High-Risk Patients Undergoing Transcatheter Aortic Valve Implantation for Symptomatic Aortic Valve Stenosis. J. Geriatr. Cardiol. 2018, 15, 261. [Google Scholar] [CrossRef]
- Van Reenen, M.; Janssen, B.; Stolk, E.; Boye, K.S.; Herdman, M.; Kennedy-Martin, M.; Kennedy-Martin, T.; Slaap, B. EQ-5D-5L-English-User-Guide. Roterdam. Available online: Https://Euroqol.Org/Wp-Content/Uploads/2019/09/EQ-5D-5L-English-User-Guide_version-3.0-Sept-2019-Secured.Pdf (accessed on 26 January 2020).
- Arnold, S.V.; Spertus, J.A.; Lei, Y.; Allen, K.B.; Chhatriwalla, A.K.; Leon, M.B.; Smith, C.R.; Reynolds, M.R.; Webb, J.G.; Svensson, L.G.; et al. Use of the Kansas City Cardiomyopathy Questionnaire for Monitoring Health Status in Patients With Aortic Stenosis. Circ. Heart Fail. 2013, 6, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, D.; Kennon, S.; Bonaros, N.; Romano, M.; Lefèvre, T.; Di Mario, C.; Stefàno, P.; Ribichini, F.L.; Himbert, D.; Urena-Alcazar, M.; et al. Trial Protocol for the Validation of the ‘Toronto Aortic Stenosis Quality of Life (TASQ) Questionnaire’ in Patients Undergoing Surgical Aortic Valve Replacement (SAVR) or Transfemoral (TF) Transcatheter Aortic Valve Implantation (TAVI): The TASQ Registry. Open Heart 2019, 6, e001008. [Google Scholar] [CrossRef] [Green Version]
- Rogers, T.; Alraies, M.C.; Moussa Pacha, H.; Bond, E.; Buchanan, K.D.; Steinvil, A.; Gai, J.; Torguson, R.; Ben-Dor, I.; Satler, L.F.; et al. Clinical Frailty as an Outcome Predictor After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2018, 121, 850–855. [Google Scholar] [CrossRef]
- Shimura, T.; Yamamoto, M.; Kano, S.; Kagase, A.; Kodama, A.; Koyama, Y.; Tsuchikane, E.; Suzuki, T.; Otsuka, T.; Kohsaka, S.; et al. Impact of the Clinical Frailty Scale on Outcomes After Transcatheter Aortic Valve Replacement. Circulation 2017, 135, 2013–2024. [Google Scholar] [CrossRef]
- Nashef, S.A.M.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rifkin, R.; Klautau, A. In Defense of One-Vs-All Classification. J. Mach. Learn. Res. 2004, 5, 101–141. [Google Scholar]
- Utgoff, P.E. Incremental Induction of Decision Trees. Mach. Learn. 1989, 4, 161–186. [Google Scholar] [CrossRef] [Green Version]
- Zisiopoulou, M.; Berkowitsch, A.; Seppelt, P.; Zeiher, A.M.; Vasa-Nicotera, M. A Novel Method to Predict Mortality and Length of Stay after Transfemoral Transcatheter Aortic Valve Implantation. Medicina 2021, 57, 1332. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dawson, E.; Moodie, J.; Martin, J.; Bagur, R.; Cheng, D.; Kiaii, B.; Hashi, A.; Bi, R.; Yeschin, M.; et al. Measurement and Prognosis of Frail Patients Undergoing Transcatheter Aortic Valve Implantation: A Systematic Review and Meta-Analysis. BMJ Open 2021, 11, e040459. [Google Scholar] [CrossRef] [PubMed]
- Church, S.; Rogers, E.; Rockwood, K.; Theou, O. A Scoping Review of the Clinical Frailty Scale. BMC Geriatr. 2020, 20, 393. [Google Scholar] [CrossRef]
- VARC-3 WRITING COMMITTEE; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef]
- Kleczynski, P.; Dziewierz, A.; Bagienski, M.; Rzeszutko, L.; Sorysz, D.; Trebacz, J.; Sobczynski, R.; Tomala, M.; Stapor, M.; Dudek, D. Impact of Frailty on Mortality after Transcatheter Aortic Valve Implantation. Am. Heart J. 2017, 185, 52–58. [Google Scholar] [CrossRef]
- Martin, G.P.; Sperrin, M.; Ludman, P.F.; deBelder, M.A.; Gunning, M.; Townend, J.; Redwood, S.R.; Kadam, U.T.; Buchan, I.; Mamas, M.A. Do Frailty Measures Improve Prediction of Mortality and Morbidity Following Transcatheter Aortic Valve Implantation? An Analysis of the UK TAVI Registry. BMJ Open 2018, 8, e022543. [Google Scholar] [CrossRef]
- Murali-Krishnan, R.; Iqbal, J.; Rowe, R.; Hatem, E.; Parviz, Y.; Richardson, J.; Sultan, A.; Gunn, J. Impact of Frailty on Outcomes after Percutaneous Coronary Intervention: A Prospective Cohort Study. Open Heart 2015, 2, e000294. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.N.; Cumming, R.G.; Hilmer, S.N. The Impact of Frailty on Mortality, Length of Stay and Re-Hospitalisation in Older Patients with Atrial Fibrillation. Heart Lung Circ. 2016, 25, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Juma, S.; Taabazuing, M.-M.; Montero-Odasso, M. Clinical Frailty Scale in an Acute Medicine Unit: A Simple Tool That Predicts Length of Stay. Can. Geriatr. J. 2016, 19, 34–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okoh, A.K.; Kambiz, K.; Unnikrishnan, D.; Fugar, S.; Sossou, C.; Thawabi, M.; Hawatmeh, A.; Haik, B.; Chen, C.; Cohen, M.; et al. Effect of Transcatheter Aortic Valve Implantation on Renal Function in Patients With Previous Renal Dysfunction. Am. J. Cardiol. 2019, 124, 85–89. [Google Scholar] [CrossRef]
- Mas-Peiro, S.; Faerber, G.; Bon, D.; Herrmann, E.; Bauer, T.; Bleiziffer, S.; Bekeredjian, R.; Böning, A.; Frerker, C.; Beckmann, A.; et al. Impact of Chronic Kidney Disease in 29 893 Patients Undergoing Transcatheter or Surgical Aortic Valve Replacement from the German Aortic Valve Registry. Eur. J. Cardiothorac. Surg. 2021, 59, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Venturi, G.; Pighi, M.; Pesarini, G.; Ferrero, V.; Lunardi, M.; Castaldi, G.; Setti, M.; Benini, A.; Scarsini, R.; Ribichini, F.L. Contrast-Induced Acute Kidney Injury in Patients Undergoing TAVI Compared With Coronary Interventions. J. Am. Heart Assoc. 2020, 9, e017194. [Google Scholar] [CrossRef] [PubMed]
- Voigtländer, L.; Schewel, J.; Martin, J.; Schewel, D.; Frerker, C.; Wohlmuth, P.; Thielsen, T.; Kuck, K.-H.; Schäfer, U. Impact of Kidney Function on Mortality after Transcatheter Valve Implantation in Patients with Severe Aortic Valvular Stenosis. Int. J. Cardiol. 2015, 178, 275–281. [Google Scholar] [CrossRef]
- Bagur, R.; Webb, J.G.; Nietlispach, F.; Dumont, E.; De Larochelliere, R.; Doyle, D.; Masson, J.-B.; Gutierrez, M.J.; Clavel, M.-A.; Bertrand, O.F.; et al. Acute Kidney Injury Following Transcatheter Aortic Valve Implantation: Predictive Factors, Prognostic Value, and Comparison with Surgical Aortic Valve Replacement. Eur. Heart J. 2010, 31, 865–874. [Google Scholar] [CrossRef] [Green Version]
- Owais, T.; Garhy, M.E.; Fuchs, J.; Schreiber, M.; Girdauskas, E.; Kuntze, T. Transcatheter Aortic Valve Replacement Without the Use of Contrast Medium: An Alternative Safe Implantation Technique. J. Invasive Cardiol. 2020, 32, 330–334. [Google Scholar]
- Mas-Peiro, S.; Fichtlscherer, S.; Walther, C.; Vasa-Nicotera, M. Current Issues in Transcatheter Aortic Valve Replacement. J. Thorac. Dis. 2020, 12, 1665–1680. [Google Scholar] [CrossRef]
- Kim, B.G.; Ko, Y.-G.; Hong, S.-J.; Ahn, C.-M.; Kim, J.-S.; Kim, B.-K.; Choi, D.; Jang, Y.; Hong, M.-K.; Lee, S.H.; et al. Impact of Peripheral Artery Disease on Early and Late Outcomes of Transcatheter Aortic Valve Implantation in Patients with Severe Aortic Valve Stenosis. Int. J. Cardiol. 2018, 255, 206–211. [Google Scholar] [CrossRef]
- Malyar, N.; Kaier, K.; Freisinger, E.; Lüders, F.; Kaleschke, G.; Baumgartner, H.; Frankenstein, L.; Reinecke, H.; Reinöhl, J. Prevalence and Impact of Critical Limb Ischaemia on In-Hospital Outcome in Transcatheter Aortic Valve Implantation in Germany. EuroIntervention 2017, 13, 1281–1287. [Google Scholar] [CrossRef]
- Krzywinski, M.; Altman, N. Classification and regression trees. Nat. Methods 2017, 14, 757–758. [Google Scholar] [CrossRef]

| Baseline Characteristic | n Inter-Quantiles (Median) Range | % of Total Participants |
|---|---|---|
| Male sex, n of participants | 156 | 51.66% of all participants |
| Age, years | 81.27 (78.19−84.24) | - |
| ES II, score values | 3.73 (2.18−6.40) | - |
| BMI, kg/m2 | 27.34 (25.37−29.58) | - |
| Post-TAVI LoS, days | 6 (5−8) | - |
| ICU-LoS, hours | 50.95 (46.82−74.67) | - |
| Mobilization time, hours | 21.12 (9.67−26.17) | - |
| LVEF, % | 50 (48−53) | - |
| CAD, n of participants | 138 | 45.54% of all participants |
| CHF, n of participants | 40 | 13.20% of all participants |
| Prior surgery, n of participants | 28 | 9.24% of all participants |
| CS, n of participants | 63 | 20.79% of all participants |
| PM/ICD implantation, n | 34 | 11.22% of all participants |
| AF, n of participants | 84 | 27.72% of all participants |
| Cardiac co-morbidity, n | 185 | 61.06% of all participants |
| Aortic aneurysm, n | 14 | 4.62% of all participants |
| PAD, n of participants | 77 | 25.41% of all participants |
| DM, n of participants | 65 | 21.45% of all participants |
| COPD, n of participants | 46 | 15.18% of all participants |
| Neurological dysfunction, n | 25 | 8.25% of all participants |
| Ranking scale score ≥ 2, score | 5 | 1.65% of all participants |
| CFS, score | 4 (3−6) | - |
| EQ-5D-5L, score | 55 (38−77) | - |
| KCCQ, score | 39 (30−48) | - |
| Hb, g/dL | 12.60 (11.40−13.75) | - |
| GFR, mL/min/1.73 m2 | 48.70 (36.78−61.73) | - |
| NTproBNP, score | 1569 (535.5−3623) | - |
| Contrast agent volume, mL | 70 (50−90) | - |
| Independent Variable | Importance | Normalized Importance | Cut-Off Values | p | HR | 95% CI |
|---|---|---|---|---|---|---|
| GFR | 0.023 | 100.0% | <33 mL/min/1.73 m2 | 0.003 | 2.252 | (1.316−3.852) |
| NT pro BNP | 0.016 | 69.9% | 0.483 | 1.174 | (0.750–1.840) | |
| CFS | 0.015 | 64.4% | >3 | 0.000 | 1.324 | (1.138−1.539) |
| KCCQ | 0.013 | 55.6% | <30 | 0.003 | 2.274 | (1.323−3.907) |
| Hb | 0.008 | 34.3% | 0.808 | 1.008 | (0.946−1.074) | |
| Age | 0.002 | 7.7% | 0.385 | 0.987 | (0.957−1.017) | |
| BMI | 0.001 | 5.0% | 0.690 | 1.010 | (0.961−1.062) | |
| PAD | 0.012 | 60.6% | 0.029 | 1.826 | (1.062−3.140) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zisiopoulou, M.; Berkowitsch, A.; Neuber, R.; Gouveris, H.; Fichtlscherer, S.; Walther, T.; Vasa-Nicotera, M.; Seppelt, P. Personalized Preoperative Prediction of the Length of Hospital Stay after TAVI Using a Dedicated Decision Tree Algorithm. J. Pers. Med. 2022, 12, 346. https://doi.org/10.3390/jpm12030346
Zisiopoulou M, Berkowitsch A, Neuber R, Gouveris H, Fichtlscherer S, Walther T, Vasa-Nicotera M, Seppelt P. Personalized Preoperative Prediction of the Length of Hospital Stay after TAVI Using a Dedicated Decision Tree Algorithm. Journal of Personalized Medicine. 2022; 12(3):346. https://doi.org/10.3390/jpm12030346
Chicago/Turabian StyleZisiopoulou, Maria, Alexander Berkowitsch, Ralf Neuber, Haralampos Gouveris, Stephan Fichtlscherer, Thomas Walther, Mariuca Vasa-Nicotera, and Philipp Seppelt. 2022. "Personalized Preoperative Prediction of the Length of Hospital Stay after TAVI Using a Dedicated Decision Tree Algorithm" Journal of Personalized Medicine 12, no. 3: 346. https://doi.org/10.3390/jpm12030346







