Circulating Endothelial Cell Levels Correlate with Treatment Outcomes of Splanchnic Vein Thrombosis in Patients with Chronic Myeloproliferative Neoplasms
Abstract
1. Introduction
2. Patients and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethic Statements
References
- Shantsila, E.; Blann, A.D.; Lip, G.Y.H. Circulating endothelial cells: From bench to clinical practice. J. Thromb. Haemost. 2008, 6, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Woywodt, A. Circulating endothelial cells: Life, death, detachment and repair of the endothelial cell layer. Nephrol. Dial. Transplant. 2002, 17, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, T.; Woywodt, A.; Beese, M.; Wyss, K.; Park, J.K.; Erdbruegger, U.; Hertel, B.; Haller, H.; Haubitz, M. Engulfment of apoptotic cells by microvascular endothelial cells induces proinflammatory responses. Blood 2007, 109, 2854–2862. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Burlini, A.; Pruneri, G.; Goldhirsch, A.; Martinelli, G.; Bertolini, F. Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood 2001, 97, 3658–3661. [Google Scholar] [CrossRef] [PubMed]
- Monestiroli, S.; Mancuso, P.; Burlini, A.; Pruneri, G.; Dell’Agnola, C.; Gobbi, A.; Martinelli, G.; Bertolini, F. Kinetics and viability of circulating endothelial cells as surrogate angiogenesis marker in an animal model of human lymphoma. Cancer Res. 2001, 61, 4341–4344. [Google Scholar] [PubMed]
- Rafii, S.; Lyden, D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 2003, 9, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Lyden, D.; Hattori, K.; Dias, S.; Costa, C.; Blaikie, P.; Butros, L.; Chadburn, A.; Heissig, B.; Marks, W.; Witte, L.; et al. Impaired recruitment of bone-marrow–derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001, 7, 1194–1201. [Google Scholar] [CrossRef]
- de Bont, E.S.; Guikema, J.E.; Scherpen, F.; Meeuwsen, T.; Kamps, W.A.; Vellenga, E.; Bos, N.A. Mobilized Human CD34+ Hematopoietic Stem Cells Enhance Tumor Growth in a Nonobese Diabetic/Severe Combined Immunodeficient Mouse Model of Human Non-Hodgkin’s Lymphoma. Cancer Res. 2001, 61, 7654–7659. [Google Scholar]
- Beerepoot, L.V.; Mehra, N.; Vermaat, J.S.P.; Zonnenberg, B.A.; Gebbink, M.F.G.B.; Voest, E.E. Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann. Oncol. 2004, 15, 139–145. [Google Scholar] [CrossRef]
- Torres, C.; Fonseca, A.M.; Leander, M.; Matos, R.; Morais, S.; Campos, M.; Lima, M. Circulating endothelial cells in patients with venous thromboembolism and myeloproliferative neoplasms. PLoS ONE 2013, 8, e81574. [Google Scholar] [CrossRef]
- Sekhar, M.; McVinnie, K.; Burroughs, A.K. Splanchnic vein thrombosis in myeloproliferative neoplasms. Br. J. Haematol. 2013, 162, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Carobbio, A.; Cervantes, F.; Vannucchi, A.M.; Guglielmelli, P.; Antonioli, E.; Alvarez-Larrán, A.; Rambaldi, A.; Finazzi, G.; Barosi, G. Thrombosis in primary myelofibrosis: Incidence and risk factors. Blood 2010, 115, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Kreher, S.; Ochsenreither, S.; Trappe, R.U.; Pabinger, I.; Bergmann, F.; Petrides, P.E.; Koschmieder, S.; Matzdorff, A.; Tiede, A.; Griesshammer, M.; et al. Prophylaxis and management of venous thromboembolism in patients with myeloproliferative neoplasms: Consensus statement of the Haemostasis Working Party of the German Society of Hematology and Oncology (DGHO), the Austrian Society of Hematology and Oncology (ÖGHO) and Society of Thrombosis and Haemostasis Research (GTH e.V.). Ann. Hematol. 2014, 93, 1953–1963. [Google Scholar] [CrossRef]
- Alessio, A.M.; Beltrame, M.P.; Nascimento, M.C.F.; Vicente, C.P.; de Godoy, J.A.; Silva, J.C.S.; Bittar, L.F.; Lorand-Metze, I.; de Paula, E.V.; Annichino-Bizzacchi, J.M. Circulating Progenitor and Mature Endothelial Cells in Deep Vein Thrombosis. Int. J. Med. Sci. 2013, 10, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Ageno, W.; Riva, N.; Schulman, S.; Beyer-Westendorf, J.; Bang, S.M.; Senzolo, M.; Grandone, E.; Pasca, S.; Di Minno, M.N.D.; Duce, R.; et al. Long-term Clinical Outcomes of Splanchnic Vein Thrombosis. JAMA Intern. Med. 2015, 175, 1474. [Google Scholar] [CrossRef]
- Kiladjian, J.J.; Cervantes, F.; Leebeek, F.W.; Marzac, C.; Cassinat, B.; Chevret, S.; Cazals-Hatem, D.; Plessier, A.; Garcia-Pagan, J.C.; Murad, S.D.; et al. The impact of JAK2 and MPL mutations on diagnosis and prognosis of splanchnic vein thrombosis: A report on 241 cases. Blood 2008, 111, 4922–4929. [Google Scholar] [CrossRef]
- Barbui, T.; Finazzi, G.; Falanga, A. Myeloproliferative neoplasms and thrombosis. Blood 2013, 122, 2176–2184. [Google Scholar] [CrossRef]
- Delhommeau, F.; Jeziorowska, D.; Marzac, C.; Casadevall, N. Molecular aspects of myeloproliferative neoplasms. Int. J. Hematol. 2010, 91, 165–173. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Kiladjian, J.J.; Griesshammer, M.; Masszi, T.; Durrant, S.; Passamonti, F.; Harrison, C.N.; Pane, F.; Zachee, P.; Mesa, R.; et al. Ruxolitinib versus Standard Therapy for the Treatment of Polycythemia Vera. N. Engl. J. Med. 2015, 372, 426–435. [Google Scholar] [CrossRef]
- Verstovsek, S.; Mesa, R.A.; Gotlib, J.; Levy, R.S.; Gupta, V.; DiPersio, J.F.; Catalano, J.V.; Deininger, M.; Miller, C.; Silver, R.T.; et al. A Double-Blind, Placebo-Controlled Trial of Ruxolitinib for Myelofibrosis. N. Engl. J. Med. 2012, 366, 799–807. [Google Scholar] [CrossRef]
- Verstovsek, S.; Passamonti, F.; Rambaldi, A.; Barosi, G.; Rumi, E.; Gattoni, E.; Pieri, L.; Zhang, H.; Cazzola, M.; Kantarjian, H.M.; et al. Long-Term Results from a Phase II Open-Label Study of Ruxolitinib in Patients with Essential Thrombocythemia Refractory to or Intolerant of Hydroxyurea. Blood 2014, 124, 1847. [Google Scholar] [CrossRef]
- How, J.; Zhou, A.; Oh, S.T. Splanchnic vein thrombosis in myeloproliferative neoplasms: Pathophysiology and molecular mechanisms of disease. Ther. Adv. Hematol. 2017, 8, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, N.; Giordano, G.; Petrilli, M.P.; Fraticelli, V.; De Gaetano, G.; Cerletti, C.; Storti, S.; Donati, M.B. Inhibition of tissue factor expression by hydroxyurea in polymorphonuclear leukocytes from patients with myeloproliferative disorders: A new effect for an old drug? J. Thromb. Haemost. 2006, 4, 2593–2598. [Google Scholar] [CrossRef] [PubMed]
- Cella, G.; Marchetti, M.; Vianello, F.; Panova-Noeva, M.; Vignoli, A.; Russo, L.; Barbui, T.; Falanga, A. Nitric oxide derivatives and soluble plasma selectins in patients with myeloproliferative neoplasms. Thromb. Haemost. 2010, 104, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.C.; Traina, F.; Almeida, C.B.; Leonardo, F.C.; Franco-Penteado, C.F.; Garrido, V.T.; Colella, M.P.; Soares, R.; Olalla-Saad, S.T.; Costa, F.F.; et al. Key endothelial cell angiogenic mechanisms are stimulated by the circulating milieu in sickle cell disease and attenuated by hydroxyurea. Haematologica 2015, 100, 730–739. [Google Scholar] [CrossRef]
- Laurance, S.; Lansiaux, P.; Pellay, F.X.; Hauchecorne, M.; Benecke, A.; Elion, J.; Lapoumeroulie, C. Differential modulation of adhesion molecule expression by hydroxycarbamide in human endothelial cells from the micro- and macrocirculation: Potential implications in sickle cell disease vasoocclusive events. Haematologica 2011, 96, 534–542. [Google Scholar] [CrossRef]
- Lucchesi, A.; Napolitano, R.; Bochicchio, M.T.; Giordano, G.; Napolitano, M. Platelets Contribution to Thrombin Generation in Philadelphia-Negative Myeloproliferative Neoplasms: The ‘Circulating Wound’ Model. Int. J. Mol. Sci. 2021, 22, 11343. [Google Scholar] [CrossRef]
- DaSilva, A.; Aronovich, E.; Nguyen, A.; Nguyen, J.; Reynolds, D.; Doak, G.D.; Vercellotti, G.M.; Wood, D.K.; Beckman, J.D. Ruxolitinib Reduces Endothelial Pro-Adhesive Interactions: Implications for JAK2V617+ MPN Thrombosis. Blood 2020, 136 (Suppl. S1), 1. [Google Scholar] [CrossRef]
- Cervantes, F.; Dupriez, B.; Pereira, A.; Passamonti, F.; Reilly, J.T.; Morra, E.; Vannucchi, A.M.; Mesa, R.A.; Demory, J.L.; Barosi, G.; et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009, 113, 2895–2901. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Fu, P.; Zhang, S.; Xiu, R. Serum C-reactive protein and circulating endothelial cells in patients with acute myocardial infarction. Clin. Hemorheol. Microcirc. 2005, 32, 287–296. [Google Scholar]
- Finazzi, G.; de Stefano, V.; Barbui, T. Are MPNs Vascular Diseases? Curr. Hematol. Malig. Rep. 2013, 8, 307–316. [Google Scholar] [CrossRef]
- Straub, R.H. The Complex Role of Estrogens in Inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [PubMed]
- Lussana, F.; Carobbio, A.; Randi, M.L.; Elena, C.; Rumi, E.; Finazzi, G.; Bertozzi, I.; Pieri, L.; Ruggeri, M.; Palandri, F.; et al. A lower intensity of treatment may underlie the increased risk of thrombosis in young patients with masked polycythaemia vera. Br. J. Haematol. 2014, 167, 541–546. [Google Scholar] [CrossRef]
- Lubberich, R.K.; Walenda, T.; Goecke, T.W.; Strathmann, K.; Isfort, S.; Brümmendorf, T.H.; Koschmieder, S.; Wagner, W. Serum of myeloproliferative neoplasms stimulates hematopoietic stem and progenitor cells. PLoS ONE 2018, 13, e0197233. [Google Scholar] [CrossRef]
- Geyer, H.L.; Kosiorek, H.; Dueck, A.C.; Scherber, R.; Slot, S.; Zweegman, S.; Te Boekhorst, P.A.; Senyak, Z.; Schouten, H.C.; Sackmann, F.; et al. Associations between gender, disease features and symptom burden in patients with myeloproliferative neoplasms: An analysis by the MPN QOL International Working Group. Haematologica 2017, 102, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Quintás-Cardama, A.; Vaddi, K.; Liu, P.; Manshouri, T.; Li, J.; Scherle, P.A.; Caulder, E.; Wen, X.; Li, Y.; Waeltz, P.; et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: Therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 2010, 115, 3109–3117. [Google Scholar] [CrossRef] [PubMed]
- Rosti, V.; Villani, L.; Riboni, R.; Poletto, V.; Bonetti, E.; Tozzi, L.; Bergamaschi, G.; Catarsi, P.; Dallera, E.; Novara, F.; et al. Spleen endothelial cells from patients with myelofibrosis harbor the JAK2V617F mutation. Blood 2013, 121, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Teofili, L.; Martini, M.; Iachininoto, M.G.; Capodimonti, S.; Nuzzolo, E.R.; Torti, L.; Cenci, T.; Larocca, L.M.; Leone, G. Endothelial progenitor cells are clonal and exhibit the JAK2V617F mutation in a subset of thrombotic patients with Ph-negative myeloproliferative neoplasms. Blood 2011, 117, 2700–2707. [Google Scholar] [CrossRef]
- Dai, H.; Zhou, H.; Sun, Y.; Xu, Z.; Wang, S.; Feng, T.; Zhang, P. D-dimer as a potential clinical marker for predicting metastasis and progression in cancer. Biomed. Rep. 2018, 9, 453–457. [Google Scholar] [CrossRef]
- Sozer, S.; Fiel, M.I.; Schiano, T.; Xu, M.; Mascarenhas, J.; Hoffman, R. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood 2009, 113, 5246–5249. [Google Scholar] [CrossRef]
- Myasoedova, V.A.; Poggio, P.; Parolari, A. A prominent role of D-dimer in inflammation and atherosclerosis. Vessel Plus 2017, 1, 96–97. [Google Scholar] [CrossRef][Green Version]
- Elli, E.M.; Baratè, C.; Mendicino, F.; Palandri, F.; Palumbo, G.A. Mechanisms Underlying the Anti-inflammatory and Immunosuppressive Activity of Ruxolitinib. Front. Oncol. 2019, 9, 1186. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, B.T.; Vesely, S.K.; Chai-Adisaksopha, C.; Scott, B.L.; Crowther, M.; Garcia, D. The impact of ruxolitinib on thrombosis in patients with polycythemia vera and myelofibrosis. Blood Coagul. Fibrinolysis 2016, 27, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Pieri, L.; Paoli, C.; Arena, U.; Marra, F.; Mori, F.; Zucchini, M.; Colagrande, S.; Castellani, A.; Masciulli, A.; Rosti, V.; et al. Safety and efficacy of ruxolitinib in splanchnic vein thrombosis associated with myeloproliferative neoplasms. Am. J. Hematol. 2017, 92, 187–195. [Google Scholar] [CrossRef]


| A (RUXOLITINIB) | B (HU) | C (VKA) | |
|---|---|---|---|
| PATIENTS, n | 4 | 5 | 6 |
| MEDIAN AGE, years (range) | 45 (35–51) | 44 (36–55) | 60 (55–65) |
| SEX (M/F) | 0/4 | 0/5 | 5/1 |
| MF, n | 4 | 1 | 0 |
| PV, n | 0 | 2 | 0 |
| ET, n | 0 | 2 | 0 |
| LC, n | 0 | 0 | 3 |
| HCC, n | 0 | 0 | 3 |
| JAK2 V617F positive, n | 4 | 5 | 0 |
| THROMBOPHILIA, n | 1 | 1 | 0 |
| MEDIAN HB g/dL (range) | 11.5 (11–12.5) | 12.5 (10–14.5) | 10 (9–11.5) |
| MEDIAN PLT/mmc (range) | 90,000 (70,000–150,000) | 150,000 (110,000–250,000) | 40,000 (30,000–60,000) |
| MEDIAN WBC/mmc (range) | 10,000 (4,–15,000) | 9000 (4000–13,000) | 6000 (3000–7500) |
| PVT, n | 4 | 5 | 6 |
| SVT, n | 2 | 3 | 3 |
| MVT, n | 1 | 0 | 0 |
| DD µg/mL (range) | 6000 (5000–11,000) | 5200 (4000–9000) | 4800 (2000–5000) |
| CEC/µL (range) | 1500 (1000–2500) | 1800 (1000–2000) | 2500 (1500–3000) |
| CRP mg/L (range) | 127 (95–150) | 80 (70–100) | 45 (15–60) |
| SYS SYM (TOT PAT n) | 4 | 4 | 6 |
| GROUP | A (RUXO) | B (HU) | C (VKA) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MONTH | 0 | 1 | 3 | 6 | 0 | 1 | 3 | 6 | 0 | 1 | 3 | 6 |
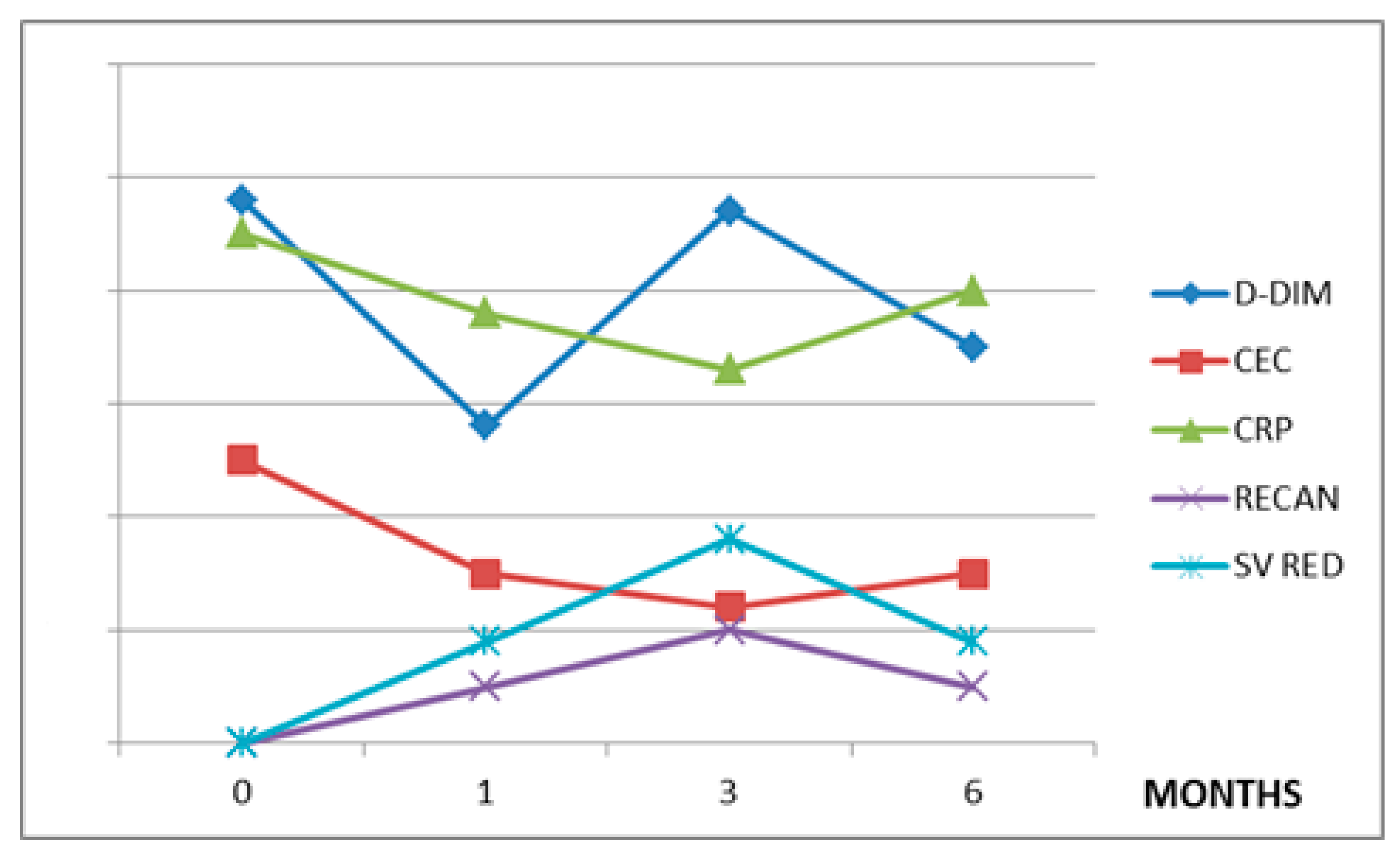
| DD µg/mL | 6000 | 5800 | 500 | 130 | 5200 | 4500 | 1800 | 700 | 4800 | 2800 | 4700 | 3500 |
| CEC/µL | 1500 (SD 407.9) | 1000 | 800 | 200 (SD 143.5) | 1800 (SD 518.6) | 1500 | 900 | 800 (SD 310.1) | 2500 | 1500 | 1200 | 1500 |
| CRP mg/L | 127 (SD 47.6) | 40 | 8 | 6 (SD 1.9) | 80 (SD 47.9) | 30 | 18 | 10 (SD 3.29) | 45 | 38 | 33 | 40 |
| RECAN % | 0 | 62,5 | 75 | 75 (SD 8.9) | 0 | 20 | 30 | 60 (SD 27.3) | 0 | 9 | 18 | 9 |
| SV RED % | 0 | 25 | 75 | 100 (SD 17.0) | 0 | 0 | 20 | 60 (SD 18.6) | 0 | 0 | 0 | 0 |
| SYS SYM (total n. pat. At each time) | 0 | 4 | 4 | 4 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 |
| A (RUXO) | B (HU) | C (VKA) | |
|---|---|---|---|
| DD | 0.8 | 0.98 | 0.3 |
| CRP | 0.7 | 0.9 | 0.9 |
| RECAN | −0.7 | −0.8 | −0.9 |
| SV RED | −0.96 | −0.9 | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, G.; Napolitano, M.; Cellurale, M.; Di Carlo, P.; Musuraca, G.; Micucci, G.; Lucchesi, A. Circulating Endothelial Cell Levels Correlate with Treatment Outcomes of Splanchnic Vein Thrombosis in Patients with Chronic Myeloproliferative Neoplasms. J. Pers. Med. 2022, 12, 364. https://doi.org/10.3390/jpm12030364
Giordano G, Napolitano M, Cellurale M, Di Carlo P, Musuraca G, Micucci G, Lucchesi A. Circulating Endothelial Cell Levels Correlate with Treatment Outcomes of Splanchnic Vein Thrombosis in Patients with Chronic Myeloproliferative Neoplasms. Journal of Personalized Medicine. 2022; 12(3):364. https://doi.org/10.3390/jpm12030364
Chicago/Turabian StyleGiordano, Giulio, Mariasanta Napolitano, Michele Cellurale, Paola Di Carlo, Gerardo Musuraca, Giorgia Micucci, and Alessandro Lucchesi. 2022. "Circulating Endothelial Cell Levels Correlate with Treatment Outcomes of Splanchnic Vein Thrombosis in Patients with Chronic Myeloproliferative Neoplasms" Journal of Personalized Medicine 12, no. 3: 364. https://doi.org/10.3390/jpm12030364
APA StyleGiordano, G., Napolitano, M., Cellurale, M., Di Carlo, P., Musuraca, G., Micucci, G., & Lucchesi, A. (2022). Circulating Endothelial Cell Levels Correlate with Treatment Outcomes of Splanchnic Vein Thrombosis in Patients with Chronic Myeloproliferative Neoplasms. Journal of Personalized Medicine, 12(3), 364. https://doi.org/10.3390/jpm12030364










